What is cellulose?
- Cellulose, a straight-chain polysaccharide made up of thousands of β-1,4-linked D-glucose residues, is commonly called the stiffest carbohydrate on Earth because those chains, which are packed side by side, won’t bend or coil.
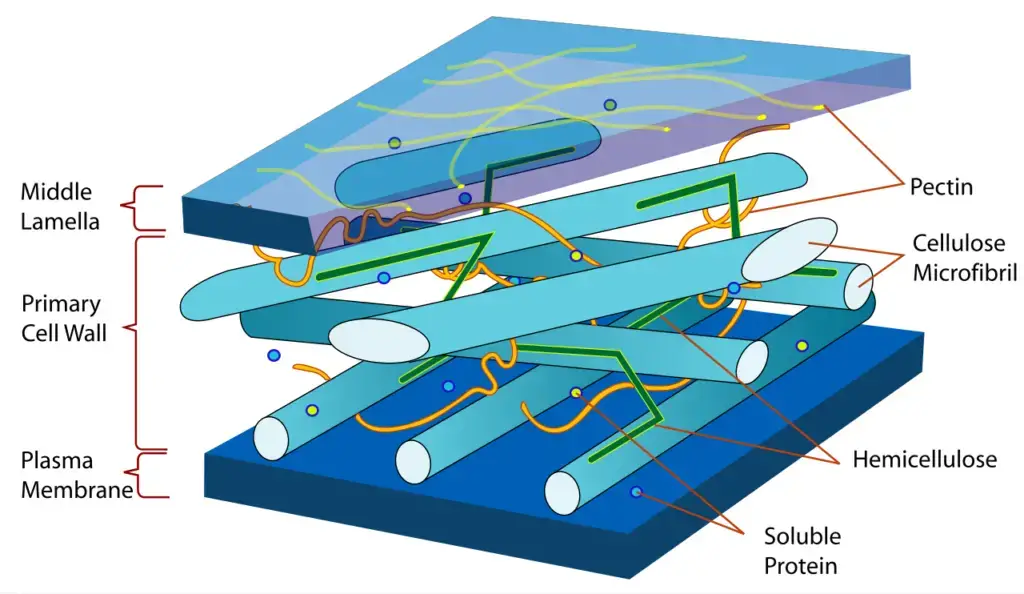
- This polymer makes up about a third of all plant matter and is the load-bearing scaffold inside almost every plant cell wall. It makes up about 90% of fluffy cotton fibers and half of the dry weight of woody tissue, so it’s pretty much everywhere green.
- Not just plants manufacture it; certain bacteria, some fungi, and even some algae quietly make cellulose as well. This shows that biosynthesis isn’t just for plants.
- The rods are very straight, and tight hydrogen bonds link them together into microfibrils that don’t absorb water, most solvents, or your digestive enzymes. This is why you can’t break it down without help from specialist cellulases.
- When you chew those veggies, the same indigestible chains go through your gut as insoluble fiber. This adds bulk, speeds up transit, and, to be honest, keeps things regular even though your own enzymes can’t break those tough β bonds.
- Cellulose is cheap, renewable, and surprisingly flexible. It can be pulped into paper, spun into rayon or lyocell, changed into cellulose acetate plastics, or ground up to make processed foods thicker without adding calories. That’s why it’s in everything from textbooks to toothpaste.
| (C6H10O5)n | Cellulose |
| Molecular Weight/ Molar Mass | 162.1406 g/mol |
| Density | 1.5 g/cm³ |
| Appears | White powder |
| Melting Point | 260–270 °C |
Properties of cellulose
- Chemical Structure: Cellulose is a straight-chain polymer made up of β-D-glucose units that are linked by β-1,4-glycosidic linkages.
- Degree of Polymerization: The degree of polymerization (DP) differs. For example, cotton and bacterial cellulose have DP values between 800 and 10,000, while wood pulp cellulose has DP values between 300 and 1700.
- Crystallinity: Cellulose has a crystalline structure, which makes it strong and resistant to breaking down.
- Melting Point: Because cellulose has a crystalline structure, it breaks down instead of melting at temperatures between 320 and 350°C.
- Density: Cellulose has a density of 1.5 to 1.6 g/cm³, which shows that its molecules are tightly packed.
- Solubility: Cellulose doesn’t dissolve in water or most organic solvents, although it does dissolve in some ionic liquids and alkaline solutions.
- Hydrophilicity: Cellulose has hydroxyl groups that make it hydrophilic, which means it can form hydrogen bonds with water molecules.
- Mechanical Strength: Cellulose fibers have a lot of hydrogen bonds between chains, which gives them tensile strength that is similar to steel.
- Biodegradability: Cellulose can be broken down by cellulase enzymes made by some microbes.
- Insolubility in Digestive Systems: Humans don’t have the enzymes needed to break down cellulose, hence it’s an indigestible fiber.
- Occurrence: Cellulose is the most common organic compound on Earth. It is mostly found in plant cell walls, and cotton has roughly 90% cellulose.
- Industrial Uses—Cellulose is used to make paper, textiles, medicines, and as a food ingredient because it has a lot of fiber in it.
Structure of cellulose
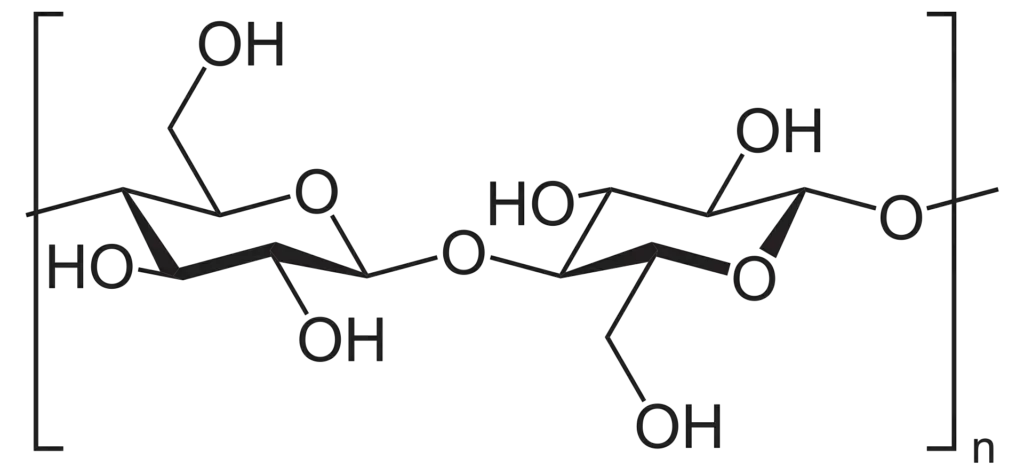
- A linear β-(1→4)-D-glucan chain makes up cellulose. It has repeating β-D-glucopyranose residues that are linked head-to-tail by β-1,4 glycosidic bonds. Every other glucose is flipped 180°, thus a cellobiose unit repeats along a very straight backbone that doesn’t bend.
- Continuous O3-H···O5 and O2-H···O6 hydrogen bridges run along each ribbon-like strand and hold the shape in place. This makes the chain much stiffer along its axis, which makes it harder to twist.
- Interchain hydrogen bonds and van-der-Waals contacts hold together roughly thirty-six parallel chains that are lined up next to each other. This makes elementary microfibrils that are about 3–4 nm thick and already act like nanocables.
- Inside each microfibril, chains pack into triclinic Iα or monoclinic Iβ lattices. The layers change by ±c/4 along the molecular axis. Algae have more Iα, whereas higher plants have more Iβ, which is a small but important difference.
