Masson’s Trichrome Staining (MTS) is a special histological staining method used to differentiate the connective tissue elements of a section.
- It is the process in which collagen fibers, muscle fibers, cytoplasm and nuclei are stained in different colours so that these structures can be separated clearly.
- It is important because the routine H&E stain cannot distinguish collagen from muscle in many tissues, and this stain is therefore used for observing fibrosis and tissue remodelling in organs like liver, kidney and heart. It is the method where three dyes are applied to the tissue, and the collagen generally appears blue or green, the muscle and cytoplasm appear red, and the nucleus appears dark brown or black.
- It is the technique based on a sequential staining mechanism. In the initial step a red plasma stain colours most tissue components, and this is referred to as the primary staining step. It is followed by treatment with phosphomolybdic or phosphotungstic acid which displaces the red dye from collagen fibers but not from the denser muscle fibers. In this step the collagen becomes free to bind a contrasting dye.
- The section is then exposed to a blue or green dye (aniline blue or light green) which stain the collagen fibers. These are important for the final differentiation of connective tissue. It is also observed that tissues are generally fixed or pre-treated with Bouin’s solution because this enhances dye binding due to the presence of picric acid.
- Historically this technique was described by Claude L. Pierre Masson in 1929. It was introduced to overcome the limitation of available staining methods which could not clearly separate collagen from other components. It is the method that was later modified in the 1950s to obtain a standardised protocol. Some of the modern versions may use alternative dyes like Biebrich scarlet, but the basic principle is the same where acid dyes and polyacids are used to selectively displace and recolour tissue elements.
Principle of the Masson’s Trichrome Staining
The principle of Masson’s Trichrome Staining is based on the differential penetration of dyes into tissue components of different density, and it is the process where electrostatic attraction and molecular size variation together determine the final colour pattern. It is observed that collagen fibers are more porous and allow easy entry of large molecules, while muscle fibers and cytoplasm are comparatively dense and do not permit the entry of larger dyes. It is the reason why a sequence of dyes of increasing molecular size is applied to separate these structures distinctly. In the first step a small acid dye like Biebrich scarlet or acid fuchsin is used which stains almost all tissue parts red because the smaller dye molecules enter every region.
In the next step a polyacid such as phosphomolybdic or phosphotungstic acid is applied. It is the process where the polyacid displaces the initial red dye from collagen but cannot remove it from the dense muscle and cytoplasm. This is referred to as the differentiation step. The collagen becomes free of the primary dye because of its porous nature and now can take up the next contrasting stain. The dense muscle fibers however retain the red dye because the larger polyacid molecules cannot penetrate them.
In the final step a very large molecular weight dye like aniline blue or light green is used. It is the process where only the cleared collagen can bind this dye due to its permeability, and the muscle remain red because they do not allow entry of the larger dye molecules. The nuclei are stained separately using Weigert’s iron hematoxylin which is resistant to the acidic steps. It is important because it keeps the nuclei sharply stained throughout the procedure. Treatment with Bouin’s solution is also important as it enhances the brightness and binding of the dyes to the tissues.
Reagents and Reagent preparation
1. Bouin’s Solution (Fixative and Pre-treatment reagent)
Bouin’s solution is the preferred fixative because it helps in improving the staining of acid dyes. It is used for secondary fixation of tissue. It is the process where picric acid, formaldehyde and acetic acid together prepare the solution.
Preparation
- Saturated picric acid – 75 ml
- 40% formaldehyde – 25 ml
- Glacial acetic acid – 5 ml
All the components is mixed carefully. Picric acid must be stored in wet condition because dry picric acid is explosive.
2. Weigert’s Iron Hematoxylin (Nuclear Stain)
It is the nuclear stain that is resistant to acidic conditions. It is prepared by mixing two stock solutions. The stain is stable because iron acts as mordant.
Stock Solution A
- Hematoxylin – 1 g
- 95% alcohol – 100 ml
The hematoxylin is dissolved in alcohol.
Stock Solution B
- 29% ferric chloride – 4 ml
- Distilled water – 95 ml
- Concentrated HCl – 1 ml
Working Solution
Equal parts of Stock A and Stock B is mixed.
