What are Macrophages?
- Macrophages, integral components of the immune system, are specialized cells primarily involved in the process of phagocytosis. Originating from monocytes, a type of white blood cell, macrophages play a pivotal role in both innate and adaptive immunity. Their primary function is to engulf and digest cellular debris, foreign substances, microbes, and cancer cells that do not possess proteins specific to healthy body cells. This process, known as phagocytosis, is a critical defense mechanism against infection and injury.
- In terms of distribution, macrophages are present in virtually all tissues. They exhibit amoeboid movement, enabling them to patrol for potential pathogens. These cells take various forms and names in different parts of the body, such as histiocytes, Kupffer cells in the liver, alveolar macrophages in the lungs, and microglia in the brain. All these forms are part of the mononuclear phagocyte system. Besides their role in phagocytosis, macrophages are crucial in initiating specific defense mechanisms by recruiting other immune cells like lymphocytes. They are particularly significant as antigen presenters to T cells.
- Macrophages are also involved in modulating immune responses. They can either stimulate or decrease inflammation through cytokine release. Functionally, they are categorized into two types: M1 macrophages, which promote inflammation, and M2 macrophages, which reduce inflammation and aid in tissue repair. This functional dichotomy is reflected in their metabolic activities; M1 macrophages metabolize arginine to nitric oxide, while M2 macrophages convert it to ornithine. However, recent studies suggest that this classification might be an oversimplification, indicating a more complex nature of macrophage functions.
- Regarding their physical characteristics, human macrophages are approximately 21 micrometres in diameter. They are identifiable through specific protein expressions such as CD14, CD40, CD11b, CD64, and others, using techniques like flow cytometry or immunohistochemical staining.
- The discovery of macrophages dates back to 1884 by Élie Metchnikoff, a Russian zoologist. Since then, the understanding of their role in the immune system has significantly evolved. Macrophages not only recognize and engulf foreign particles through toll-like receptors but also play a vital role in presenting these particles to T cells, thus orchestrating the immune response. They are also involved in producing reactive oxygen species to destroy engulfed cells.
- In summary, macrophages are large, specialized cells capable of phagocytosing foreign particles and are found predominantly in body tissues, especially during infection. They originate from monocytes in the bone marrow, circulate in the blood, and differentiate into macrophages in various tissues. Their functions extend beyond simple phagocytosis, playing a crucial role in bridging innate and adaptive immunity and participating actively in immune regulation and tissue homeostasis.
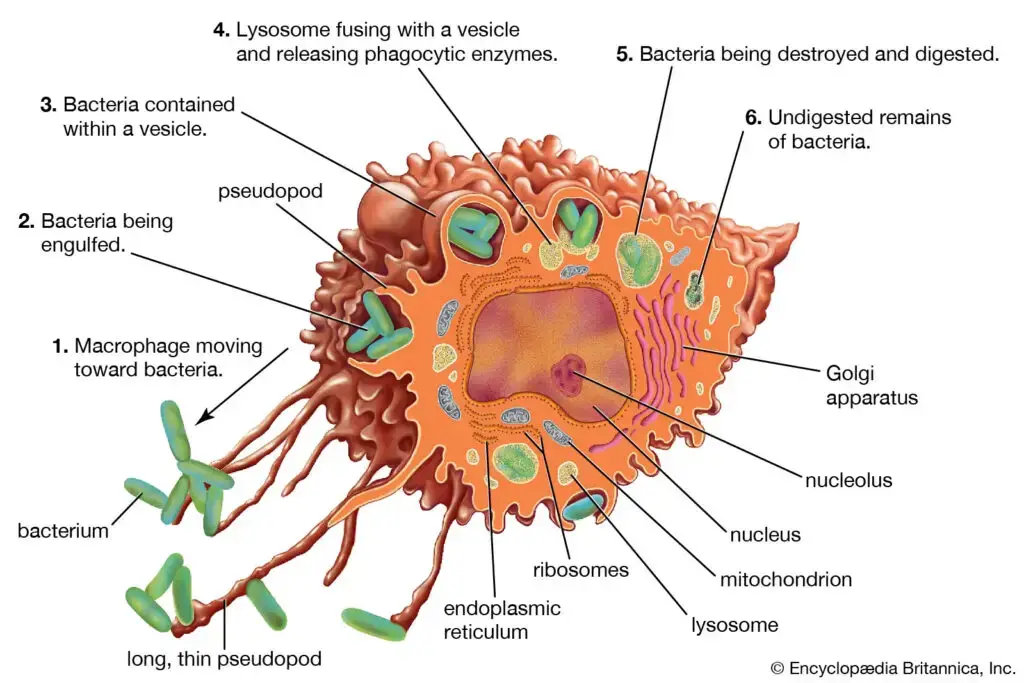
Definition of Macrophage
A macrophage is a type of white blood cell found in the immune system, known for its ability to engulf and digest cellular debris, foreign substances, and pathogens. It plays a crucial role in both innate and adaptive immunity, aiding in defense against infections and in tissue repair.