- Crystalline blocks that are tens of nanometers long are broken up by disordered amorphous stretches that cluster around fibril surfaces. This makes it easier for chemicals and enzymes to attack those looser, more accessible areas.
- The chain lengths in bacterial cellulose are about 300 sugar units long, whereas in cotton they can be more than 10,000 sugar units long. This means that one molecule can go through numerous crystalline domains before it stops, giving it a lot of tensile strength.
- When elementary fibrils come together to form macrofibrils, they may twist and cross each other and then embed themselves in hemicellulose-lignin matrices. This makes the macrofibrils layer differentially between wall lamellae, which gives them anisotropic mechanical behavior.
- There are a lot of C-H···O interactions and dispersion forces that push glucan backbones together, making it hard for water to get in. This is why cellulose doesn’t dissolve in most solvents.
- Packing changes—alkali mercerization or regeneration turns chains into antiparallel cellulose II, whereas liquid-ammonia treatments turn chains into cellulose III. This shows that packing can change without severing the β-1,4 spine.
- Plant cell walls get amazing tensile strength, low stretchability, and durability from stiff chains that group together to create crystalline nanocables that weave into macroscale networks. These are the same properties that make wood and fiber strong.
What are cellulases?
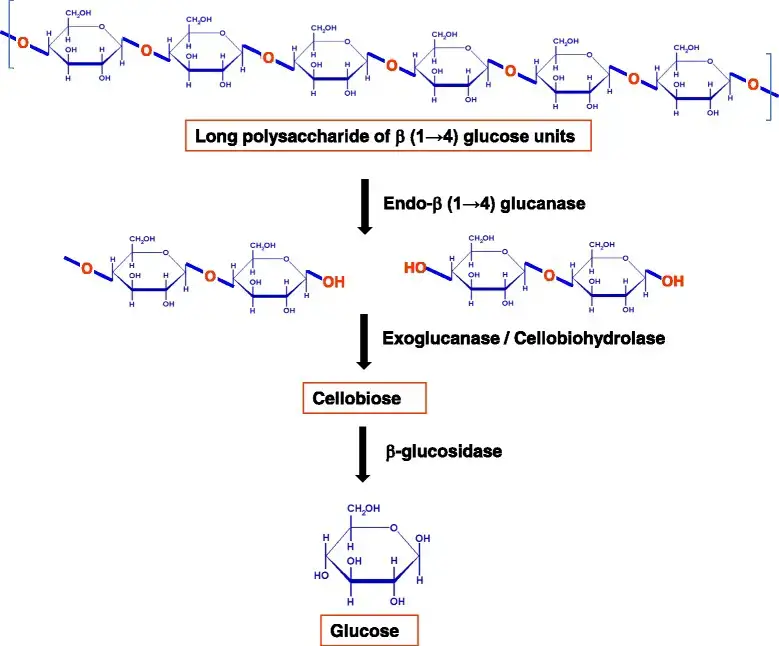
Cellulases are a group of enzymes that catalyze the hydrolysis of cellulose, a complex polysaccharide present in plant cell walls. These enzymes are largely produced by fungus, bacteria, and certain animals, including termites and some mollusks, which rely on them to digest plant materials. In industrial applications, cellulases are utilized to break down cellulose into simpler sugars like glucose, aiding operations such as biofuel manufacturing, textile finishing, and food processing. The cellulase enzyme complex normally comprises three main types: endoglucanases, which cleave internal bonds inside the cellulose chain; exoglucanases, which remove cellobiose units from the chain ends; and β-glucosidases, which hydrolyze cellobiose into glucose molecules. This coordinated activity permits efficient decomposition of cellulose, making cellulases crucial in both natural ecosystems and numerous industrial processes.
Factors affecting cellulose degradation
- Temperature – Elevated temperatures enhance microbial and enzymatic activities, thereby accelerating cellulose degradation. However, extreme heat can denature enzymes, halting the process.
- Moisture Content – Adequate moisture is crucial for microbial metabolism and enzyme function; excessive moisture may lead to anaerobic conditions, slowing degradation.
- pH Levels – Optimal pH ranges between 6 and 7 favor cellulase activity; deviations can reduce enzyme efficiency and microbial growth.
- Aeration – Sufficient oxygen levels support aerobic microbes that produce cellulases, while low oxygen can hinder the degradation process.
- Nutrient Availability – The presence of nitrogen and other nutrients can stimulate microbial growth and cellulase production, enhancing cellulose breakdown.
- Lignin Content – High lignin concentrations in plant material act as a physical barrier, making cellulose less accessible to degrading enzymes.
- Enzyme Concentration – The abundance of cellulases directly correlates with the rate of cellulose degradation; higher enzyme levels accelerate the process.
- Microbial Community Composition – Diverse microbial populations can synergistically degrade cellulose more efficiently than monocultures.
- Chemical Inhibitors – Presence of toxic substances or pollutants can inhibit microbial activity and enzyme function, slowing degradation.
- Substrate Accessibility – The physical structure of cellulose, such as crystallinity and particle size, affects enzyme access and degradation rates.
Microorganisms involved in cellulose degradation/cellulolytic microorganisms
Cellulolytic microorganisms are diverse groups of bacteria, fungi, and other microbes capable of degrading cellulose into simpler sugars through enzymatic activity. These organisms play a crucial role in the carbon cycle by breaking down plant biomass in various environments.
Bacteria:
- Bacillus species – Commonly found in soil, they produce extracellular cellulases and are utilized in industrial applications.
- Pseudomonas species – Known for their versatility, some strains can degrade cellulose under aerobic conditions.
- Cellulomonas species – Gram-positive bacteria that secrete cellulases, aiding in cellulose degradation.
- Clostridium species – Anaerobic bacteria like Clostridium cellulovorans and Clostridium phytofermentans possess cellulosomes, complex enzyme systems that efficiently break down cellulose.
- Ruminococcus species – These bacteria are predominant in the rumen of herbivores, specializing in cellulose degradation.
- Fibrobacter species – Anaerobic bacteria found in the intestines of herbivores, contributing significantly to cellulose breakdown
Fungi:
- Trichoderma reesei – A filamentous fungus renowned for its high cellulase production, widely used in industrial applications.
- Myceliophthora thermophila – A thermophilic fungus that produces thermostable enzymes, beneficial for industrial processes requiring high temperatures.
- Aspergillus species – These fungi secrete cellulases and are involved in decomposing plant materials in various ecosystems.
- Penicillium species – Known for producing cellulolytic enzymes, aiding in the breakdown of cellulose in organic matter.
Other Microorganisms:
- Termites – These insects harbor a complex microbiota in their gut, including bacteria and protozoa, that assist in cellulose digestion.
- Earthworms – Their digestive systems contain cellulolytic microorganisms that help decompose plant material in soil.
- Ruminants – Animals like cows and sheep rely on a consortium of microorganisms in their rumen to digest cellulose.