It is used freshly but can stay active for some months.
3. Biebrich Scarlet–Acid Fuchsin Solution (Plasma Stain)
It stains cytoplasm, muscle and keratin. It is the process where red acid dyes are mixed.
Preparation
- 1% aqueous Biebrich Scarlet – 90 ml
- 1% aqueous Acid Fuchsin – 10 ml
- Glacial acetic acid – 1 ml
Some protocols use Xylidine Ponceau in place of these dyes.
4. Phosphomolybdic–Phosphotungstic Acid Solution (Differentiator)
This reagent is used to differentiate collagen from other tissue parts. It displaces the plasma stain from collagen fibres. These are polyacid mordants.
Preparation
- 5% Phosphomolybdic acid – 25 ml
- 5% Phosphotungstic acid – 25 ml
Both solutions is mixed in equal volumes. The reagent must be kept cold because phosphotungstic acid becomes unstable at higher pH.
5. Aniline Blue Solution (Collagen Stain)
It stains collagen fibres blue. This process gives clear differentiation of muscle and collagen.
Preparation
- Aniline Blue – 2.5 g
- Distilled water – 100 ml
- Glacial acetic acid – 2 ml
Alternative (Light Green Solution)
- Light Green SF Yellowish – 2 g
- Distilled water – 100 ml
- Dilute acetic acid – 2 ml
6. 1% Acetic Acid Solution (Rinse/Differentiation Solution)
This step helps in preventing fading of the collagen stain. It removes extra dye and improves colour quality.
Preparation
- Glacial acetic acid – 1 ml
- Distilled water – 99 ml
It is used just before dehydration of stained sections.
Procedure of Masson’s Trichrome Staining
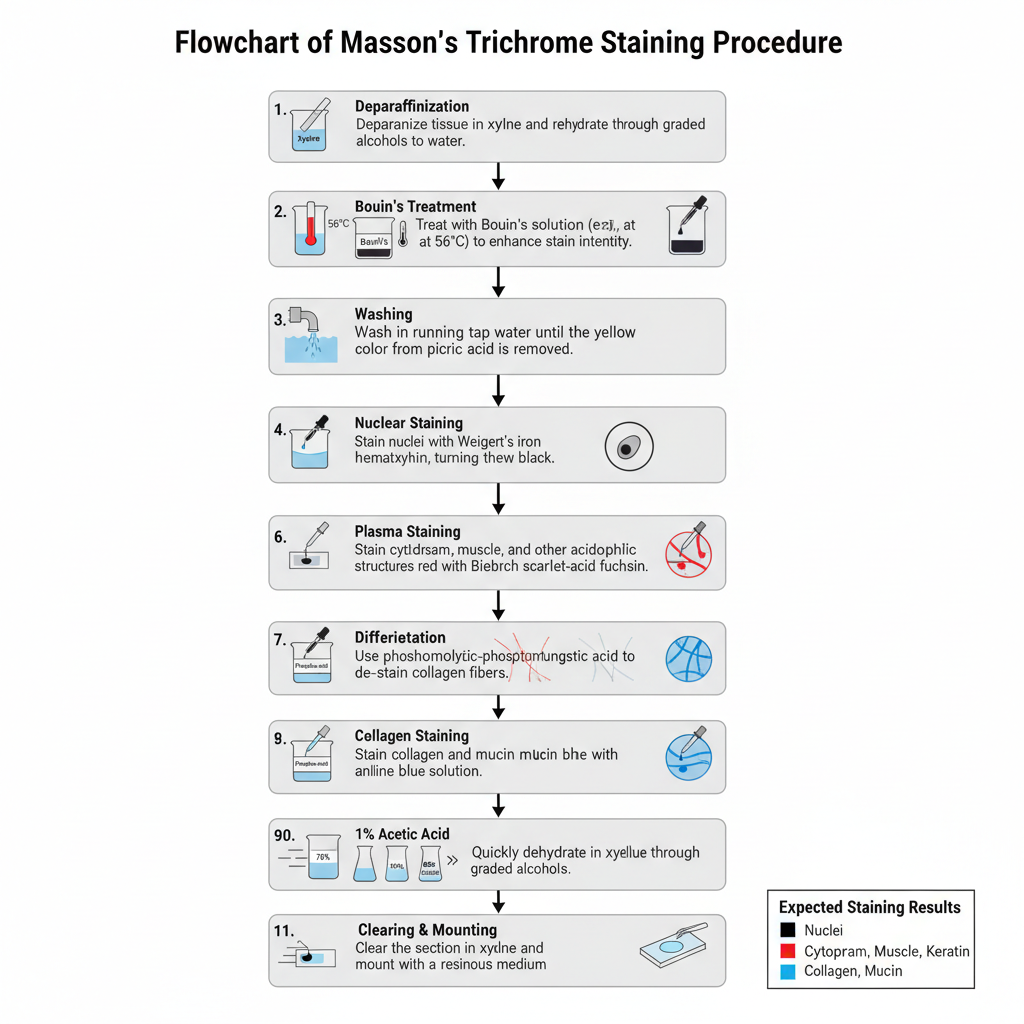
- Deparaffinization of the tissue section– The paraffin sections (4–5 μm) are first deparaffinized in xylene and then rehydrated through graded alcohols to distilled water.