Structure of Macrophages
- Morphological Characteristics: Macrophages exhibit a variable morphology, primarily influenced by their state of activity. Typically, these cells range in size from 10 to 30 micrometers in diameter. The structural adaptability of macrophages allows them to efficiently perform their role in the immune system.
- Cytoplasmic Composition: The cytoplasm of a macrophage is characterized by the presence of vacuoles and granules, which exhibit a basophilic nature. This aspect of the cytoplasm is crucial for the cell’s phagocytic function. The nucleus of the macrophage is ovoid in shape, measuring approximately 6 to 12 micrometers in diameter, which is a significant aspect of its structural identity.
- Microscopic Appearance: Under a phase-contrast microscope, peritoneal macrophages display a distinct appearance. Their cytoplasm appears light gray and diffuse, containing dark gray, rod-shaped mitochondria. This microscopic observation is essential for identifying and studying macrophages.
- Cytoplasmic Periphery: The peripheral region of the macrophage’s cytoplasm is finely granular. Notably, it lacks structures such as endoplasmic reticula and attached ribosomes, which differentiates it from other cell types.
- Vesicular Structures: Within the cytoplasm, three distinct types of vesicles are observable. These include pinocytic vesicles containing variously sized organelles and diner granular material. This vesicular diversity is indicative of the macrophage’s active role in material uptake and processing.
- Nuclear and Ribosomal Association: Ribosomes are often found attached to the external portion of the nuclear membrane. This membrane is continuous with the endoplasmic reticula, highlighting a structural relationship crucial for protein synthesis and processing within the cell.
- Presence of Dense Granules: The cytoplasm of macrophages contains dense granules, predominantly secondary lysosomes derived from endocytic vacuoles. These granules are integral to the macrophage’s digestive and defensive functions.
- Inflammatory Macrophages: In inflammatory conditions, macrophages exhibit slim cytoplasmic extensions. These extensions are often tightly intertwined with adjacent epithelioid cells, a characteristic feature in inflammatory responses.
- Formation of Giant Cells: In certain conditions, such as in granulomas, there is evidence suggesting the formation of giant cells. These are believed to result from the fusion of preexisting macrophages, indicating a unique adaptive structural response to specific pathological conditions.
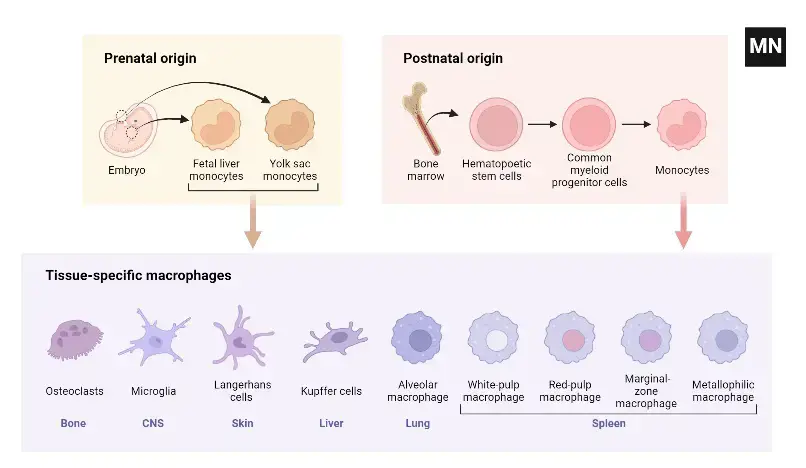
Development of macrophages
- Early Embryonic Development: The genesis of macrophages commences during the initial stages of embryogenesis. This process, known as primitive hematopoiesis, occurs between embryonic days 6.5 and 8.5. Precursor cells within the extraembryonic yolk sac are the first to differentiate into macrophages. As embryonic development progresses, hematopoiesis transitions to the fetal liver around day 10.5 and eventually establishes in the bone marrow in adult organisms, including humans.