Enzymes involved in the degradation of cellulose
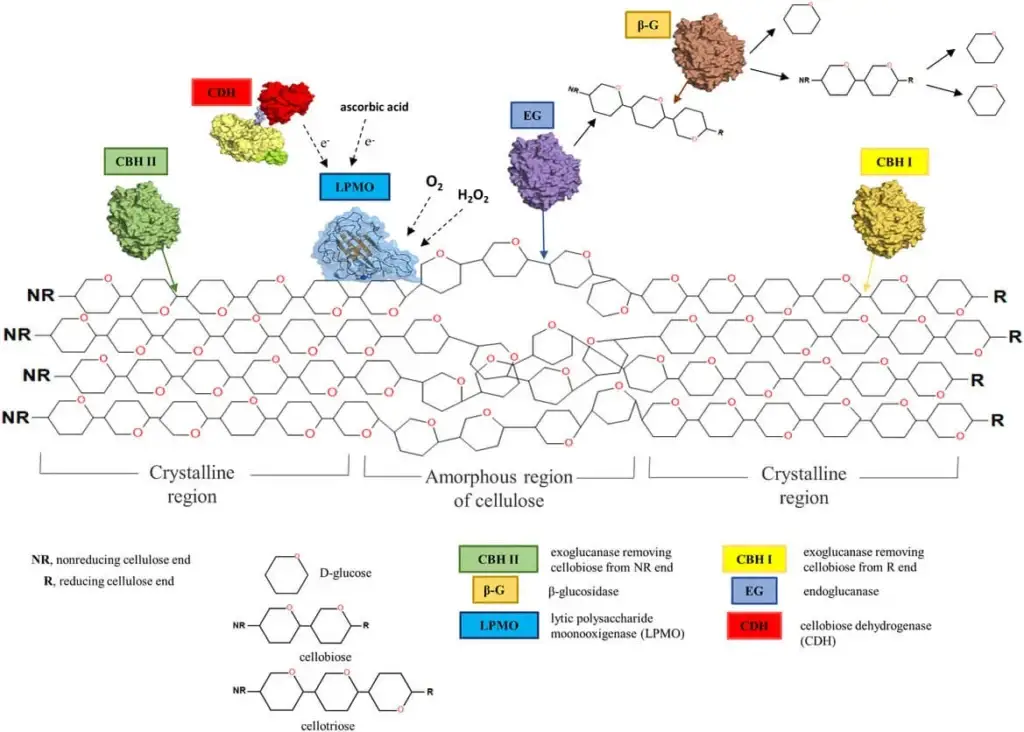
- Endoglucanases (EGs) – These enzymes cleave internal β-1,4-glycosidic bonds within the cellulose chain, producing oligosaccharides. They act randomly along the cellulose molecule, creating new chain ends for further degradation.
- Exoglucanases (Cellobiohydrolases, CBHs) – These enzymes hydrolyze cellobiose units from the non-reducing or reducing ends of cellulose chains. They play a crucial role in breaking down crystalline cellulose.
- β-Glucosidases – These enzymes hydrolyze cellobiose into two glucose molecules. They are essential for preventing the accumulation of cellobiose, which can inhibit other cellulases.
- Lytic Polysaccharide Monooxygenases (LPMOs) – These enzymes introduce oxidative cleavage into cellulose chains, enhancing the accessibility of cellulose to hydrolytic enzymes. They require electron donors and metal ions for activity.
- Feruloyl Esterases – These enzymes hydrolyze ester bonds between ferulic acid and polysaccharides, disrupting the lignocellulosic matrix and facilitating cellulose degradation.
- Cellobiose Dehydrogenases (CDHs) – These enzymes oxidize cellobiose to cellobionic acid, generating reducing equivalents that can enhance the activity of other cellulases. Wikipedia
- Cellulosomes – These are multi-enzyme complexes found in certain anaerobic bacteria. They contain various cellulases and accessory proteins, working synergistically to degrade cellulose efficiently.
- Processive Endoglucanases – These enzymes can degrade cellulose in a continuous manner without releasing the substrate, increasing the efficiency of cellulose breakdown.
- Hemicellulases – These enzymes degrade hemicellulose, a component of plant cell walls, which is often associated with cellulose. Their activity can indirectly enhance cellulose degradation by disrupting the cell wall structure.
- Xylanases – These enzymes break down xylan, a major hemicellulose component, into xylose. Their action can facilitate the removal of hemicellulose, making cellulose more accessible to other degradative enzymes.
Mechanism of cellulose decomposition
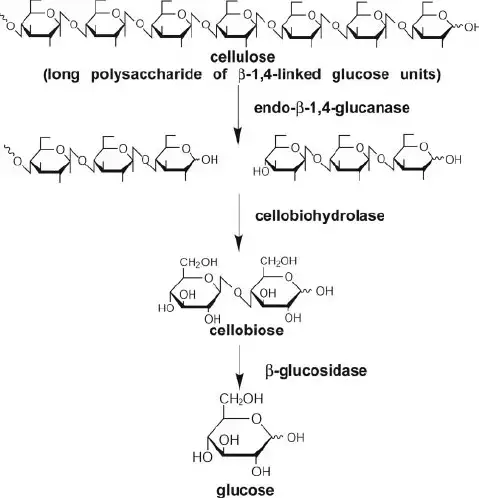
- Endoglucanases (EGs) – These enzymes cleave internal β-1,4-glycosidic bonds within the cellulose chain, producing oligosaccharides. They act randomly along the cellulose molecule, creating new chain ends for further degradation.
- Exoglucanases (Cellobiohydrolases, CBHs) – These enzymes hydrolyze cellobiose units from the non-reducing or reducing ends of cellulose chains. They play a crucial role in breaking down crystalline cellulose.
- β-Glucosidases – These enzymes hydrolyze cellobiose into two glucose molecules. They are essential for preventing the accumulation of cellobiose, which can inhibit other cellulases.
- Lytic Polysaccharide Monooxygenases (LPMOs) – These enzymes introduce oxidative cleavage into cellulose chains, enhancing the accessibility of cellulose to hydrolytic enzymes. They require electron donors and metal ions for activity.
- Feruloyl Esterases – These enzymes hydrolyze ester bonds between ferulic acid and polysaccharides, disrupting the lignocellulosic matrix and facilitating cellulose degradation.
- Cellobiose Dehydrogenases (CDHs) – These enzymes oxidize cellobiose to cellobionic acid, generating reducing equivalents that can enhance the activity of other cellulases.
- Cellulosomes – These are multi-enzyme complexes found in certain anaerobic bacteria. They contain various cellulases and accessory proteins, working synergistically to degrade cellulose efficiently.
- Processive Endoglucanases – These enzymes can degrade cellulose in a continuous manner without releasing the substrate, increasing the efficiency of cellulose breakdown. Microbe Notes
- Hemicellulases – These enzymes degrade hemicellulose, a component of plant cell walls, which is often associated with cellulose. Their activity can indirectly enhance cellulose degradation by disrupting the cell wall structure.
- Xylanases – These enzymes break down xylan, a major hemicellulose component, into xylose. Their action can facilitate the removal of hemicellulose, making cellulose more accessible to other degradative enzymes.
Simple Steps of cellulose degradation
Step 1 – Random cleavage by endoglucanases
- Endoglucanases first attack internal β-1,4-glycosidic bonds within cellulose chains, randomly breaking down long fibrils into shorter fragments.
- This internal hydrolysis lowers the polymer size, producing oligosaccharides and exposing chain ends for further action.
Step 2 – Progressive trimming by exoglucanases
- Exoglucanases then act on the newly exposed ends of the cellulose fragments, mainly the reducing ends.
- These enzymes remove cellobiose (a disaccharide) or tetrasaccharide units sequentially from the chain ends.
Step 3 – Final breakdown by β-glucosidase
- β-glucosidase hydrolyzes cellobiose or small sugar units into glucose monomers.
- This terminal hydrolysis step completes cellulose degradation, releasing simple, fermentable glucose.
