What is Mannitol Salt Agar (MSA)?
- Mannitol Salt Agar (MSA) is a specialized culture medium widely used in microbiology laboratories for the isolation and identification of Staphylococcus aureus, a pathogenic bacterium commonly associated with various infections. MSA is recommended for use in both clinical and non-clinical settings and can be employed for detecting and enumerating coagulase-positive Staphylococci in milk, food, and other specimens.
- The development of MSA can be traced back to the early work of researchers such as Gordon, Dudgeon, and Winslow. Gordon initially proposed the fermentation of mannitol as a means of differentiating pathogenic staphylococci from nonpathogenic ones. This concept was later confirmed and expanded upon by other investigators. However, the diagnostic value of mannitol fermentation was initially questioned by Winslow and his colleagues, who considered it to be of limited use.
- In addition to the challenges associated with differentiating staphylococci, the isolation of Staphylococcus species itself posed difficulties due to the presence of contaminants and the lack of suitable growth media. To address these issues, George Chapman and his coworkers at the Clinical Research Laboratory in New York developed a series of media in the 1930s and 1940s. One of these media was bromthymol-blue lactose agar, which, when combined with phenol-red mannitol agar, proved to be reliable for the isolation and differentiation of potential pathogenic staphylococci. Chapman further modified the formula by incorporating 7.5% sodium chloride, a concentration that inhibited the growth of most organisms except staphylococci. This modification led to the creation of Mannitol Salt Agar.
- The principle of MSA lies in its selective and differential properties. The high concentration of salt in the agar creates a selective environment that inhibits the growth of many bacteria, except for Staphylococcus species that can tolerate high salt concentrations. This selectivity allows for the isolation of Staphylococcus colonies from mixed cultures. Additionally, MSA differentiates Staphylococcus species based on their ability to ferment mannitol, a type of sugar present in the medium. Staphylococcus aureus, in particular, is known to ferment mannitol, leading to a change in the pH indicator (phenol red) and resulting in a color change from red to yellow.
- When using Mannitol Salt Agar, microbiologists observe the growth of colonies and the color changes that occur. The formation of yellow colonies indicates the fermentation of mannitol and suggests the presence of Staphylococcus aureus. However, further confirmatory tests are typically performed to definitively identify the species, as other bacteria, though inhibited, may also produce similar results. These additional tests may include coagulase tests, which help distinguish Staphylococcus aureus from other coagulase-negative Staphylococci.
- In conclusion, Mannitol Salt Agar is a widely employed selective and differential medium used for the isolation and identification of Staphylococcus aureus. Its high salt concentration inhibits the growth of many bacteria while allowing for the growth of salt-tolerant Staphylococcus species. The ability of Staphylococcus aureus to ferment mannitol and produce a characteristic color change on the agar plate helps presumptively identify this pathogenic bacterium. However, confirmatory tests are necessary to definitively identify the species.
Purpose of Mannitol Salt Agar (MSA)
The purpose of Mannitol Salt Agar (MSA) is twofold: it serves as a selective and differential medium for the isolation and identification of staphylococci.
- Selective purpose: MSA contains a high concentration of sodium chloride (7.5%), making it a selective medium. This high salt concentration creates an environment that selectively inhibits the growth of many bacteria except for staphylococci. Staphylococci are known to be able to tolerate and grow in high-salt conditions. Therefore, MSA favors the growth of staphylococci while inhibiting the growth of other bacteria, aiding in their isolation.
- Differential purpose: MSA allows for the differentiation of bacteria based on their ability to ferment the sugar mannitol, which is the sole carbohydrate present in the medium. Staphylococcus aureus, a pathogenic staphylococcus species, possesses the enzyme necessary to ferment mannitol. As a result, if S. aureus is present and can ferment mannitol, it produces acid as a byproduct, which lowers the pH of the medium. This acidification of the medium causes a color change due to the pH indicator (phenol red) present in MSA, turning the colonies yellow and creating a yellow zone surrounding them. Non-pathogenic staphylococci and other bacteria that cannot ferment mannitol do not cause this color change.
Therefore, the purpose of MSA is to provide a medium that selectively favors the growth of staphylococci while allowing for the differentiation of bacteria based on their mannitol fermentation capabilities. This combination of selectivity and differential properties makes MSA a valuable tool in the isolation and preliminary identification of staphylococci, particularly the pathogenic Staphylococcus aureus.