- Bouin’s treatment– The slides are kept in Bouin’s solution, usually heated at about 56°C for one hour or kept overnight at room temperature. It is the process that enhances the staining intensity of the tissue.
- Washing step– The sections are washed in running tap water for about 5–15 minutes till the yellow colour of picric acid is removed.
- Nuclear staining– The slides are stained with Weigert’s iron hematoxylin for around 10 minutes. This stain keeps the nuclei sharply stained in acidic conditions.
- Rinsing– The slides are rinsed briefly in water to remove excess hematoxylin.
- Plasma staining– The slides are stained in Biebrich scarlet–acid fuchsin solution for 10–15 minutes. In this step all acidophilic structures (cytoplasm, muscle, collagen) take a uniform red colour.
- Differentiation step– The sections are placed in phosphomolybdic–phosphotungstic acid solution for about 10–15 minutes. It removes the red dye from collagen fibers but keeps the colour in muscle and cytoplasm.
- Collagen staining– Without rinsing, the slides are transferred to aniline blue (or light green) solution for 5–10 minutes. This stains the collagen fibers blue or green.
- Acid rinse– The sections are treated in 1% acetic acid for about 2–5 minutes to improve the contrast and remove excess stain.
- Dehydration– The slides are dehydrated quickly through graded alcohols because slow dehydration can remove the red colour from the tissue.
- Clearing and mounting– The sections are cleared in xylene and mounted with a resinous medium.
Expected staining pattern
- Nuclei appear black or dark brown.
- Cytoplasm, muscle and keratin appear red.
- Collagen and mucin appear blue with aniline blue or green with light green.