- Mononuclear Phagocyte System (MPS): The MPS, formerly known as the reticuloendothelial system (RES), comprises monocytes, macrophages, and dendritic cells (DCs). This system plays a pivotal role in maintaining tissue and organismal homeostasis. It is also involved in various physiological and pathological processes, including inflammation, cancer, autoimmune diseases, infection, and immune responses in organ transplantation.
- Morphological Stages of Development: Macrophage development involves distinct morphological stages. Common myeloid progenitors (CMPs) evolve into monoblasts, promonocytes, and ultimately monocytes. These cells then migrate to various tissues. This differentiation process is regulated by specific gene expression programs, controlled by lineage-determining transcription factors (TFs).
- Role of Transcription Factors: The transcription factor PU.1 is critical in macrophage development. Its concentration influences the development of either macrophages or B cells. Other TFs, such as C/EBP-α, -β, -ε, IRF8, ZEB2, and MAFB, are also instrumental in macrophage lineage commitment and differentiation.
- Monocyte Subtypes in Mice: In mice, there are two primary subtypes of monocytes: classical Ly6chi monocytes and non-classical Ly6clow monocytes. Classical monocytes, characterized by high CCR2 and low CX3CR1 expression, rapidly migrate to infection and inflammation sites. Non-classical monocytes, with high CX3CR1 and low CCR2 expression, patrol vasculature and maintain endothelial integrity.
- Monocyte Differentiation in Humans: Human monocytes are differentiated into classical (CD14++CD16−), intermediate (CD14+CD16+), and non-classical (CD14lowCD16+) subsets. Non-classical monocytes in humans, akin to Ly6clow monocytes in mice, patrol vasculature and play roles in immune defense, particularly through TLR7 and TLR8 receptors.
- Regulation by Colony-Stimulating Factors: The development of mononuclear phagocytes from monocyte/macrophage progenitor cells is regulated by colony-stimulating factors such as M-CSF, GM-CSF, and Flt3-ligand. M-CSF specifically controls the quantity of tissue and organ monocytes/macrophages, while GM-CSF influences both activation and differentiation into DCs.
- Tissue-Resident Macrophages: Tissue-resident macrophages, including Kupffer cells in the liver, alveolar macrophages in the lungs, and macrophages in the spleen and peritoneum, are established prenatally. These cells can self-maintain independently of blood monocyte replenishment in adulthood. The characteristics and functions of macrophages in other tissues, such as endocrine and reproductive organs, adipose tissue, and musculoskeletal tissues, are less well-defined but equally significant.
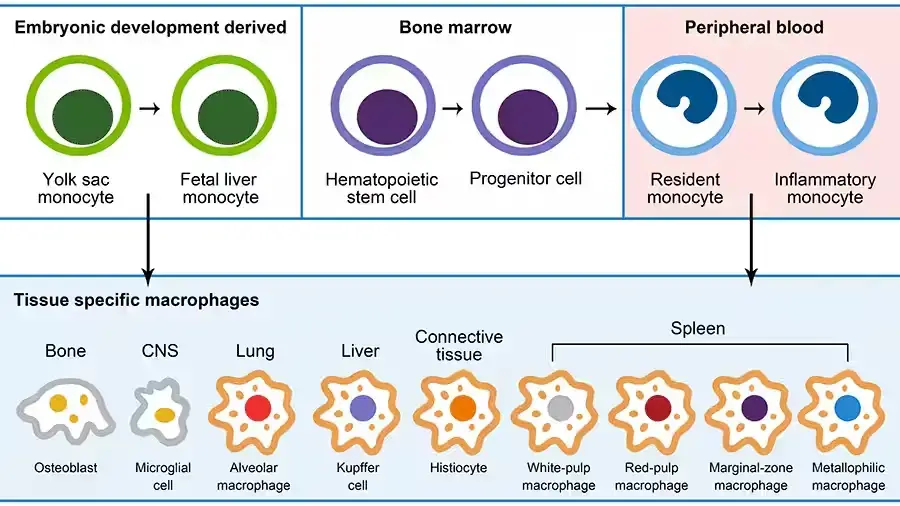
Origins of Macrophages
Macrophages, versatile immune cells, are found throughout the body, including the liver, brain, bones, and lungs. Each organ influences their differentiation and imparts specific functions. For example, alveolar macrophages in the lungs process surfactants, while those in the gastrointestinal tract and adipose tissue are crucial for maintaining homeostasis.