Principle of Mannitol Salt Agar (MSA)
The principle of Mannitol Salt Agar (MSA) revolves around its composition and the reactions it elicits from different bacteria. MSA contains beef extract and proteose peptone, which provide essential growth factors, trace nutrients, and nitrogen, vitamins, minerals, and amino acids necessary for bacterial growth. The medium’s key component is a 7.5% concentration of sodium chloride, which serves as a selective agent by inhibiting the growth of bacterial organisms other than staphylococci.
Mannitol, a fermentable carbohydrate source present in MSA, plays a crucial role in the medium. Bacteria capable of fermenting mannitol produce acid as a byproduct. Staphylococcus aureus, a pathogenic species, can grow on MSA and readily ferment mannitol, leading to the production of yellow colonies. On the other hand, most coagulase-negative Staphylococci and Micrococci do not possess the ability to ferment mannitol and, therefore, appear as small red colonies on the agar.
The color changes observed in both the colonies and the medium are due to the presence of a pH indicator called phenol red. Phenol red is red at a pH of 8.4 and turns yellow at a pH of 6.8. When Staphylococcus aureus ferments mannitol, the production of acid lowers the pH, causing the phenol red to shift from red to yellow.
Agar, a solidifying agent, is also present in MSA, providing a gel-like consistency to the medium, allowing for the growth and observation of bacterial colonies.
In some cases, a 5% v/v Egg Yolk Emulsion may be added to MSA. This addition enables the detection of lipase activity exhibited by staphylococci in conjunction with mannitol fermentation. The salt present in MSA helps to clear the egg yolk emulsion, and if the bacteria produce lipase, a yellow opaque zone appears around the colonies, indicating the presence of this enzyme.
In summary, the principle of Mannitol Salt Agar lies in its selective and differential properties. The high concentration of sodium chloride selectively inhibits the growth of bacteria other than staphylococci, while the inclusion of mannitol allows for the differentiation of fermentative and non-fermentative Staphylococcus species. The pH indicator, phenol red, helps identify the acid production resulting from mannitol fermentation by Staphylococcus aureus. The addition of Egg Yolk Emulsion further enables the detection of lipase activity. These components and reactions form the basis of the principle behind Mannitol Salt Agar.
Composition of Mannitol Salt Agar (MSA)
| Ingredients | Gms/Litre |
| Proteose peptone | 10.0 |
| Sodium chloride | 75.0 |
| D- mannitol | 10.0 |
| Beef extract | 1.0 |
| Phenol red | 0.025 |
| Agar | 15.0 |
Final pH 7.4 +/- 0.2 at 25ºC.
Selective medium
The inclusion of 7.5 percent sodium chloride in the medium allows for selection of only the bacteria that are able to endure high salt concentrations. MSA can demonstrate the capacity for a bacterium’s growth in an 7.5 percent salt-based environment (growth signifies tolerance to the high salt conditions but no growth does not mean intolerance). Staphylococci species can take this salt level, but other pathogenic bacteria might not. This salt concentration hinders the growth of a majority of other gram positive and gram negative bacteria. Therefore, MSA specifically isolates Staphylococcus species i.e. specific media specifically for Staphylococcus spp.
Differential Medium
Staphylococci that are pathogenic, i.e. Staphylococcus aureus can ferment mannitol, but other (coagulase-negative staphylococci) are not. Therefore, if a particular specimen is contaminated with S. aureus that ferments mannitol, it alters in the pH. This can be acidic. Because MSA includes the color red phenol as an indicator of pH for pH levels that are less than 6.9 it is colored yellow. However, if coagulase-negative staphylococci (CONS) develops and they are unable to ferment mannitol. As a result, it’s color for the medium surrounding the colony of bacteria is not changed to yellow and appears to be pink. Thus, MSA is also a different medium.
Take note that in the pH range of neutral (6.9 and 8.4) that the hue of the phenol red is red when it is above pH 8.4 the hue of phenol red is pink. Other media commonly used to contain Phenol Red as a pH indicator include TSI Agar Urea base agar, and XLD Agar.