Results and Interpretation of Masson’s Trichrome Staining
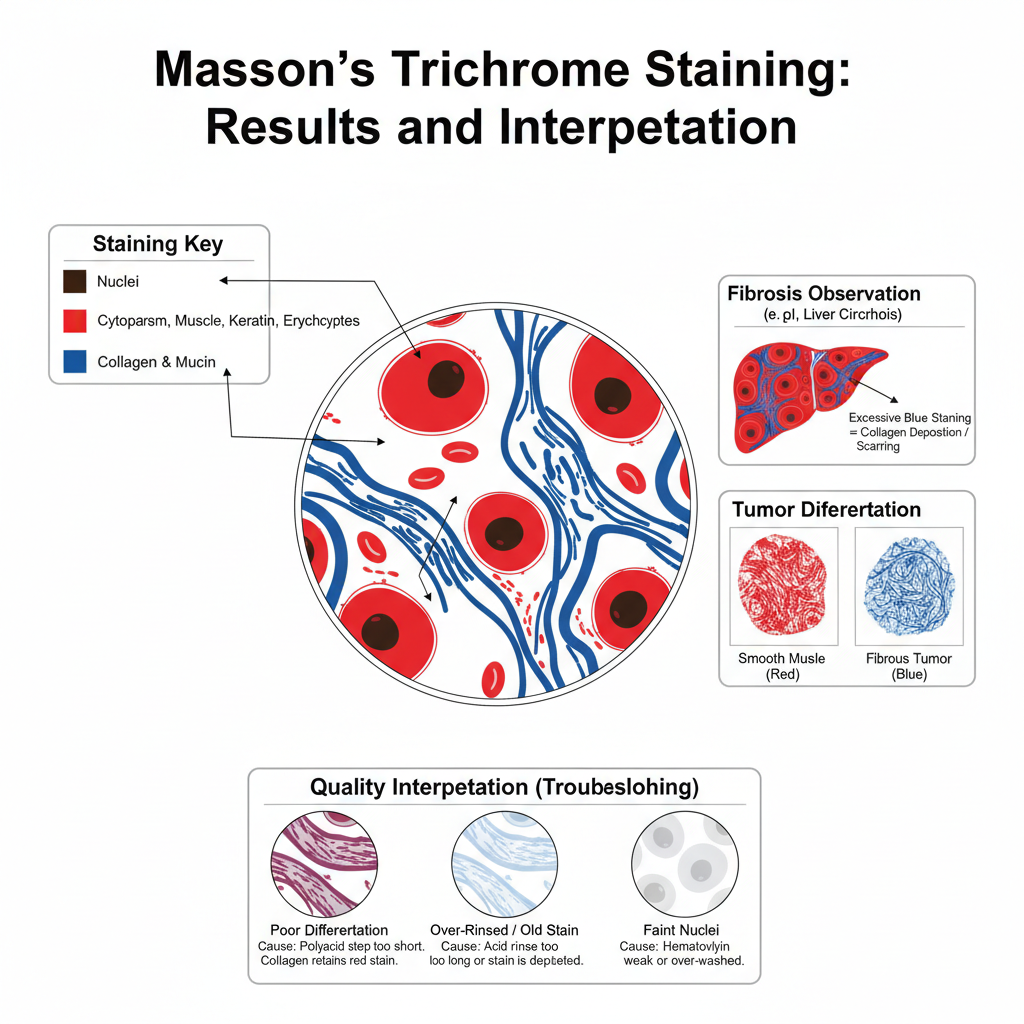
- Nuclei appearance– The nuclei appear black or dark brown. It is due to the use of Weigert’s iron hematoxylin which remains stable in acidic conditions and gives a sharp nuclear outline.
- Cytoplasm, muscle and keratin– These structures appear bright red or pink because they retain the plasma stain. It is the process where the dense cytoplasm and muscle fibers do not allow the polyacid to remove the red dye. Erythrocytes also show a red colour.
- Collagen and mucin fibres– The collagen fibres take blue colour with aniline blue or green colour with light green. This happens because collagen is porous and allows the polyacid to displace the red dye, preparing it to bind the fibre stain.
- Observation of fibrosis– Areas that show blue or green staining indicate collagen deposition. It is useful for studying fibrosis or scarring in tissues like liver, kidney and heart. In liver sections this stain is used for staging cirrhosis, and the scoring systems like METAVIR (F0–F4) or Ishak score (0–6) are used to estimate the extent of fibrosis.
- Tumour differentiation– The stain helps to separate smooth muscle tumours from fibrous tumours. Smooth muscle tumours retain the red stain while fibrous tumours take the blue stain.
- Quality interpretation– If collagen appears red it means under-differentiation, and the polyacid step was insufficient. If collagen is pale blue or green the acid rinse may have been too long or the staining solution may be old. If nuclei are faint it indicates that the hematoxylin was washed away or not properly prepared.


Applications of Masson’s Trichrome Staining
- It is used for identifying collagen distribution in tissues, and it is the major method for separating collagen fibers from muscle and cytoplasm in histology. It is the process where collagen is stained blue or green while muscle and cytoplasm appear red, helping in detecting fibrosis clearly.