Hydrolytic Mechanism of cellulose degradation
- General Acid/Base Catalysis by Carboxylate Pair
- Cellulose hydrolysis by glycosyl hydrolases involves general acid/base catalysis, requiring two critical residues: a proton donor (HA) and a nucleophile/base (B−).
- These catalytic activities are typically provided by two aspartic or glutamic acid residues within the enzyme’s active site.
- The proton donor protonates the glycosidic oxygen, while the nucleophile/base facilitates the departure of the leaving group and the attack by water.
- Inverting Mechanism
- In this mechanism, the enzyme’s active site features two carboxylate residues positioned approximately 10 Å apart.
- One residue acts as a general acid, protonating the glycosidic oxygen, while the other serves as a nucleophile/base.
- A water molecule attacks the anomeric carbon in an SN2-type displacement reaction, leading to inversion of the configuration at the anomeric carbon (C1).
- This mechanism results in the formation of a product with an inverted stereochemistry compared to the substrate.
- Retaining Mechanism
- Enzymes utilizing this mechanism also possess two carboxylate residues, but they are situated about 5 Å apart.
- The first step involves the nucleophilic attack on the anomeric carbon, forming a covalent glycosyl-enzyme intermediate.
- The second step entails hydrolysis of this intermediate by water, with the general acid/base residue facilitating the departure of the leaving group and the attack by water.
- This two-step process leads to retention of the anomeric configuration in the product.
- Glycosidase Mechanism (GH4 Enzymes)
- A fundamentally different mechanism is observed in NAD+ and divalent metal ion-dependent GH4 glycosidases.
- The process begins with hydride abstraction at C3, generating a ketone intermediate.
- Deprotonation of C2 occurs, accompanied by acid-catalyzed elimination of the glycosidic oxygen, forming a 1,2-unsaturated intermediate.
- This intermediate undergoes base-catalyzed attack by water, resulting in the formation of a 3-keto derivative.
- The final step involves reduction by NADH, completing the reaction cycle.
Example of Hydrolytic Mechanism
- Trichoderma reesei
- Trichoderma reesei, a mesophilic filamentous fungus, is renowned for its substantial cellulase production, including endoglucanases (EGI, EGII, EGIII, EGIV, EGV), exoglucanases (CBHI, CBHII), and β-glucosidases (BGLI, BGLII).
- These enzymes synergistically degrade cellulose by cleaving β-1,4-glycosidic bonds, facilitating the conversion of complex polysaccharides into fermentable sugars like glucose.
- The cellulase system of T. reesei has been extensively studied for its efficiency in industrial applications, such as biofuel production and textile processing.
- Clostridium thermocellum
- Clostridium thermocellum employs a complexed cellulase system known as the cellulosome, which is anchored to the bacterial cell surface.
- The cellulosome comprises various cellulases and related enzymes, including endoglucanases and exoglucanases, that work synergistically to degrade cellulose into simpler sugars.
- This system is particularly effective in anaerobic conditions, making C. thermocellum a subject of interest for biofuel production processes.
- Cellulomonas fimi
- Cellulomonas fimi, an aerobic bacterium, produces a cellulase system similar to that of T. reesei, including endoglucanases, exoglucanases, and β-glucosidases.
- These enzymes act in concert to hydrolyze cellulose, initiating the breakdown at amorphous regions and progressing to crystalline areas, thereby enhancing the efficiency of cellulose degradation.
- The cellulolytic capabilities of C. fimi are of interest for industrial applications involving cellulose-rich substrates.
- Aspergillus niger
- Aspergillus niger, a filamentous fungus, produces a range of cellulolytic enzymes, including endoglucanases, exoglucanases, and β-glucosidases.
- These enzymes function synergistically to degrade cellulose, with endoglucanases initiating the breakdown by cleaving internal β-1,4-glycosidic bonds, followed by exoglucanases and β-glucosidases acting on the resulting oligosaccharides.
- A. niger’s cellulolytic enzymes are utilized in various industrial processes, such as the production of biofuels and the treatment of cellulose-containing waste.
- Fusarium oxysporum
- Fusarium oxysporum, a soil-borne fungus, produces cellulolytic enzymes that contribute to the degradation of cellulose in the environment.
- These enzymes include endoglucanases and exoglucanases, which work together to break down cellulose into simpler sugars, facilitating nutrient cycling in soil ecosystems.
- The cellulolytic activity of F. oxysporum plays a role in the decomposition of plant materials, contributing to soil health and fertility.
- Neurospora crassa
- Neurospora crassa, a model organism in fungal biology, produces cellulolytic enzymes that degrade cellulose into glucose.
- The enzymes involved include endoglucanases, exoglucanases, and β-glucosidases, which act sequentially to hydrolyze cellulose.
- N. crassa’s cellulolytic system has been extensively studied for understanding the molecular mechanisms of cellulose degradation and for potential applications in biofuel production.
Oxidative Mechanism of cellulose degradation
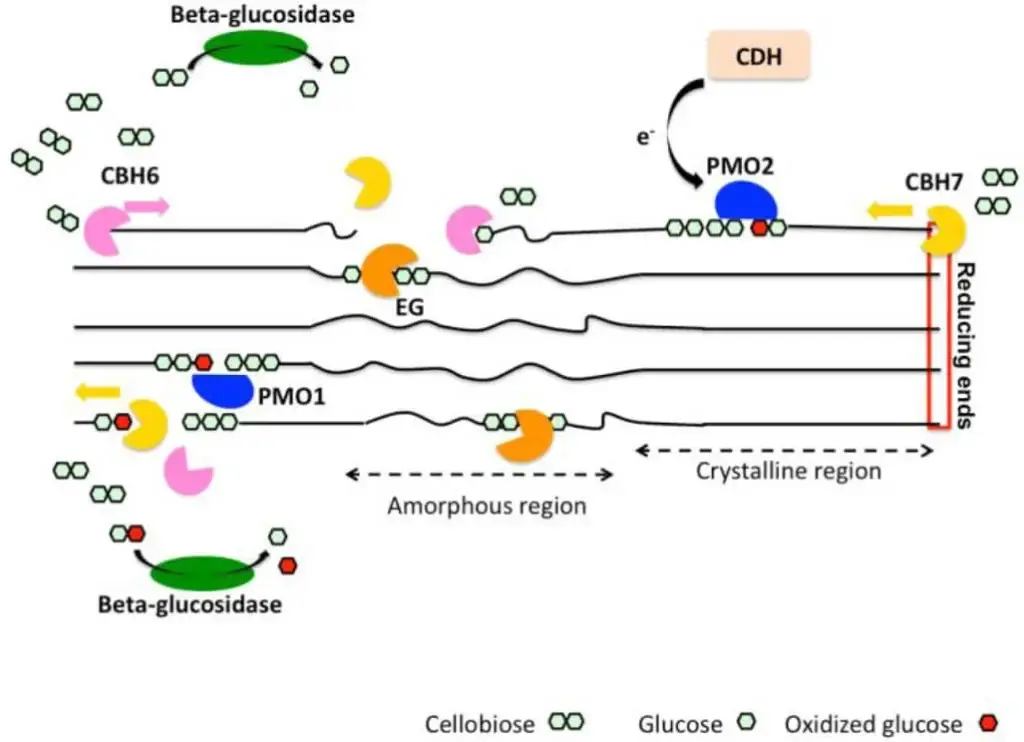
Role of Cellobiose Dehydrogenase (CDH)
CDH is a flavocytochrome enzyme that is very important for breaking down cellulose by oxidizing cellobiose to cellobionolactone.