Preparation of Mannitol Salt Agar (MSA)
The preparation of Mannitol Salt Agar (MSA) involves a few simple steps to ensure the medium is properly dissolved, sterilized, and poured into sterile petri dishes. Here is a step-by-step guide for preparing MSA:
- Begin by measuring out 111 grams of Mannitol Salt Agar and add it to 1000 ml of distilled water. The water should be of good quality to prevent any contamination.
- Mix the agar and water mixture thoroughly to ensure the powder is evenly dispersed.
- Heat the mixture while stirring to dissolve the medium completely. It is recommended to use a Bunsen burner or a heating source that allows for controlled heating.
- Once the medium is completely dissolved, it needs to be sterilized to eliminate any unwanted microorganisms. Autoclaving is the preferred method of sterilization for MSA. Place the medium in the autoclave and set the pressure to 15 lbs (pounds per square inch). The temperature should reach 121°C (250°F). Allow the medium to autoclave for 15 minutes, ensuring proper sterilization.
- After autoclaving, if desired, sterile Egg Yolk Emulsion (E7899) can be added to the medium. This addition is optional and can be done to a final concentration of 5% v/v. The Egg Yolk Emulsion can be sterilely added to the medium after autoclaving by aseptically transferring it and thoroughly mixing.
- Once the medium is prepared and any optional additions have been made, it needs to be poured into sterile petri dishes. Ensure that the medium has cooled down to room temperature before pouring. This helps prevent condensation and allows the agar to solidify properly.
- Sterile technique is crucial during the pouring process. Use a sterile technique to transfer the cooled MSA into the petri dishes. Pour enough medium to cover the bottom of each dish evenly.
- After pouring, allow the Mannitol Salt Agar in the petri dishes to cool and solidify completely at room temperature. Once solidified, the dishes can be used for bacterial culture and analysis.
Prepared medium
To prepare the Mannitol Salt Agar (MSA) medium, follow these steps:
- Start by melting the content of the bottle in a water bath at 100°C. Loosen the cap partially to allow for the escape of steam and prevent pressure buildup. Heat the medium until it is completely dissolved. This heating process helps to liquefy the agar and other components present in the medium.
- Once the medium is completely dissolved, securely screw the cap back on the bottle. This ensures the medium remains sterile and prevents contamination.
- Check the homogeneity of the dissolved medium by gently turning the bottle upside down. This step helps verify that the medium is well-mixed and free of any clumps or sedimentation. If the medium appears uniform and homogeneous, it is ready for the next step.
- Allow the medium to cool down to a temperature of 45-50°C. It is important to cool the medium to this specific temperature range to avoid damaging the agar or inhibiting bacterial growth. Use a thermometer to monitor the temperature during the cooling process.
- While the medium is cooling, mix it well to ensure even distribution and to prevent the formation of foam. Stir gently to avoid introducing air bubbles into the medium.
- Once the medium has reached the desired temperature and has been thoroughly mixed, it is ready to be aseptically distributed into Petri dishes. Aseptic technique is crucial to maintain the sterility of the medium. Carefully pour or pipette the medium into sterile Petri dishes, ensuring each dish is evenly filled with the medium.
Shelf life of Mannitol Salt Agar (MSA):
A few weeks provided there isn’t any changes in the appearance or color of the medium which could suggest the presence of contamination, degradation or change in the pH.
pH of Mannitol Salt Agar (MSA):
It should fall within the pH range of 7.3 up to 7.7 at temperatures at room temperature.
Physical Properties of Mannitol Salt Agar (MSA)
- Appearance: Light yellow to pink homogeneous free flowing powder
- Gelling: Firm,comparable with 1.5% Agar gel
- Colour and Clarity of prepared medium: Red coloured clear to slightly opalescent gel forms in Petri plates
- Reaction: Reaction of 11.1% w/v aqueous solution at 25°C. pH : 7.4±0.2
- pH: 7.20-7.60
Cultural Response: The cultural aspects that are observed following an incubation period of 35-37°C for 18-72 hours. The rate of recovery is considered to be 100% for the growth of bacteria upon Soybean casein digest agar.
Storage of Mannitol Salt Agar (MSA)
The powder is extremely hygroscopic, keep the powder at 10-30 degrees C in a dry area and keep it in the container that was tightly sealed. Keep the prepared plates and bottles in a temperature of 10-25 degrees, far from light. Don’t use the product after the expiry date as indicated on the label, or if the product has any indication of contamination or signs of deterioration.
