What is Methyl Red (MR) Test?
- The Methyl Red (MR) test is a crucial biochemical analysis used to detect the production of sufficient acid during the fermentation of glucose and to maintain conditions that sustain a pH below approximately 4.5. This is achieved by observing a change in the color of the methyl red indicator added at the end of the incubation period.
- Developed by Clark and Lubs, MR-VP Broth is utilized to perform both the MR and VP tests on the same inoculated medium by dividing portions into different tubes.
- The primary purpose of the Methyl Red (MR) test is to determine whether an organism undergoes mixed acid fermentation and produces stable acid end products. This test employs methyl red as an indicator to measure the pH after the completion of glucose fermentation by an enteric Gram-negative rod.
- The test differentiates organisms based on their ability to undergo mixed acid fermentation. Organisms capable of fermenting glucose will yield a positive MR test result by producing a significant amount of acid, while some organisms will yield a negative MR test result.
- Furthermore, the MR test assists in identifying and categorizing different genera of Enteric bacteria based on their glucose fermentation abilities. In simpler terms, the test assesses the amount of acid produced by an organism after glucose fermentation, causing the pH of the medium to drop from 6.9 to 4.5.
- The presence of acid formation is detected by adding a methyl red pH indicator. If a test organism in the medium undergoes glucose fermentation, the methyl red solution imparts a red color to it. This description covers the definition, principle, procedure, and results of the methyl red test. Additionally, it provides information about the composition of the test media and test reagent, along with some key points to remember.
- The Methyl Red test is sometimes abbreviated as MR-test and is part of a biochemical analysis called the IMViC test, where ‘M’ stands for the “Methyl red test.” It is a quantitative test, and the appearance of a red color in the medium confirms its positive result, depending on the amount of acid production.
- Before delving into the theory of the methyl red test, it’s essential to understand some key concepts:
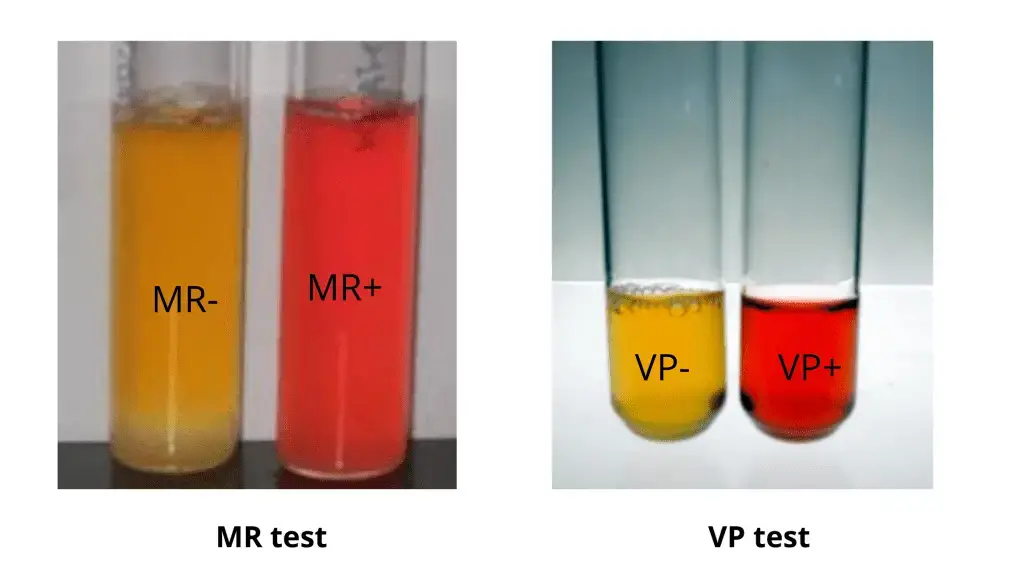
- Methyl Red Indicator: This is an acidic pH indicator containing p-dimethylaminobenzene-O-carboxylic acid, which quantifies the concentration of H+ ions in the medium. If the medium contains a sufficient amount of acids, methyl red imparts a red color to it. In the absence of acid formation, the medium remains yellow even after adding methyl red, indicating no acid production.