- Developmental Origins: Historically, macrophages were thought to originate from hematopoietic stem cells, differentiating into tissue-specific macrophages. However, research by Takahashi et al. revealed a dual origin: some macrophages derive from embryonic yolk sacs and self-renew in tissues, while others originate from hematopoietic stem cells. Van Furth et al.’s 1968 hypothesis suggested that tissue-resident macrophages are replenished by circulating monocytes from adult bone marrow. Advanced techniques like flow cytometry and DNA microarray analysis have further elucidated macrophage subsets, revealing diverse origins and maintenance mechanisms across different tissues.
- Macrophage Activation and Categorization: Macrophages are broadly categorized into M1 and M2 types. M1 macrophages, activated by pathogens, are involved in direct destruction and are pro-inflammatory. In contrast, M2 macrophages are associated with chronic inflammation, wound healing, and cancer metastasis. The activation of these phenotypes is influenced by various factors, including PAMPs, DAMPs, and cytokines like TNF-α and IFN-γ for M1, and anti-inflammatory cytokines such as IL-10, IL-4, and IL-13 for M2.
- Tissue-Specific Functions of Macrophages
- Lung Macrophages: In the lungs, macrophages derived from different sources (yolk sac, fetal liver, bone marrow) play distinct roles. Alveolar macrophages, crucial for pathogen recognition and immune response, are influenced by factors like PPAR-γ and TGF-β, which regulate their differentiation and function.
- Liver Macrophages (Kupffer Cells): Kupffer cells, liver-resident macrophages, are vital for clearing pathogens and managing iron and bilirubin metabolism. They also play a role in cholesterol metabolism and tissue repair, particularly in liver injuries.
- Brain Macrophages (Microglia): Microglia, derived from yolk sac progenitors, are responsible for sensing and responding to brain damage and neurodegenerative diseases. They produce neuroprotective factors and can also release nerve injury factors under certain conditions like Alzheimer’s disease.
- Intestinal Tract Macrophages: Intestinal macrophages, replaced by bone marrow-derived macrophages after birth, are central to host defense against infections. They maintain a unique balance, being phagocytic yet unresponsive to bacterial antigens, and play a role in immunosuppression and tissue repair.
- Spleen Macrophages: The spleen hosts various macrophage types, each with specific functions like clearing damaged blood cells, recycling iron, and participating in immune responses.
- Adipose Tissue Macrophages: In adipose tissue, macrophages are involved in regulating insulin sensitivity and glucose uptake, with a notable difference in function between obese and healthy individuals.
- Tumor-Associated Macrophages (TAMs): TAMs significantly influence tumor growth and expansion. They can promote vascularization, suppress antitumor immunity, and support cancer stem cell survival.
Mechanism of Macrophage
- Antigen Recognition: Macrophages play a pivotal role in the immune system, beginning with antigen recognition. They identify antigens, such as bacteria, through their toll-like receptors system (TLRs). These receptors are adept at recognizing pathogen-specific characteristics like lipopolysaccharides, nucleic acids, or proteins like flagellin from bacterial flagella. The binding of an antigen to TLRs triggers an alarm signal, activating and mobilizing other immune cells to combat the antigen.
- Microbial Killing: Primarily, macrophages mediate innate immune responses against bacteria, as they are not equipped to eliminate viral infections independently. In the case of viruses, T cells generate an antiviral mechanism to destroy the virus, after which macrophages remove the dead virus particles. Macrophages utilize either oxygen-dependent or oxygen-independent modes of pathogen destruction. Upon pathogen recognition, they become activated, exhibiting an increased propensity for phagocytosis and emitting inflammatory factors.