Test Procedure on Mannitol Salt Agar (MSA)
- Inoculated plates through straight streaking substance to be examined on the surface of the agar.
- Incubate at an aerobic temperature of 35 degrees Celsius +/- 24 to 48 hours.
- The Harmonized USP/EP/JP microbiological method for testing of non-sterile items recommends inoculating the product into Tryptic Soy Broth.
- Subculture on a dish of Mannitol Salt Agar , and then keep incubating at 30-35degC for 18 to 72 hours.
Results and Colony Characteristics in Mannitol Salt Agar (MSA)
Results on Mannitol Salt Agar (MSA) provide valuable information for the identification of different bacterial species, particularly Staphylococcus aureus. Here are the expected results and interpretations on MSA:
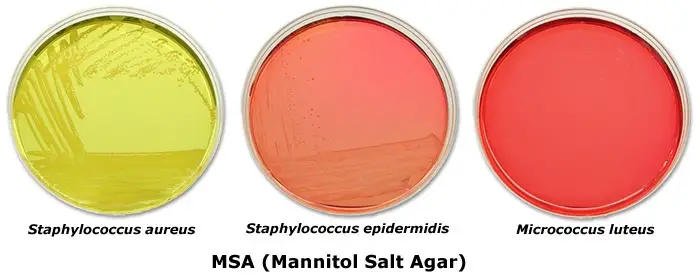
- Staphylococcus aureus: This pathogenic bacterium ferments mannitol, resulting in the production of acid. On MSA, Staphylococcus aureus forms lush, pigmented colonies surrounded by a yellow halo. The yellow halo is indicative of the fermentation of mannitol, and it is a characteristic feature used to presumptively identify Staphylococcus aureus.
- Non-pathogenic staphylococci: Non-pathogenic staphylococci, such as Staphylococcus epidermidis, typically form small red colonies on MSA. However, they do not have the ability to ferment mannitol, and therefore, do not cause a change in the color of the medium surrounding the colonies.
- Micrococci: Micrococci are another group of bacteria that can grow on MSA. They appear as red colonies on the medium. It is important to note that the color of the colony alone is not a reliable indicator of mannitol fermentation, as many micrococci are pigmented.
- Escherichia coli: Escherichia coli, a member of the Enterobacteriaceae family, does not grow on MSA due to the inhibitory effect of the high salt concentration. Therefore, no growth is expected for this organism on MSA plates.
| Organisms | Results |
| Staphylococcus aureus | Yellow colonies surrounded by yellow zone |
| Staphylococcus epidermidis | Pink or Red colonies |
| Micrococci | Red colonies |
| Escherichia coli | No growth |
Quality Control on Mannitol Salt Agar
The quality control of Mannitol Salt Agar (MSA) involves checking various parameters to ensure the medium’s performance and reliability. Here are the quality control measures and their expected results for MSA:
- Appearance: The MSA powder should have a light yellow to pink color and should be a free-flowing homogeneous powder.
- Gelling: When prepared, the agar gel should have a firm consistency comparable to a 1.5% agar gel. This ensures that the medium solidifies properly for bacterial culture.
- Color and Clarity of prepared medium: The prepared MSA should form a red-colored clear to slightly opalescent gel in the Petri plates. This indicates the correct formulation and proper mixing of the medium.
- Reaction: A 11.1% w/v aqueous solution of MSA at 25°C should have a pH of 7.4 ± 0.2. This pH range is essential for the medium to support the growth of specific bacteria.
- pH: The pH of the MSA medium should be within the range of 7.20 to 7.60. This range ensures the appropriate conditions for bacterial growth.
- Cultural Response: After incubation at 35-37°C for 18-72 hours, the MSA should exhibit specific cultural responses for different organisms. The expected results for selected organisms are as follows:
- Staphylococcus aureus subsp. aureus ATCC 6538: Luxuriant growth of yellow/white colonies surrounded by a yellow zone.
- Escherichia coli ATCC 8739: Inhibited growth (no growth observed).
- Staphylococcus aureus subsp. aureus ATCC 25923: Luxuriant growth of yellow/white colonies surrounded by a yellow zone.