- MRVP Broth: This serves as the standard medium for performing the Methyl Red and Voges-Proskauer (VP) tests. It is supplemented with glucose, and test organisms utilize the glucose to carry out anaerobic fermentation or mixed acid fermentation, resulting in the formation of various end products.
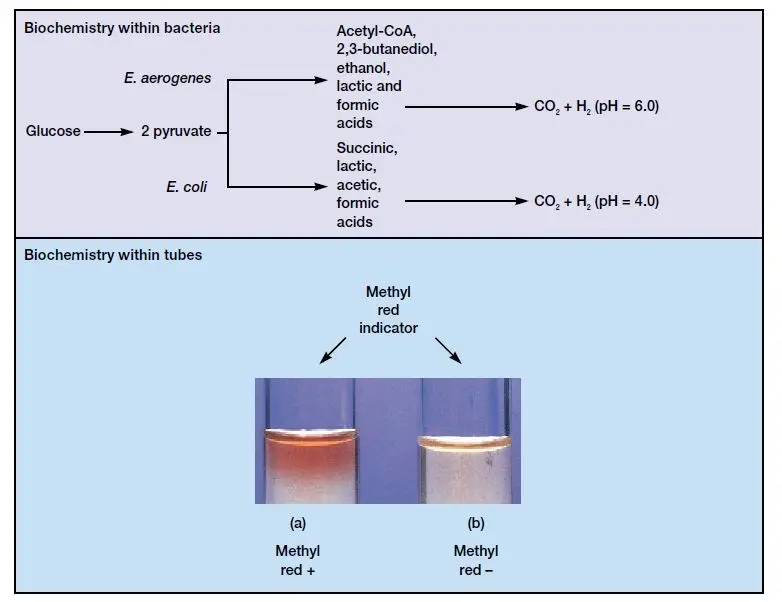
- Gram-negative bacteria belonging to the Enterobacteriaceae family typically undergo mixed acid fermentation. This pathway involves breaking down glucose into products like lactic, acetic, formic, and succinic acids, as well as ethanol, carbon dioxide, and hydrogen.
Objectives of Methyl Red (MR) Test
The objectives of the Methyl Red (MR) Test can be outlined as follows:
- Differentiation of Facultative Anaerobic Enteric Bacteria: One of the primary objectives of the MR Test is to differentiate between two major types of facultative anaerobic enteric bacteria. Facultative anaerobic bacteria are those that can thrive in both aerobic (oxygen-rich) and anaerobic (oxygen-limited) environments. By subjecting these bacteria to the MR Test, it becomes possible to categorize them based on their metabolic capabilities.
- Assessment of Acid Production: The MR Test aims to assess the bacterial ability to produce stable acid end products. This is achieved through a process known as mixed-acid fermentation of glucose. The test determines whether the bacteria under examination can efficiently ferment glucose and generate substantial amounts of acid as a metabolic byproduct.
Principle of Methyl Red test
The principle of the Methyl Red (MR) test is based on assessing the ability of an organism to produce and maintain stable acid end products through the fermentation of glucose. This test is typically performed in conjunction with the Voges-Proskauer (VP) test, and both tests are conducted using MRVP broth due to their physiological relationship.
Members of the Enterobacteriaceae family share the capability to convert glucose into pyruvic acid through the Embden-Meyerhof pathway. However, they can further metabolize pyruvic acid through two distinct pathways. Organisms that metabolize pyruvic acid via the mixed acid pathway produce a greater quantity of acid end products, including lactic acid, acetic acid, and others, while maintaining an acidic environment. The Methyl Red test is designed to detect mixed acid fermentation, which results in a decrease in the pH of the broth.
Here is a detailed explanation of the principle:
- Fermentation Pathways: All Enterobacteriaceae members can initially convert glucose into pyruvic acid. This is a common step among these bacteria.
- Mixed Acid Fermentation: Some bacteria, however, metabolize pyruvic acid through the mixed acid fermentation pathway. In this pathway, they produce various organic acids, including formic acid, acetic acid, lactic acid, and succinic acid, along with carbon dioxide and water. These acids significantly lower the pH of the medium, which is initially close to neutral.