- Oxygen-independent Killing: This process involves the absorption of vesicles containing bacterial invaders into the macrophage (phagosome). Lysosomes, which contain hydrolytic enzymes, then fuse with the phagosome, leading to the destruction of the target organism. This method employs a series of enzymes: electrically charged proteins disrupt the cell membrane, lysozymes degrade the bacterial cell wall, lactoferrins extract iron from bacteria, and lysosomal proteolytic and hydrolytic enzymes degrade the proteins of dead bacteria. The remnants of the bacterial cell are then expelled from the macrophage.
- Oxygen-dependent Killing
- By Superoxide Dismutase: Following phagocytosis, macrophages experience a respiratory burst, consuming more oxygen and generating reactive oxygen species (ROS) or superoxides. Superoxide dismutase catalyzes the conversion of superoxide into hydrogen peroxide and singlet oxygen, which, along with hydroxyl radicals formed from superoxide and hydrogen peroxide, destroy the invading microorganism.
- By Nitric Oxide Synthase: Activation of macrophages increases the synthesis of nitric oxide synthase, enhancing the production of peroxynitrite radicals through the reaction of nitric oxide with hydrogen peroxide.
- By Myeloperoxidase: Found in neutrophil granules, this enzyme complex, upon contact with a phagosome, forms a phagolysosome and releases myeloperoxidase. This enzyme, using hydrogen peroxide and chlorine, produces hypochlorite, a highly toxic antimicrobial compound.
- Present Antigen to T Cell: In addition to releasing oxidative radicals, macrophages also secrete cytotoxic substances such as TNF-alpha, IL-1, 8, and 12 to induce an inflammatory response. As antigen-presenting cells, macrophages manufacture MHC class II molecules to present antigens to T helper cells. This collaboration between macrophages and T lymphocytes is crucial in combating foreign bodies.
Types of Macrophages
The macrophage reaches the target cell’s tissues, where it assists foreign body invasion or phagocytosis and removes dead cells. Therefore, macrophages comprise the mononuclear phagocyte system, a collection of phagocytic cells. Previously, macrophages were referred to as the reticuloendothelial system.
Types of Macrophages Based on Location
- Alveolar Macrophages: Located in the alveoli of the lungs, alveolar macrophages play a crucial role in respiratory defense. They are responsible for consuming tiny particles, dead cells, and germs that enter the respiratory system. Additionally, these macrophages are instrumental in stimulating the immune system upon encountering respiratory pathogens, thereby safeguarding the pulmonary system.
- Kupffer Cells: Found within the liver tissues, Kupffer cells are specialized macrophages that initiate immunological responses and contribute to hepatic tissue remodeling. Their strategic location in the liver enables them to filter and remove pathogens and toxins from the blood, playing a vital role in maintaining liver health and systemic immunity.
- Microglia: Microglia are the resident macrophages of the central nervous system. They are primarily involved in removing old or dead neurons and regulating brain immunity. These cells are essential for maintaining neural health, responding to injury or disease, and supporting the overall homeostasis of the brain and spinal cord.
- Splenic Macrophages: The spleen hosts several types of macrophages, including marginal zone, metallophilic, and red pulp macrophages. These cells are situated in the marginal zone of the spleen, which contains both red and white pulp. Their primary function is to dispose of non-functional and aged erythrocytes, thereby playing a critical role in blood filtration and immune response.
- Testicular Macrophages: Testicular macrophages are found in association with Leydig cells in the testes. They contribute to establishing an immune-privileged environment in the testis. One of their key functions includes the conversion of 25-hydroxycholesterol, an oxysterol, into testosterone, which is crucial for male reproductive health.
- Cardiac Resident Macrophages: Cardiac resident macrophages are located in the heart and are involved in electrical conduction. They interact with cardiac myocytes through gap junctions, playing a role in maintaining cardiac function and health. These macrophages are integral to the heart’s immune defense and contribute to the organ’s response to injury or infection.