- It is used in assessment of liver fibrosis, and it is applied in chronic liver diseases where staging of fibrosis and cirrhosis is required. These are done by scoring systems like METAVIR and Ishak which depend on the pattern of collagen deposition.
- It is used for studying myocardial fibrosis, and in heart tissues it helps in observing the surviving muscle fibers and the fibrotic areas after myocardial infarction.
- It is used for analyzing vascular wall structure, especially in venous insufficiency conditions where the arrangement of smooth muscle fibers and collagen is examined. It also helps when combined with elastic stains to show vessel wall damage.
- It is used in kidney pathology, and it is applied for identifying glomerular and interstitial fibrosis. The blue-green collagen regions indicate chronic and irreversible damage in kidney tissues.
- It is used for tumor differentiation, since it can clearly identify collagen-rich stroma and distinguish tumors arising from muscle tissues from those arising from fibroblasts.
- It is used to observe desmoplastic reactions, and it is important in tumors like pancreatic ductal adenocarcinoma where abundant fibrous tissue surrounds the tumor cells.
- It is used in quantitative digital pathology, and MTS stained slides are used to generate objective measurement of fibrosis percentage by image analysis software. This gives numerical data rather than subjective scoring.
- It is used for validating non-invasive diagnostic tools, and the quantitative fibrosis values obtained from MTS serve as reference for calibrating technologies like Magnetic Resonance Elastography (MRE) and FibroScan.
- It is used in general histological teaching, because it provides a clear demonstration of connective tissue organization, muscle arrangement, and tissue repair processes in different organs.
Advantages of Masson’s Trichrome Staining
- It is used for clear differentiation of tissue components, and it separates collagen fibers from muscle and cytoplasm with very high contrast. It is the process where collagen becomes blue or green while muscle and cytoplasm stain red, so the structural arrangement of different tissues is seen more clearly than in routine H&E staining.
- It is helpful in diagnosing fibrosis, and it is the major method used for staging fibrotic diseases. In liver sections it is used for identifying cirrhosis and earlier fibrotic stages, and it is also applied in cardiac fibrosis and renal glomerulosclerosis.
- It is used for tumor differentiation, since the stain separates collagen and muscle in distinct colors. Smooth muscle tumors and fibroblast-origin tumors can be identified in a reliable manner when the stroma and muscle arrangement is clearly visible.
- It is used for objective digital quantification, because the non-overlapping colors help in separating collagen pixels from muscle pixels in image analysis software. It is the process that makes fibrosis measurable in percentage values rather than only by subjective observation.
- It helps in validating diagnostic tools, because the quantitative collagen data obtained from MTS stained slides is used as reference for instruments like elastography devices.
- It offers good procedural control, and the multi-step staining allows the technician to adjust the differentiation step according to the tissue type. It helps in obtaining consistent contrast since polyacids remove excess dyes in a controlled manner.
- It gives reproducible results across laboratories, and it is preferred in diagnostic scoring systems because the staining pattern remains stable when the protocol is followed correctly.
- It is suitable for a wide range of tissues, and it works effectively in liver, heart, kidney, skin and vessel wall studies where connective tissue needs to be identified clearly.
- It is easy to interpret under a microscope, and the strong color separation helps students and pathologists in understanding the relation between muscle, collagen, and nuclei without confusion.
- It is useful in research settings, since the stain provides consistent visualization of extracellular matrix changes during disease progression, healing, or experimental fibrosis induction.
Disadvantages of Masson’s Trichrome Staining
- It is technically sensitive, and the staining result depends on correct timing in each step. The differentiation step is critical because if the polyacid treatment is too short the collagen retains red dye, and if it is too long the red colour from muscle is also removed. It is the process that makes the method difficult to standardize.
- It is less suitable for automation, since the multi-step procedure often needs manual adjustment. The stain does not behave uniformly across tissues, so strict supervision is required to maintain reproducibility.
- It requires specific fixation conditions, and the best staining needs pretreatment with Bouin’s solution. Bouin’s solution contains picric acid which is an explosive chemical when dry and needs special storage and handling.