- Methyl Red Indicator: The key component of the MR test is the methyl red indicator. This indicator changes color based on pH. It is red at pH 4.4 and yellow at pH 6.2.
- Detection of Acid Production: After the incubation period, the methyl red indicator is added to the medium. If the test organism has produced a substantial amount of organic acids, such as those mentioned earlier, as a result of glucose fermentation, the medium remains red after the addition of the methyl red indicator. This red color indicates a positive MR test.
- MR-Negative Organisms: Conversely, MR-negative organisms metabolize the initial fermentation products through decarboxylation, leading to the production of neutral acetyl methylcarbinol (acetoin). This process reduces the acidity in the medium and raises the pH to a more neutral level (pH 6.0 or above). As a result, the broth medium changes to a yellow color, indicating a negative MR test.
Procedure of Methyl Red test
- Inoculation: The first step involves inoculating MRVP (Methyl Red Voges-Proskauer) broth with a pure culture of the organism under examination. This means introducing a known, uncontaminated culture of the bacteria into the broth. This is a critical step to ensure that the observed results are attributed solely to the tested organism.
- Incubation: Following inoculation, the MRVP broth is incubated at a specific temperature range of 35°C to 37°C for a minimum duration of 48 hours. The incubation is carried out under ambient air conditions, without the addition of any specialized gases. During this incubation period, the bacteria metabolize glucose and produce various metabolic byproducts, including acids.
- Methyl Red Reagent: After the required incubation time, the next step is to add the methyl red reagent to the broth. The methyl red reagent is added in a specific proportion, typically 5 or 6 drops per 5 mL of the broth. This reagent contains the methyl red indicator, which is crucial for detecting the pH changes in the medium.
- Observation: Following the addition of the methyl red reagent, the MRVP broth is observed for any color change. The methyl red indicator has a pH range of 4.4 to 6.2. Therefore, the color change in the broth medium is indicative of the pH level. Specifically, if the organism has produced a significant amount of organic acids through glucose fermentation, the medium will remain red after the addition of the methyl red reagent. This is a positive MR test result, indicating the organism’s ability to maintain an acidic environment through mixed acid fermentation.
Result of Methyl Red test
- Positive MR Test (Bright Red Color): A positive MR test result is characterized by a bright red coloration of the medium. This indicates that the organism being tested has produced a significant amount of organic acids as end products of glucose fermentation. These organic acids include lactic acid, acetic acid, formic acid, and succinic acid, among others. The presence of these acids significantly lowers the pH of the medium, causing the methyl red indicator to turn bright red. This color change signifies the organism’s capability to maintain an acidic environment through mixed acid fermentation.
- Weakly Positive MR Test (Red-Orange Color): In some cases, the MR test may yield a red-orange coloration of the medium. This result suggests a weaker but still detectable production of organic acids from glucose fermentation. While the acid production is not as substantial as in a strongly positive test, it indicates that the organism has some capacity for mixed acid fermentation.
- Negative MR Test (Yellow Color): A negative MR test result is represented by a yellow coloration of the medium. This outcome occurs when the organism being tested either does not produce significant acid end products or produces other neutral end products. The absence of substantial acid production results in the medium’s pH remaining relatively unchanged, and the methyl red indicator shows a yellow color. This indicates that the organism cannot maintain an acidic environment through mixed acid fermentation.
In summary, the Methyl Red (MR) Test produces three possible results:
- Positive MR Test (Bright Red Color): Signifies the organism’s strong ability to produce organic acids and maintain an acidic environment.
- Weakly Positive MR Test (Red-Orange Color): Indicates a weaker but still detectable acid production capability.
- Negative MR Test (Yellow Color): Suggests the absence of significant acid production, with the medium remaining at a neutral pH.
List of Methyl Red Positive and negative Organisms
| Organism Name | Result |
|---|---|
| Escherichia coli | Positive (MR+) |
| Klebsiella pneumoniae | Negative (MR-) |
| Shigella species | Positive (MR+) |
| Enterobacter species | Negative (MR-) |
| Salmonella species | Positive (MR+) |
| Hafnia species | Negative (MR-) |
| Proteus species | Positive (MR+) |
| Citrobacter species | Positive (MR+) |
| Serratia marcescens | Negative (MR-) |
| Yersinia species | Positive (MR+) |
Limitation of Methyl Red test
- 48-Hour Incubation Requirement: One limitation of the MR test is that the results should not be read before 48 hours of incubation. This is because some organisms may require this extended incubation period to produce a sufficient amount of products from the fermentation of glucose. Reading the test too early could yield inaccurate results, as some organisms may not have had adequate time to complete their metabolic processes.