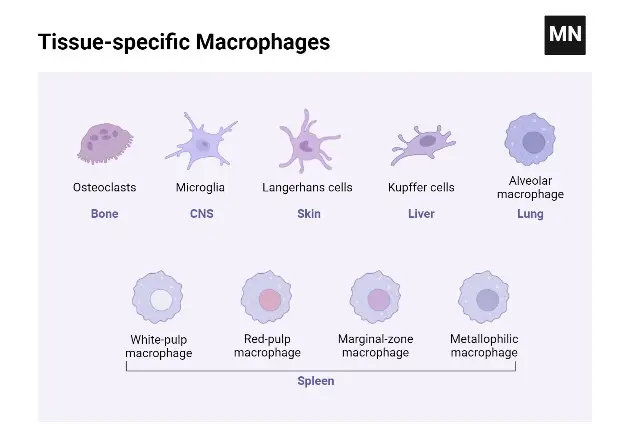
Types of Macrophages Based on Function & Activation
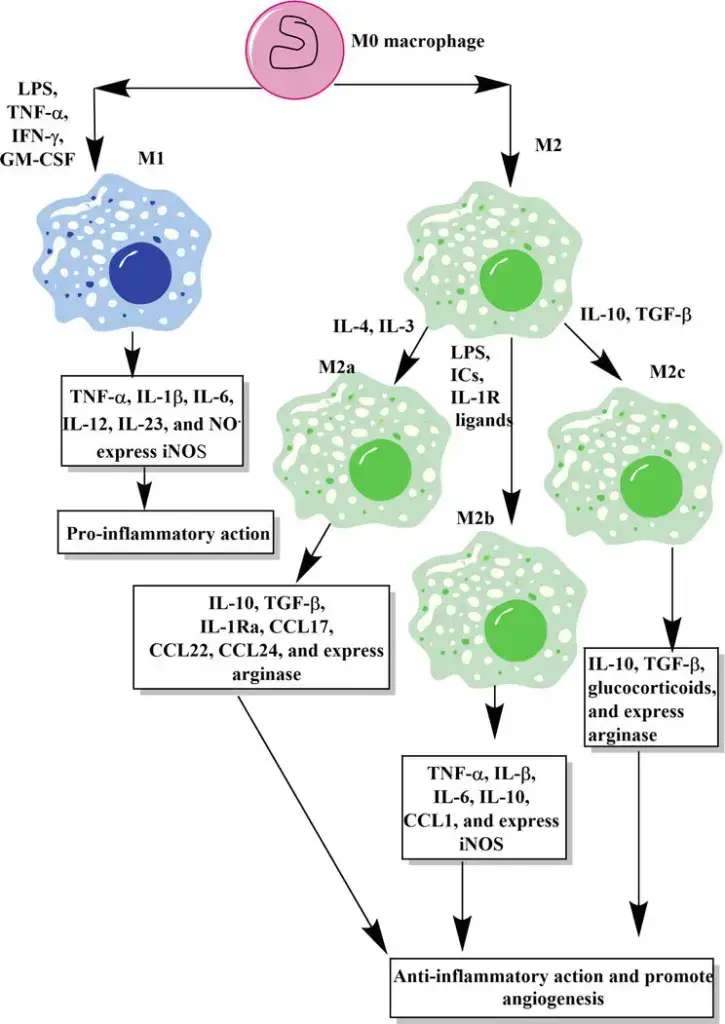
Macrophages, versatile cells of the immune system, exhibit significant heterogeneity in function and activation. This diversity is influenced by their location, morphology, infection recognition patterns, and cytokine production. They can be broadly classified into two main phenotypes: classically activated (M1) and alternatively activated (M2) macrophages. Additionally, a third subtype known as Mregs also plays a crucial role in immune regulation.
- Classically Activated Macrophages (M1)
- Activation Process: M1 macrophages are stimulated through toll-like receptors in the presence of interferon-γ.
- Functional Characteristics: These macrophages have an enhanced capacity for antigen presentation and produce significant amounts of nitric oxide and chemokines.
- Role in Immune Response: M1 macrophages are pivotal in defending against bacterial infections. However, their defense mechanisms can sometimes cause collateral damage to the host.
- Cytokine Production: They are known for producing inflammatory cytokines like IL-1, IL-6, and tumor necrosis factor-alpha.