This oxidation happens by a “ping-pong” process that uses electron acceptors such quinones, chelated Fe(III), O₂ (which makes H₂O₂), and phenoxyl radicals.
The enzyme has two parts: a dehydrogenase domain that binds to flavin adenine dinucleotide (FAD) and a cytochrome domain that contains heme. These two parts help electrons move between them.
CDH also stops cellulose chains from re-condensing by oxidizing the free ends that endo-acting cellulases make.
CDH removes cellobiose, which is a strong inhibitor of cellulases. This makes the product less likely to inhibit cellulases, which increases their overall activity.
Also, CDH can change Fe(III) to Fe(II) and O₂ to H₂O₂, starting a Fenton-type reaction that makes hydroxyl radicals (·OH), which can break down or change cellulose.
Fungal Examples Exhibiting Oxidative Mechanism
Phanerochaete chrysosporium
- This white-rot basidiomycete produces CDH, which is essential for efficient cellulose degradation.
- CDH is secreted into the growth medium and binds strongly to cellulose, facilitating oxidative cleavage of cellulose chains.
- The enzyme’s activity is upregulated in the presence of cellulose, highlighting its role in lignocellulose degradation.
- CDH’s oxidative actions complement the hydrolytic activities of cellulases, enhancing the overall efficiency of cellulose breakdown.
Sporotrichum thermophile
- Formerly classified as Chrysosporium thermophilum, this thermophilic fungus produces CDH and other oxidative enzymes.
- The enzyme’s activity is optimal at elevated temperatures, aligning with the organism’s thermophilic nature.
- CDH contributes to the degradation of cellulose by generating reactive oxygen species that cleave cellulose fibers.
Poria placenta
- This brown-rot basidiomycete employs an oxidative mechanism for cellulose degradation.
- The fungus produces laccase, a copper-containing enzyme that oxidizes phenolic compounds, generating reactive oxygen species.
- These reactive species facilitate the breakdown of cellulose and lignin in the wood matrix.
Lenzites trabea
- Another white-rot basidiomycete, L. trabea produces CDH and laccase.
- The synergistic action of these enzymes leads to efficient degradation of cellulose and lignin in wood substrates.
- The oxidative cleavage of cellulose by CDH is complemented by the oxidative modification of lignin by laccase, enhancing the overall degradation process.
Commercial applications of cellulose
- Food Industry – Cellulose serves as a bulking agent, dietary fiber supplement, thickener, emulsifier, and anti-caking agent in various food products, enhancing texture and stability without adding calories.
- Pharmaceuticals – Microcrystalline cellulose (MCC) and hydroxypropyl cellulose (HPC) function as binders, disintegrants, and stabilizers in tablet formulations, improving drug delivery and stability.
- Cosmetics and Personal Care – Cellulose derivatives like hydroxyethyl cellulose (HEC) are utilized as thickeners, film-formers, and stabilizers in lotions, shampoos, and other personal care products.
- Textiles – Regenerated cellulose fibers such as lyocell and viscose are employed in clothing and home textiles, offering biodegradable alternatives to synthetic fibers.
- Paper and Packaging – Cellulose is the primary material in paper, cardboard, and biodegradable packaging, providing strength and recyclability.
- Building Materials – Cellulose insulation, derived from recycled paper, offers eco-friendly thermal and acoustic insulation properties in construction.
- Electronics – Cellulose nanocrystals are explored for use in flexible electronics, sensors, and displays due to their mechanical strength and optical properties.
- Energy – Cellulose is a potential source for biofuels, such as cellulosic ethanol, contributing to renewable energy solutions.
- Aerospace – Cellulose-based composites are investigated for lightweight, strong materials in aircraft construction, aiming to reduce fuel consumption.
- Medical Applications – Bacterial cellulose is utilized in wound dressings and tissue engineering due to its biocompatibility and structural properties.
- Nanotechnology – Cellulose nanomaterials are developed for use in hydrogels, aerogels, and as stabilizers in various industrial applications.
- Welding – Cellulose electrodes are used in welding for deep penetration and strong arc stability, particularly in vertical-down positions.
- Water Treatment – Modified cellulose serves as an effective adsorbent in water purification processes, removing contaminants efficiently.
- Antimicrobial Coatings – Cellulose-based thin films are developed for antimicrobial surfaces, offering sustainable and effective pathogen control.
- Optical Filters – Cellulose nanocrystals are used to create reflective films for optical filtering and solar gain regulation in building materials.
- Sustainable Packaging – Cellophane, a biodegradable film made from cellulose, is used in packaging, offering an eco-friendly alternative to plastic.
- Food Additives – Cellulose derivatives like carboxymethyl cellulose (CMC) are employed as emulsifiers, stabilizers, and thickeners in processed foods.
- Construction Chemicals – Hydroxyethyl methyl cellulose (HEMC) and hydroxypropyl methyl cellulose (HPMC) are used to enhance water retention and workability in cement-based materials.
- Biodegradable Films – Cellulose-based films are developed for use in biodegradable packaging, reducing environmental impact.
- Recycled Materials – Cellulose from recycled paper is utilized in various applications, promoting sustainability and reducing waste.
- Hydrogels – Cellulose-based hydrogels are used in drug delivery systems, wound care, and tissue engineering due to their biocompatibility and water retention properties.
- Smart Textiles – Cellulose nanocrystals are incorporated into textiles to create fabrics with responsive properties, such as moisture sensing.
- Food Packaging – Cellulose films are used in food packaging to provide a biodegradable alternative to plastic wraps.
- Environmental Applications – Cellulose-based materials are developed for use in environmental applications, such as water filtration and pollution control.
- Advanced Composites – Cellulose nanocrystals are used to reinforce composites, enhancing their mechanical properties for various industrial applications.
- Medical Devices – Bacterial cellulose is utilized in the production of medical devices like artificial skin and wound dressings due to its high purity and biocompatibility.
- Biodegradable Plastics – Cellulose is used as a raw material in the production of biodegradable plastics, offering an alternative to petroleum-based plastics.
- Textile Recycling – Cellulose from recycled textiles is processed into new fibers, promoting circular economy practices in the fashion industry.
- Food Processing – Cellulose derivatives are used in food processing to improve texture, stability, and shelf life of food products.
- Agricultural Products – Cellulose is used in the production of agricultural products like pesticides and fertilizers, enhancing their effectiveness and sustainability.
- Water-Based Lubricants – Hydroxyethyl cellulose is used in the formulation of water-based lubricants, offering environmental benefits over petroleum-based alternatives.
- Adhesives – Cellulose derivatives are used in the production of adhesives for various applications, providing strong bonding properties.
- Paints and Coatings – Cellulose derivatives are used in paints and coatings to improve viscosity, stability, and application properties.
- Ceramics – Cellulose derivatives are used in ceramics to enhance processing and final product properties.
- Inks – Cellulose derivatives are used in ink formulations to improve flow and stability.
- Pencils – Cellulose derivatives are used in the production of pencils, contributing to their hardness and writing properties.
- Joint Fillers – Cellulose derivatives are used in joint fillers to improve workability and adhesion.
- Welding Rods – Cellulose is used in the production of welding rods, providing deep penetration and strong arc stability.
- Drilling Fluids – Hydroxyethyl cellulose is used in drilling fluids to improve viscosity and stability in oil and gas operations.
- Personal Lubricants – Hydroxyethyl cellulose is used in personal lubricants to provide smooth application and stability.