- Potential for MR-Negative Organisms to Appear MR-Positive: In some cases, MR-negative organisms may not have had enough time to convert the fermentation products into the end products detectable by the MR test. Consequently, they may initially appear MR-positive. This highlights the importance of the full 48-hour incubation period to ensure accurate results.
- Inconclusive Results (Orange Color): If the methyl red test results are inconclusive and exhibit an orange color after the 48-hour incubation period, it is recommended to continue incubation of the broth for an additional three days and then retest the broth culture. This additional incubation allows for further metabolic processes to take place, potentially resolving the ambiguity in the results.
- Complementary Confirmatory Tests: MR-VP (Methyl Red-Voges-Proskauer) testing should be used in conjunction with other confirmatory tests to differentiate organisms within the Enterobacteriaceae family. While the MR test provides valuable information, it may not be sufficient on its own to definitively identify certain bacterial species or strains.
- Avoidance of Extremely Turbid Broth: It is advisable to avoid testing an extremely turbid broth-inoculum mixture. Excessive turbidity can complicate the interpretation of the test results. It is essential to maintain clear and observable conditions to ensure accurate readings.
- Standardization of Variables: To obtain optimal and reproducible results, several variables should be standardized, including the inoculum density, the total volume of broth, and the size of the test tube used. Deviating from standardized conditions can lead to inconsistent results. For example, using too large a volume of broth may result in an orange color reaction, which can be misleading.
Quality Control of Methyl Red (MR) Test
- Avoidance of Extremely Turbid Broth: It is crucial to avoid testing an extremely turbid broth-inoculum mixture. Excessive turbidity can interfere with the interpretation of test results, making it challenging to accurately assess the color change in the medium. Maintaining clear and observable conditions is essential for obtaining valid results.
- Inoculum Density: Standardization of the inoculum density is critical for quality control. The number of bacterial cells introduced into the MRVP broth should be within an appropriate range to ensure consistent and reproducible results. Exceeding the maximum cell concentration of approximately 10^9 viable cells/ml can inhibit bacterial growth and affect the test outcome. Therefore, it is essential to use an inoculum with an appropriate cell density.
- Standardization of Variables: To achieve optimal and reproducible results, several variables should be standardized:
- Inoculum Density: The number of bacterial cells introduced into the broth should be consistent across tests to ensure uniform starting conditions.
- Total Volume of Broth: The total volume of MRVP broth used in the test should be standardized. Deviating from this standard can impact the test outcome.
- Size of the Test Tube: The size of the test tube used for incubation is also a critical variable. Using tubes of consistent size ensures that the same incubation conditions are applied to all samples.
- Positive and Negative Control Strains: Quality control in the MR Test includes the use of known positive and negative control strains. These control strains serve as reference organisms to validate the test results. Escherichia coli (ATCC25922) is commonly used as a positive control strain, as it is known to produce stable acid end products through mixed acid fermentation, yielding a positive MR test result. Conversely, Enterobacter aerogenes (ATCC13048) is used as a negative control strain, as it typically produces neutral end products and yields a negative MR test result.
Examples of Methyl Red (MR) Test Organism
Methyl Red (MR) Positive Organisms:
- Escherichia coli: Escherichia coli, often referred to as E. coli, is a well-known MR-positive organism. It has the capability to perform mixed acid fermentation of glucose, producing stable acid end products that lead to a positive MR test result.
- Shigella species: Shigella species, which include various pathogenic bacteria causing shigellosis (a type of foodborne illness), are MR positive. They also undergo mixed acid fermentation, resulting in a positive MR test.
- Salmonella species: Salmonella species, including Salmonella enterica, exhibit mixed acid fermentation and produce stable acid end products. Hence, they yield a positive MR test result.