- It has reagent stability issues, because reagents like phosphotungstic acid lose activity when the pH rises, and collagen colours may fade if the acetic acid rinse is not controlled. These factors reduce the consistency of long-term staining quality.
- It may give uneven staining in dense tissues, and tissues having hydrophobic lipids sometimes resist dye penetration. It results in areas that appear less stained and may not represent true pathological changes.
- It can obscure fine structures, since intense colours in thicker tissue sections can hide delicate cellular detail. Sections more than 5 microns often show reduced clarity.
- It is subjective without digital tools, and human estimation of fibrosis varies because visual judgement is not fully accurate. It is the reason why digital image analysis is now widely used to convert the colour data into objective measurements.
- It has limited flexibility in some laboratories, because the multi-reagent system requires frequent monitoring, and small variations in pH, fixation, or timing cause major shifts in the final appearance of the stained slide.
- It is more time consuming than routine stains, and this reduces its use in high-volume diagnostic settings where rapid staining is required.
- It depends strongly on tissue preparation quality, and overstaining, incomplete fixation, or delayed processing can easily disturb the contrast between collagen and muscle, leading to unreliable interpretation.
Precautions
- It is necessary to handle picric acid with care, because dried picric acid is explosive. It must always be stored in wet condition and the container threads should not be allowed to dry. If crystals are seen, the container is placed in water instead of opening it.
- It is important to use protective clothing, since the acids and reagents like Weigert’s solution are corrosive and can irritate skin or eyes. Gloves and proper ventilation are required while preparing the staining solutions.
- It is required to ensure proper tissue fixation, and tissues give better staining when they are mordanted in Bouin’s solution. After mordanting, washing the sections thoroughly is essential to remove yellow discoloration, otherwise the final stain may appear altered.
- It is needed to remove mercury pigments, and iodine followed by sodium thiosulfate is used when mercury fixatives were applied earlier. This prevents dark deposits from interfering with the stain.
- It is necessary to stain nuclei with Weigert’s iron hematoxylin, because alum hematoxylin is removed by the acidic solutions in the next steps. The iron mordant helps the nuclei remain black.
- It is essential to monitor the differentiation step, since insufficient time leaves collagen red, and excessive time removes red colour from muscle. This step controls the final contrast of the stained section.
- It is required to check the final acetic acid rinse, and over-rinsing leads to fading of the blue or green collagen colour. The concentration and timing must be kept controlled.
- It is important to dehydrate quickly, because the red cytoplasmic stain is sensitive to alcohol and may be removed if dehydration is slow. Graded alcohol steps should be performed without delay.
- It is required to prepare Weigert’s hematoxylin fresh, since the working solution deteriorates with time and becomes muddy due to oxidation. Using older solutions can give weak or uneven nuclear staining.
- It is necessary to maintain reagent stability, and the phosphotungstic acid solution should be kept at low pH and stored properly. If the pH rises above 2 the reagent loses effectiveness and the differential staining becomes poor.
FAQ
What is Masson’s Trichrome staining?
Masson’s Trichrome staining is a histological staining method used to visualize the different components of biological tissues, such as connective tissue, muscle fibers, and cells.
Why is Masson’s Trichrome staining used?
Masson’s Trichrome staining is used to assess the degree of fibrosis in tissues, evaluate muscle fibers, diagnose tumor tissue, study cardiovascular disease, and study degenerative diseases.
What type of tissue can Masson’s Trichrome staining be used on?
Masson’s Trichrome staining can be used on a wide range of tissues, including liver, kidney, lung, muscle, heart, brain, and tumor tissue.
How does Masson’s Trichrome staining work?
Masson’s Trichrome staining uses a combination of dyes to differentiate the different components of a tissue sample. The dyes are absorbed into the tissue and then visualized under a microscope.
What dyes are used in Masson’s Trichrome staining?
The dyes used in Masson’s Trichrome staining include aniline blue, acid fuchsin, and light green.
Is Masson’s Trichrome staining reliable?
Masson’s Trichrome staining is a well-established and reliable staining method, widely used in histology and pathology for the analysis of tissue samples.