- Soap Bubbles – Hydroxyethyl cellulose is used in the production of soap bubbles, enhancing their stability and size.
- Slime – Hydroxyethyl cellulose is used in the production of slime, contributing to its texture and stretchability.
- Paint Thickeners – Hydroxyethyl cellulose is used as a thickener in paint formulations, improving application properties.
- Emulsion Polymerization – Hydroxyethyl cellulose is used in emulsion polymerization processes to stabilize emulsions.
- Surface Coatings – Hydroxyethyl cellulose is used in surface coatings to improve adhesion and durability.
- Adhesive Formulations – Hydroxyethyl cellulose is used in adhesive formulations to enhance bonding strength and stability.
- Pulp and Paper Industry – Cellulose is the primary raw material in the pulp and paper industry, forming the basis of paper products.
- Textile Industry – Cellulose is used in the textile industry to produce fibers and fabrics.
- Packaging Industry – Cellulose is used in the packaging industry to produce biodegradable and recyclable materials.
- Construction Industry – Cellulose is used in the construction industry to produce insulation materials and additives.
- Chemical Industry – Cellulose is used in the chemical industry to produce various derivatives and compounds.
- Energy Sector – Cellulose is used in the energy sector to produce biofuels and other energy sources.
- Aerospace Industry – Cellulose is used in the aerospace industry to produce lightweight and strong materials.
- Medical Industry – Cellulose is used in the medical industry to produce various devices and materials.
- Nanotechnology – Cellulose is used in nanotechnology to produce nanomaterials with unique properties.
Biotechnological Applications of Cellulose Degradation
- Cellulases are important for making biofuels like bioethanol because they turn lignocellulosic material into fermentable sugars.
- Cellulases are used in the textile industry to bioston denim, make fabric softer, and make colors brighter.
- Cellulases help deink recycled paper and make the fibers stick together better, which makes the paper better quality.
- Detergent formulation, cellulases are added to laundry detergents to get rid of stains and make fabrics seem brighter again.
- Agriculture: Cellulases are used to break down plant debris and improve soil quality by speeding up the composting process.
- Animal feed, cellulases are added to animal feed to make it easier for them to digest fibrous plant elements.
- In medicine, cellulases are used to treat phytobezoars and to break down biofilms in wounds.
- Bioremediation, Cellulases help break down cellulose in polluted areas, which helps manage pollution.
- Bio-based materials, such aerogels made from cellulose, are used in a number of ways, including as insulation.
- Synthetic biology is the field of study that creates genetically altered microbes that make cellulases with better qualities for use in industry.
FAQ
What is microbial degradation of cellulose?
Microbial degradation of cellulose is the process by which microorganisms break down cellulose fibers into simpler compounds, such as glucose and other sugars, through the action of enzymes.
Which microorganisms are involved in the degradation of cellulose?
A wide range of microorganisms, including bacteria, fungi, and protozoa, are capable of degrading cellulose.
What are the enzymes involved in the degradation of cellulose?
The primary enzymes involved in the degradation of cellulose are endoglucanases, exoglucanases, and cellobiohydrolases, which break down cellulose into smaller oligosaccharides, and β-glucosidases, which hydrolyze these oligosaccharides into glucose.
How does microbial degradation of cellulose affect the environment?
Microbial degradation of cellulose is an important process in the carbon cycle, as it helps to release carbon trapped in cellulose fibers back into the ecosystem.
What are the applications of microbial degradation of cellulose?
Microbial degradation of cellulose has a wide range of applications, including in the production of biofuels, biodegradation of agricultural and industrial waste, and the production of paper and textiles.
How is microbial degradation of cellulose measured?
Cellulose degradation can be measured using a variety of methods, including gravimetric analysis, spectrophotometric analysis, gas chromatography, and nucleic acid sequencing.
What factors affect the rate of microbial degradation of cellulose?
Factors that affect the rate of microbial degradation of cellulose include temperature, pH, oxygen concentration, and the presence of inhibitors or other substances that may interfere with the degradation process.
What is the role of microbial consortia in cellulose degradation?
Microbial consortia, or communities of microorganisms working together, can increase the rate of cellulose degradation by providing a range of enzymes and metabolic pathways.
What are some challenges in the microbial degradation of cellulose?
Some challenges in the microbial degradation of cellulose include slow rates of degradation, susceptibility to inhibition by toxic substances, and the limited range of microorganisms capable of degrading cellulose.
How can the efficiency of microbial cellulose degradation be improved?
Efforts to improve the efficiency of microbial cellulose degradation include the identification and selection of cellulose-degrading microorganisms, optimization of environmental conditions, and genetic engineering of microorganisms to enhance their cellulose-degrading capabilities.
What is cellulose and why is its degradation important?
Cellulose is the most abundant organic compound on Earth, a complex linear polysaccharide primarily found in plant cell walls. It’s composed of thousands of β-1,4-linked D-glucose residues, forming strong, stiff chains packed into microfibrils. This crystalline structure makes cellulose resistant to degradation by most solvents and enzymes, including those in the human digestive system.
The degradation of cellulose is crucial for several reasons:
Carbon Cycle: It is a vital process in the global carbon cycle, releasing carbon stored in plant biomass back into the ecosystem.
Nutrient Cycling: In natural environments like soil and the guts of herbivores, its breakdown by microorganisms recycles nutrients.
Industrial Applications: Its degradation into simpler sugars (glucose) is a bottleneck in producing sustainable biofuels (like bioethanol) from lignocellulosic biomass. It’s also critical for various applications in the textile, paper and pulp, food and feed, and pharmaceutical industries.
Waste Management: Efficient cellulose degradation can help manage agricultural and industrial cellulosic waste by converting it into valuable products.
Which types of microorganisms are primarily involved in cellulose degradation, and how do their roles differ?
A wide range of microorganisms, including bacteria, fungi, and protozoa, are capable of degrading cellulose. They can be broadly categorized based on their environment and mechanisms:
Bacteria:Aerobic Bacteria: Examples include Bacillus species and Pseudomonas species, which produce extracellular cellulases.
Anaerobic Bacteria: Such as Clostridium species (C. cellulovorans, C. thermocellum) and Ruminococcus species. Many anaerobic bacteria, particularly those in the Clostridia class, produce sophisticated multi-enzyme complexes called cellulosomes for highly efficient degradation of insoluble cellulose. These are often found in environments with decaying plant material, like soils, sediments, aquatic environments, and animal guts (e.g., ruminants, termites, humans).
Fungi:Filamentous Fungi: Including Trichoderma reesei, Aspergillus species, and Penicillium species, known for their high cellulase production. Many are aerobic and secrete enzymes freely.
Wood-Degrading Fungi: These are further classified into:
White-rot fungi (Phanerochaete chrysosporium, Trametes versicolor): Capable of degrading all lignocellulose components (lignin, cellulose, hemicellulose) more efficiently, especially lignin. They employ a diverse set of oxidative enzymes.
Brown-rot fungi (Gloeophyllum trabeum, Postia placenta): Primarily degrade cellulose and hemicellulose, only slightly modifying lignin, often through non-enzymatic Fenton reactions.
Soft-rot fungi (Aspergillus, Neurospora): Mostly ascomycetes that degrade polysaccharides in the surface layers of plants, leading to darkening and softening of wood.
Anaerobic Fungi: Found in herbivore gastrointestinal systems (e.g., Neocallimastigomycetes phylum), they also produce cellulosomes, which can have an even more extensive collection of carbohydrate-active enzymes than bacterial cellulosomes.