- Citrobacter species: Citrobacter species possess the metabolic capacity for mixed acid fermentation, leading to the production of stable acids. As a result, they are classified as MR positive organisms.
- Proteus species: Proteus species, known for their swarming motility, are MR positive. They can produce stable acid end products when fermenting glucose, leading to a positive MR test outcome.
- Yersinia species: Yersinia species, such as Yersinia enterocolitica and Yersinia pseudotuberculosis, undergo mixed acid fermentation of glucose and generate stable acid end products. Therefore, they are MR positive organisms.
Methyl Red (MR) Negative Organisms:
- Enterobacter species: Enterobacter species, including various members of the Enterobacteriaceae family, are typically MR negative. They may not produce sufficient stable acid end products during glucose fermentation, resulting in a negative MR test.
- Hafnia species: Hafnia species, another group within the Enterobacteriaceae family, are often MR negative. They may not exhibit significant mixed acid fermentation, leading to a negative MR test result.
- Serratia marcescens: Serratia marcescens is known to be MR negative. This bacterium may produce different metabolic byproducts that do not lower the pH sufficiently to yield a positive MR test.
- Klebsiella pneumoniae: Klebsiella pneumoniae, a common member of the Enterobacteriaceae family, is typically MR negative. Its glucose fermentation pathway may result in neutral end products rather than stable acids.
Uses of Methyl Red (MR) Test
- Differentiation of Enteric Bacteria: One of the main uses of the MR Test is to differentiate between different genera of enteric bacteria. Enteric bacteria are a group of Gram-negative bacteria commonly found in the intestines of humans and other animals. The MR Test helps classify these bacteria based on their ability to ferment glucose and produce stable acid end products.
- Identification of Mixed Acid Fermentation: The MR Test specifically identifies whether an organism can perform mixed acid fermentation. Mixed acid fermentation is a metabolic pathway in which glucose is fermented to produce stable acid end products, including lactic acid, acetic acid, formic acid, and succinic acid. Detection of these acids is a key characteristic assessed by the MR Test.
- Assessment of Glucose Fermentation: The MR Test evaluates an organism’s ability to ferment glucose completely, leading to the production of a large amount of stable acids. The test helps determine if glucose fermentation results in a significant decrease in pH, indicating a positive MR Test result.
- Confirmation of Enteric Bacterial Species: In clinical and diagnostic microbiology, the MR Test can be used as part of a battery of tests to confirm the identity of specific enteric bacterial species. It is often used in combination with other biochemical tests like the Voges-Proskauer (VP) Test, Citrate Utilization Test, and Triple Sugar Iron (TSI) Test to differentiate between closely related species.
- Research and Taxonomy: The MR Test is valuable in research and taxonomy, where it aids in classifying and characterizing bacteria at the genus and species levels. It helps researchers understand the metabolic diversity of bacteria within the Enterobacteriaceae family.
- Quality Control in Microbiology Laboratories: Microbiology laboratories use the MR Test as a quality control measure to ensure that bacterial cultures are correctly identified and classified. It helps verify the accuracy of bacterial species identification in diagnostic and research settings.
- Monitoring Environmental Samples: In environmental microbiology, the MR Test can be employed to assess the metabolic activities of bacteria present in environmental samples. This information can be useful in studies related to microbial ecology and environmental monitoring.
- Teaching and Education: The MR Test is commonly used in microbiology education and training programs to teach students about bacterial metabolic pathways and the principles of biochemical testing.
Quiz
References
- https://www.onlinebiologynotes.com/methyl-red-mr-test-objective-principle-procedure-and-result/
- https://microbeonline.com/methyl-red-mr-test-principle-procedure-results/
- https://biologyreader.com/methyl-red-test.html
- https://biokimicroki.com/methyl-red-mr-test/
- https://assets.thermofisher.com/TFS-Assets/LSG/manuals/IFU21236.pdf
- https://universe84a.com/methyl-red-test/
- https://microbialnotes.com/methyl-red-test-principle-procedure-result
- https://senthilprabhu.blogspot.com/2017/09/methyl-red-test-mixed-acid-fermentation.html
Thank you very much, Sourav.
Most Welcome,…stay with us.