- Staphylococcus epidermidis ATCC 12228: Fair to good growth with 30-40% of colonies appearing red.
- Proteus mirabilis ATCC 12453: None to poor growth with 0-10% of colonies appearing yellow.
- Escherichia coli ATCC 25922: Inhibited growth (no growth observed).
- Enterobacter aerogenes ATCC 13048: Inhibited growth (no growth observed).
By comparing the observed cultural responses with the expected results, the quality control ensures that the MSA medium performs as intended and supports the growth of specific organisms under standardized conditions.
- If grown on mannitol in agar, certain kinds that belong to Micrococcus (Micrococcus is a common human skin flora that can be found in the mucosa and oropharynx) including M. Lutus (yellow) can form colonies of yellow. The M. roseus (red) produces pink colonies on MSA. Find out the difference among Micrococcus and Staphylococcus here.
- Enterococcus Faecalis and Enterococcus Faecium (the most prevalent enterococcal species which has been identified from human infection) are salt-tolerant bacteria that can tolerate salt. They are able to ferment mannitol and produce lactic acid, resulting in white-colored colonies that are visible on MSA. Catalase tests can help identify the difference between Enterococcus (-ve) as well Staphylococcus (+ve).
Uses of Mannitol Salt Agar (MSA)
Mannitol Salt Agar (MSA) has various applications in microbiology due to its selective and differential properties. Here are some common uses of MSA:
- Selective isolation and differentiation of Staphylococcus aureus: MSA is primarily used for the selective isolation and differentiation of Staphylococcus aureus from clinical samples. The high salt concentration in the medium inhibits the growth of many bacteria, allowing for the selective growth of staphylococci. Staphylococcus aureus, in particular, ferments mannitol, resulting in the production of acid and the formation of yellow colonies surrounded by a yellow zone on MSA.
- Enumeration of staphylococci in food and dairy products: MSA is employed for the enumeration of staphylococci in food and dairy products. It provides a selective environment that promotes the growth of staphylococci, including Staphylococcus aureus, which is commonly associated with foodborne illnesses.
- Cosmetics testing: MSA is included in the Bacteriological Analytical Manual for cosmetics testing. It can be used to detect and identify Staphylococcus aureus in cosmetic products, ensuring their safety and quality.
- Bacteriological examination of water: MSA is utilized in the bacteriological examination of swimming pool water, spas, and drinking water using membrane filtration. It serves as a selective medium for the isolation and identification of staphylococci, including Staphylococcus aureus, which can be indicators of water contamination and potential health hazards.
Limitations of Mannitol Salt Agar (MSA)
Mannitol Salt Agar (MSA) has some limitations that should be considered when using the medium. These limitations include:
- Cross-reactivity with other Staphylococcus species: Several Staphylococcus species, such as S. capitis, S. xylosus, S. cohnii, S. sciuri, S. simulans, and others, can also ferment mannitol and produce yellow colonies surrounded by yellow zones on MSA. Therefore, MSA alone cannot definitively identify Staphylococcus aureus. Further biochemical tests are necessary to differentiate S. aureus from other species.
- Inhibition of non-staphylococcal organisms: The high salt concentration in MSA inhibits the growth of most organisms other than staphylococci. However, some halophilic marine organisms, which can tolerate high salt concentrations, may still grow on MSA. This can be a limitation in certain environmental samples or when dealing with organisms that are adapted to high salt conditions.
- Delayed fermentation of mannitol: While most strains of Staphylococcus aureus ferment mannitol and produce yellow colonies surrounded by yellow zones, a few strains may exhibit a delayed fermentation. In such cases, negative plates should be re-incubated overnight to allow for the detection of delayed mannitol fermentation.
- Confirmation of Staphylococcus aureus: Presumptive identification of Staphylococcus aureus based solely on MSA results is not sufficient. It is necessary to confirm the presence of Staphylococcus aureus using additional tests, such as a coagulase test, which is considered a definitive test for the identification of this pathogenic species.
Troubleshooting
Troubleshooting Mannitol Salt Agar (MSA) involves addressing some potential issues and differentiating between bacterial species that may produce similar colony appearances. Here are some troubleshooting tips for MSA:
- Differentiating Micrococcus from Staphylococcus: When using MSA, it’s important to note that certain species of Micrococcus, such as M. luteus and M. roseus, can produce colonies that resemble those of Staphylococcus aureus. M. luteus typically forms yellow colonies, while M. roseus forms pink colonies on MSA. To distinguish between Micrococcus and Staphylococcus, further tests and biochemical identification methods should be employed.