- Alternatively Activated Macrophages (M2)
- Activation Process: M2 macrophages are activated in response to IL-4 and IL-13 produced by CD4+ T cells.
- Functional Characteristics: These macrophages express high levels of cytosolic arginase and proteins related to the extracellular matrix.
- Role in Immune Response: M2 macrophages are typically involved in responses to parasites and fungi. They play a crucial role in limiting inflammation and are essential for tissue repair and wound healing.
- Macrophage Regulators (Mregs)
- Function: Mregs are a subtype of macrophages that regulate the activity of other immune cells.
- Role in Immune System: They contribute to the fine-tuning of immune responses, ensuring a balanced reaction to pathogens and preventing excessive immune activity that could harm the host.
Factors Contributing to Macrophage Heterogeneity
- Diverse Locations: Macrophages are found in various tissues, each adapting to the specific needs of its environment.
- Morphological Differences: Their physical structure varies depending on their location and function.
- Infection Recognition: Different macrophages have unique patterns for recognizing infections, contributing to their functional diversity.
- Cytokine Production: The type and amount of cytokines produced by macrophages vary, influencing their role in the immune response.
Classically activated M1 macrophages
- Activation and Formation
- Stimulation Process: M1 macrophages are formed when macrophages are stimulated with toll-like receptors in the presence of interferon-γ.
- Resulting Phenotype: This stimulation leads to the development of macrophages with enhanced antigen-presenting capabilities and the ability to produce significant amounts of nitric oxide and chemokines.
- Role in Immune Defense
- Primary Function: M1 macrophages are crucial in defending against bacterial infections. However, their defense mechanisms can sometimes lead to collateral damage to the host.
- Immune Response Involvement: They are actively involved in both innate and adaptive immune responses, aiding in Th1 cell recruitment, pathogen resistance, and tumor management.
- Stimuli for Polarization
- Inducing Factors: Pathogens, lipopolysaccharides (LPS), granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) from Th1 cells typically stimulate the polarization of macrophages into M1 cells.
- Pathways Influencing Polarization
- Key Pathways: The transition to M1 polarization involves several pathways, including IRF/STAT, LPS/TLR4, and NF-κB/PI-3 kinase.
- Characteristic Features
- Antigen Presentation and Cytokine Synthesis: M1 macrophages are characterized by their high antigen presentation activity and the production of pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, TNF-α, nitric oxide (NO), and reactive oxygen species (ROS).
- Cytokine Profile: They exhibit overexpression of IL-12 and IL-23 and downregulation of IL-10. Upon stimulation, M1 macrophages produce significant levels of IL-1b, TNF-α, IL-12, IL-18, and IL-23.
- Expression Markers
- Surface and Secretory Markers: The M1 macrophage phenotype expresses high levels of major histocompatibility complex class II (MHC II), CD68, CD80, and CD86.
- Chemokine Production: They also produce Th1 cell-attracting chemokines such as CXCL9 and CXCL12.
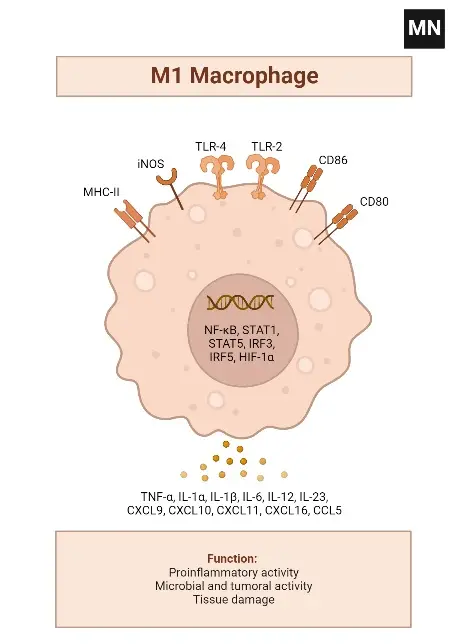
Alternatively activated M2 macrophages
- Activation Mechanism
- Stimuli for Activation: M2 macrophages are activated in response to IL-4 and IL-3, produced by CD4+ T cells. This activation typically occurs in response to parasites and fungi.