How long does Masson’s Trichrome staining take?
Masson’s Trichrome staining usually takes 2-3 hours, depending on the specific protocol being used.
Can Masson’s Trichrome staining be performed on fixed tissue samples?
Yes, Masson’s Trichrome staining is typically performed on fixed tissue samples, such as those preserved in formalin.
What are the limitations of Masson’s Trichrome staining?
The limitations of Masson’s Trichrome staining include the potential for tissue over-staining or under-staining, as well as the need for specialized equipment, such as a microscope, to visualize the stained tissue.
How can the results of Masson’s Trichrome staining be interpreted?
The results of Masson’s Trichrome staining can be interpreted by a trained histologist or pathologist, who will analyze the stained tissue sample under a microscope and determine the degree of fibrosis or muscle damage, or identify the type of tumor tissue present.
- Anderson, J., & Rolls, G. (2025). An introduction to routine and special staining. Leica Biosystems. https://www.leicabiosystems.com/us/knowledge-pathway/an-introduction-to-routine-and-special-staining/
- Creative Bioarray. (2025). Masson’s trichrome staining protocol. https://www.creative-bioarray.com/support/masson-s-trichrome-staining-protocol.htm
- Daniel, C. (2025, October 21). METAVIR score uses and results. Verywell Health. https://www.verywellhealth.com/what-is-your-metavir-score-1759917
- Expert-level technical report: Masson’s trichrome staining – Principle, procedure, result, and advanced diagnostic applications [Technical report]. (n.d.).
- Hernández-Morera, P., Castaño-González, I., Travieso-González, C. M., Mompeó-Corredera, B., & Ortega-Santana, F. (2016). Quantification and statistical analysis methods for vessel wall components from stained images with Masson’s trichrome. PLOS ONE, 11(1), e0146954. https://doi.org/10.1371/journal.pone.0146954
- IHC World. (2009, August 21). Masson’s trichrome staining protocol for collagen fibers. http://www.ihcworld.com/_protocols/special_stains/masson_trichrome.htm
- iHisto. (2025). Masson’s trichrome stain service: Reveal fibrosis and collagen with precision. https://www.ihisto.io/routine-histology/massons-trichrome-stain
- Kiernan, J. A. (2015). Methods for connective tissue. In Histological and histochemical methods: Theory and practice (5th ed., pp. 198–205). Scion. https://med.emory.edu/departments/medicine/divisions/cardiology/research/labs/microscopy-in-medicine/_documents/kiernan-book-masson-trichrome-stain.pdf
- Saffron Scientific Histology Services. (2017, December 1). Masson trichrome technique & tricks. https://www.saffronscientific.com/masson-trichrome-technique-tricks/
- Shiha, G. (2011, September). Bridging fibrosis , Masson trichrom stain [Figure]. ResearchGate. https://www.researchgate.net/figure/Bridging-fibrosis-Masson-trichrom-stain_fig3_221915618
- StainsFile. (2025). Comparison guide: Trichrome staining variants. https://www.stainsfile.com/theory/methods/trichrome-staining/comparison-guide-trichrome-variants/
- StainsFile. (2025). Factors affecting trichrome staining. https://www.stainsfile.com/theory/methods/trichrome-staining/factors-how-it-works/
- StainsFile. (2025). Masson’s trichrome an original variant. https://www.stainsfile.com/protocols/massons-trichrome-an-original-variant/
- StainsFile. (2025). Picric acid – Chemical fixing agent. https://www.stainsfile.com/fixing-agents/picric-acid/
- StainsFile. (2025). Trichrome staining. https://www.stainsfile.com/theory/methods/trichrome-staining/
- Tufts Comparative Medicine Services. (n.d.). Special stains in histology. https://cms.tufts.edu/services/histologypathology/animal-histology-core/special-stains-histology
- Wikipedia. (2025). Masson’s trichrome stain. https://en.wikipedia.org/wiki/Masson%27s_trichrome_stain
- Wikipedia. (2025). Trichrome staining. https://en.wikipedia.org/wiki/Trichrome_staining