The efficiency of degradation often relies on synergistic interactions between different microbial groups and their diverse enzymatic systems.
What are the key enzymes involved in cellulose degradation, and how do they work together?
Cellulose degradation is a complex enzymatic reaction involving several types of enzymes, often acting synergistically:
Cellulases (Glycoside Hydrolases – GHs): These are the primary enzymes that hydrolyze the β-1,4-glycosidic bonds in cellulose. They are typically classified into three major types:
Endoglucanases (EGs) / Carboxymethylcellulases (CMCases): These enzymes randomly cleave internal β-1,4-glycosidic bonds in amorphous (disordered) regions of the cellulose chain. This reduces the polymer size, producing oligosaccharides and creating new chain ends.
Exoglucanases / Cellobiohydrolases (CBHs): These enzymes cleave two to four units (primarily cellobiose) from the ends (reducing or non-reducing) of the cellulose chains exposed by endoglucanases. They are crucial for breaking down crystalline cellulose.
β-Glucosidases (BGLs): These enzymes hydrolyze the cellobiose (a disaccharide) or short oligosaccharides produced by endo- and exoglucanases into individual glucose monomers. This final step is essential as cellobiose can inhibit the activity of other cellulases.
Lytic Polysaccharide Monooxygenases (LPMOs) / Auxiliary Activities (AAs): A more recently discovered class of copper-dependent enzymes that perform oxidative cleavage of cellulose chains. They require electron donors and metal ions. LPMOs significantly enhance the accessibility of crystalline cellulose to hydrolytic cellulases, thereby “boosting” the overall degradation efficiency. They work in synergy with cellobiose dehydrogenases (CDHs).
Accessory Enzymes:Hemicellulases (e.g., Xylanases, β-Mannanases): These enzymes degrade hemicellulose, which often encases cellulose fibers in plant cell walls. By breaking down this protective barrier, they increase the accessibility of cellulose to cellulases.
Cellobiose Dehydrogenases (CDHs): These flavocytochrome enzymes oxidize cellobiose, generating reducing equivalents that can be used by LPMOs and preventing cellobiose inhibition of other cellulases. They can also produce reactive oxygen species that aid in degradation.
Esterases (e.g., Feruloyl Esterases): These break down ester bonds that link hemicellulose and lignin, further loosening the lignocellulosic matrix.
This synergistic action is critical because of cellulose’s compact and recalcitrant structure, requiring a coordinated attack from multiple enzymes with different modes of action.
What is a “cellulosome,” and how does it enhance cellulose degradation efficiency?
A “cellulosome” is a highly efficient, multi-enzyme complex produced primarily by certain anaerobic bacteria (e.g., Clostridium species) and some anaerobic fungi. Unlike freely secreted enzymes that diffuse independently, cellulosomes are typically assembled on the cell surface, tethering multiple lignocellulolytic enzymes together.
Key features and efficiency enhancements of cellulosomes include:
Multi-enzyme Complex: They comprise various carbohydrate-active enzymes (CAZymes), including endoglucanases, exoglucanases, and β-glucosidases, along with accessory proteins.
Scaffolding Structure: Enzymes are attached to a large, non-catalytic “scaffoldin” subunit. This scaffoldin often contains carbohydrate-binding modules (CBMs) that attach the entire complex to the cellulose substrate, ensuring close proximity of the enzymes to their target.
Cohesin-Dockerin Interactions: The complex is assembled through high-affinity interactions between “cohesin” domains on the scaffoldin and “dockerin” domains on the enzymes. These interactions can be species- and type-specific in bacteria, allowing for precise assembly and enzyme composition.
Enhanced Synergism: The spatial proximity of multiple enzymes within the cellulosome promotes highly effective synergistic interactions between the catalytic units. This dramatically increases the overall rate and efficiency of cellulose hydrolysis, with studies showing up to a 50-fold increase compared to free enzymes.
Increased Stability: Anchoring enzymes within the cellulosome can enhance their stability, particularly in challenging environments.
Selective Enzyme Synthesis: Some cellulosome-producing bacteria can vary the enzyme composition of their cellulosomes based on the specific lignocellulosic substrate available, optimizing degradation for different plant materials.
While fungal cellulosomes exist, they are less well-characterized and may exhibit different specificities (e.g., low species-specificity in dockerin-cohesin interactions, potentially broader enzyme repertoires) compared to their bacterial counterparts.
What factors affect the rate of microbial cellulose degradation, and why are some important for industrial applications?
Several factors significantly influence the rate and efficiency of microbial cellulose degradation:
Substrate Characteristics:Lignin Content: Lignin acts as a physical barrier, protecting cellulose from enzymatic access. Higher lignin concentrations make degradation more difficult.
Crystallinity: Cellulose’s highly crystalline regions are resistant to hydrolysis. Amorphous regions are degraded more easily.
Degree of Polymerization (DP): Longer cellulose chains are harder to break down.
Particle Size and Surface Area: Smaller particle size and increased surface area allow for greater enzyme access, accelerating hydrolysis.
Inhibitors: The presence of compounds like cellobiose (product of initial breakdown) can inhibit cellulase activity.
Environmental Conditions:Temperature: Elevated temperatures generally enhance microbial and enzymatic activity, but extreme heat can denature enzymes. Thermostable enzymes are highly desired for industrial applications.
pH Levels: Each enzyme and microbial community has an optimal pH range for activity. Deviations can reduce efficiency.
Moisture Content: Adequate moisture is essential for microbial metabolism and enzyme function; excessive moisture can lead to anaerobic conditions (unless anaerobic microbes are desired).
Aeration (Oxygen Concentration): Crucial for aerobic microbes that produce cellulases; anaerobic conditions suit other microbial groups.
Nutrient Availability: Essential nutrients like nitrogen stimulate microbial growth and cellulase production.
Enzyme and Microbial Community Characteristics:Enzyme Concentration: Higher concentrations of active enzymes directly accelerate degradation.
Microbial Community Composition: Diverse microbial populations, especially those forming consortia, can synergistically degrade cellulose more efficiently by providing a broad range of enzymes and metabolic pathways.
Chemical Inhibitors: Presence of toxic substances or pollutants can inhibit microbial activity and enzyme function.
For industrial applications, optimizing these factors is critical to achieve cost-effective and efficient biomass conversion. For instance, developing thermostable enzymes reduces processing costs, and understanding substrate recalcitrance guides effective pre-treatment strategies.
How is lignocellulose degradation different from general cellulose degradation, and what unique challenges does it present?
Lignocellulose is the complex, composite structure found in plant cell walls, primarily composed of three main polymers: cellulose (60%), hemicellulose (17-32%), and lignin (10-25%). While cellulose degradation focuses specifically on breaking down the cellulose polymer, lignocellulose degradation involves the breakdown of all three interconnected components.
Unique challenges and differences arise due to the presence of hemicellulose and, especially, lignin:
Lignin Recalcitrance: Lignin is a complex, cross-linked phenyl propane polymer that forms an outer barrier, contributing significantly to biomass recalcitrance (resistance to degradation). It physically obstructs enzyme access to cellulose and hemicellulose.
Hemicellulose Interweaving: Hemicellulose, a branched heteropolysaccharide, acts as a linking agent between lignin and cellulose and forms a physical barrier that further obstructs enzyme access to cellulose.
Enzyme Specificity: Lignin degradation requires a distinct set of oxidative enzymes (e.g., laccases, peroxidases like LiP, MnP, VP, DyP) that break down alkyl-aromatic polymers. These are different from the hydrolytic enzymes (cellulases and hemicellulases) needed for carbohydrate breakdown.