- Salt-tolerant Enterococcus species: Enterococcus faecalis and Enterococcus faecium are examples of salt-tolerant bacteria that can ferment mannitol and produce lactic acid. This fermentation can result in the formation of yellow-colored colonies on MSA, similar to Staphylococcus aureus. To differentiate between Enterococcus and Staphylococcus, a catalase test can be conducted. Enterococcus species are typically catalase-negative, meaning they do not produce catalase, while Staphylococcus species are catalase-positive and produce catalase.
FAQ
What is Mannitol Salt Agar (MSA)?
Mannitol Salt Agar (MSA) is a selective and differential medium used in microbiology for the isolation and identification of staphylococci, particularly Staphylococcus aureus, from various sources.
How does MSA differentiate Staphylococcus aureus from other staphylococci?
Staphylococcus aureus can ferment mannitol, producing acid as a byproduct. This acidification of the medium causes a pH change, resulting in a color change of the colonies and the surrounding medium to yellow. Other staphylococci that cannot ferment mannitol do not cause this color change.
What is the differential component in MSA?
The differential component in MSA is mannitol, which is the only carbohydrate present in the medium. It allows for the differentiation of bacteria based on their ability to ferment mannitol.
How does MSA select for staphylococci?
MSA contains a high concentration of sodium chloride (7.5%), which creates a selective environment inhibiting the growth of many bacteria except for staphylococci, which can tolerate high salt concentrations.
Can organisms other than staphylococci grow on MSA?
Most organisms other than staphylococci are inhibited by the high salt concentration in MSA. However, some halophilic marine organisms and a few other bacteria can tolerate the high salt content and grow on MSA.
Are all yellow colonies on MSA indicative of Staphylococcus aureus?
No, some Micrococcus species and Enterococcus faecalis/faecium can also produce yellow colonies on MSA. Therefore, further tests and identification methods are necessary to differentiate between these organisms and Staphylococcus aureus.
How can Micrococcus species be differentiated from Staphylococcus on MSA?
Further tests and biochemical identification methods are needed to differentiate Micrococcus from Staphylococcus species. Colony morphology, Gram staining, and additional biochemical tests can help in the differentiation process.
Can Enterococcus species ferment mannitol and produce yellow colonies on MSA?
Yes, Enterococcus faecalis and Enterococcus faecium are salt-tolerant bacteria that can ferment mannitol and produce lactic acid, resulting in yellow-colored colonies on MSA. A catalase test can help differentiate Enterococcus (-ve) from Staphylococcus (+ve).
Is MSA used only for clinical samples?
No, MSA has various applications beyond clinical samples. It can be used for the enumeration of staphylococci in food and dairy products, testing cosmetics, and the bacteriological examination of water sources like swimming pool water and drinking water.
Can MSA be used as a definitive test for identifying Staphylococcus aureus?
No, MSA is a selective and differential medium that provides presumptive identification of Staphylococcus aureus based on mannitol fermentation. Definitive identification of Staphylococcus aureus requires further confirmatory tests, such as a coagulase test or molecular methods.
References
- https://exodocientifica.com.br/_technical-data/M118.pdf
- https://microbiologie-clinique.com/Mannitol-Salt-Agar.html
- https://asm.org/ASM/media/Protocol-Images/Mannitol-Salt-Agar-Plates-Protocols.pdf?ext=.pdf
- https://microbeonline.com/mannitol-salt-agar-msa-composition-uses-and-colony-characteristics/
- https://www.uwyo.edu/molb2021/additional_info/summ_biochem/msa.html
- https://www.humeau.com/media/blfa_files/__TC_Mannitol-oealt-Agar_EN_280618_55016010054.pdf
- https://www.austincc.edu/microbugz/mannitol_salt_agar.php#:~:text=Mannitol%20Salt%20Agar%20(MSA)%20is,they%20typically%20grow%20very%20weakly.
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/264/373/m9052dat.pdf
- https://www.scientistcindy.com/mannitol-salt-agar-msa-test.html