Pre-treatment Requirement: Due to the compact and recalcitrant nature of lignocellulose, a pre-treatment step is often necessary before enzymatic hydrolysis to reduce its resistance and make cellulose and hemicellulose more accessible. This can involve thermochemical or physical methods, which contribute significantly to the overall cost of biofuel production.
Synergistic Enzyme Systems: Effective lignocellulose degradation often requires a broader “enzymatic cocktail” that includes enzymes targeting lignin and hemicellulose, in addition to cellulases. The microbial communities involved in lignocellulose degradation (e.g., white-rot fungi) possess these diverse enzyme sets.
Industrial Cost: The high cost of lignocellulosic-degrading enzymes and the need for energy-intensive pre-treatments are major economic barriers to widespread use of plant biomass for biofuels.
In essence, lignocellulose degradation is a more complex and energetically demanding process than cellulose degradation alone because it must overcome the protective ligno-hemicellulose matrix.
What are some current and emerging industrial applications of microbial cellulases?
Microbial cellulases have a wide and growing array of industrial applications, driven by their ability to break down cellulose into valuable products:
Biofuels Production: This is a major application, as cellulases hydrolyze lignocellulosic biomass into fermentable sugars (glucose), which are then converted into bioethanol and other advanced biofuels. This offers a sustainable alternative to fossil fuels.
Textile Industry:Bio-stoning (Stone Washing): Cellulases provide a more environmentally friendly alternative to traditional pumice stones for achieving a “stone-washed” look on denim, without damaging the fabric or machinery.
Bio-polishing: They remove fuzz and pills from fabric surfaces, improving softness, brightness, and appearance.
De-fibrillation: Used for fabrics like Lyocell.
Pulp and Paper Industry:Deinking: Cellulases help remove ink and other contaminants from recycled paper pulp, improving paper brightness and quality.
Pulping and Bleaching: They can enhance these processes, reducing the need for harsh chemicals and saving energy.
Fiber Modification: Improve fiber strength, cleanliness, and overall paper properties.
Laundry and Detergent Industry: Cellulases in detergents remove dirt, restore fabric color, improve brightness, and impart smoothness, offering a “fabric care” benefit.
Food and Feed Industry:Food Processing: Improve stabilization, extraction, and clarification of fruit and vegetable juices, and enhance the texture and stability of various food products (e.g., as thickeners or emulsifiers).
Animal Feed: Supplement animal feed to improve its nutritional value, enhance digestion and absorption of fibrous materials, and promote weight gain.
Agricultural Industries:Disease Control: Degrading the cell walls of plant pathogens.
Crop Improvement: Enhancing seed germination, root development, and overall plant growth.
Soil Quality: Improving soil health, which can reduce reliance on mineral fertilizers.
Waste Management: Converting cellulosic agricultural, forestry, and industrial wastes into valuable products (e.g., food, feed, organic acids, enzymes), contributing to circular economy practices.
Pharmaceutical Applications: Microcrystalline cellulose (MCC) and other derivatives are used as binders, disintegrants, and stabilizers in tablet formulations. Cellulases are also being explored for treating phytobezoars and degrading bacterial biofilms in medical contexts.
Flavonoids Extraction: Assisting in the extraction and modification of beneficial flavonoids from plants.
Ongoing research focuses on enhancing enzyme stability and efficiency to make these applications even more economically viable.
What are the main strategies for improving the efficiency and cost-effectiveness of microbial cellulose degradation for industrial use?
Improving the efficiency and cost-effectiveness of microbial cellulose degradation is crucial for unlocking the full potential of lignocellulosic biomass. Key strategies involve:
Strain Development and Improvement:Discovery of Novel Species and Enzymes: Continuously identifying and characterizing new lignocellulose-degrading microorganisms (bacteria, fungi, and their unique enzymes) from diverse ecosystems (e.g., gut microbiomes, thermophilic environments) that possess superior degradation capabilities.
Genetic Modification (Strain Engineering):Random Mutagenesis: Using chemical mutagens or radiation (UV, gamma-rays) to induce random mutations in microbial genomes, followed by high-throughput screening to select hyper-producing or more efficient strains.
Recombinant DNA Technology: Introducing specific cellulase-producing genes from different sources into host organisms to enhance enzyme yield or introduce desirable traits. This can involve increasing gene copy numbers.
Site-Directed Mutagenesis: Precisely altering specific amino acid sequences in enzymes to improve properties like activity, thermostability, pH profile, or substrate specificity.
Protoplast Fusion: Fusing the protoplasts of two genetically different strains to combine desirable characteristics, such as disease resistance, rapid growth, or enhanced enzyme production.
Optimization of Fermentation Technology:Solid-State Fermentation (SSF): Often preferred for fungal cellulase production as it mimics natural habitats, potentially leading to higher enzyme yields with lower substrate and energy utilization.
Submerged Fermentation (SmF): Allows for easier control of environmental factors (temperature, pH, aeration) and simpler product recovery, making it suitable for large-scale production.
Biofilm Fermentation: An advanced form where microbial cells adhere to surfaces, potentially leading to different physiological states and enhanced enzyme production.
Process Parameter Optimization: Systematically adjusting physical (temperature, pH, incubation time, moisture) and chemical (carbon and nitrogen sources, mineral supplements) conditions to maximize cellulase production and activity. Using cheap and abundant cellulosic waste as a carbon source is particularly important for cost-effectiveness.
Enzyme Engineering and Immobilization:Enhancing Enzyme Stability: Engineering enzymes for improved thermostability (e.g., active at higher temperatures) allows for more robust industrial processes and efficient enzyme recycling.
Immobilization: Attaching cellulases to solid support materials (e.g., synthetic polymers, nanoparticles) to improve their catalytic activity, thermal stability, reusability, and easier separation from the product, significantly reducing processing costs.
Designer Cellulosomes: Constructing artificial multi-enzyme complexes that mimic natural cellulosomes. These “designer cellulosomes” can integrate diverse enzymes from multiple species (both cellulosomal and non-cellulosomal) to enhance synergistic degradation. Improving the structural stability and compatibility of cohesin-dockerin interactions in these designer complexes is an active area of research.
Interdisciplinary Approaches: Integrating insights from ecology, microbiology, biochemistry, and engineering to gain a comprehensive understanding of microbial carbon cycling and develop holistic solutions.
- Lakhundi S, Siddiqui R, Khan NA. Cellulose degradation: a therapeutic strategy in the improved treatment of Acanthamoeba infections. Parasit Vectors. 2015 Jan 14;8:23. doi: 10.1186/s13071-015-0642-7. PMID: 25586209; PMCID: PMC4300153.
- de Souza, W. R. (2013). Microbial Degradation of Lignocellulosic Biomass. InTech. doi: 10.5772/54325
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/cellulose-decomposition
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/cellulose-decomposition
- https://bioenergycenter.org/besc/publications/wilson_microbial.pdf
- https://pubs.acs.org/doi/10.1021/acs.estlett.1c00843
- https://www.jstor.org/stable/4353503
- https://www.frontiersin.org/articles/10.3389/fmicb.2019.00204/full
- https://www.onlinebiologynotes.com/cellulose-decomposition-microbial-decomposition-of-cellulose-in-soil/
helped me understand it better than my textbook.
Thank You
your blog is one of the few that explain things so well, thnks a ton.
Thank You
Test coment