- Characteristic Expression: These macrophages express high levels of cytosolic arginase and proteins related to the extracellular matrix.
- Functional Role
- Inflammation and Healing: M2 macrophages play a crucial role in limiting inflammation and are instrumental in tissue repair and wound healing.
- Additional Activation Triggers
- Diverse Stimuli: Activation can also occur due to parasite or fungal infections, immune complexes, apoptotic cells, macrophage colony-stimulating factor (M-CSF), IL-13, TGF-b, and Th2 cytokines like IL-4, IL-33, and IL-25.
- Signaling Pathways: The transition to the M2 state involves signaling through STAT6, IRF4, PPARδ, and PPARγ.
- Cytokine Profile
- Inverse Expression: Contrasting with M1 macrophages, M2 macrophages exhibit downregulation of IL-12 and IL-23 and overexpression of IL-10 and IL-1RA.
- Pro-inflammatory Cytokines: They produce lower levels of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α.
- Broad Spectrum of Functions
- Versatile Roles: Beyond pathogen clearance, M2 macrophages are involved in anti-inflammatory responses, metabolism, wound healing, tissue remodeling, immunoregulation, tumor growth, and malignancies.
- Phenotypic Markers
- Surface Markers: The M2 phenotype is indicated by the expression of CD206, CD163, CD209, FIZZ1, and Ym1/2.
- Phagocytic and Scavenging Receptors: They demonstrate high expression of receptors crucial for the phagocytosis and scavenging of mannose and galactose.
- Ornithine and Polyamine Synthesis: A notable feature is the high level of ornithine and polyamine synthesis via the arginase pathway.
- Chemokine Production
- Chemokine Expression: M2 macrophages express chemokines such as CCL1, CCL17, CCL18, CCL22, and CCL24, which are essential for various immune functions.
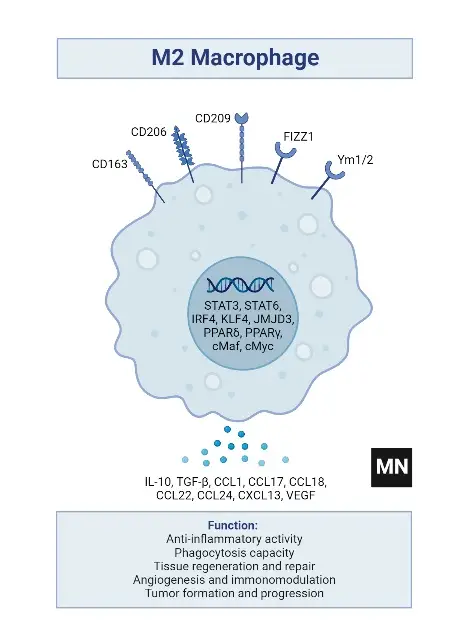
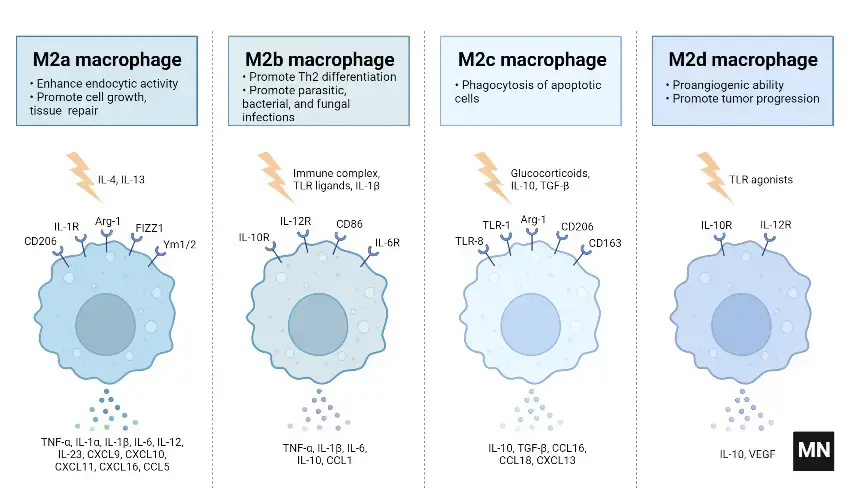
Subtypes of M2 macrophages
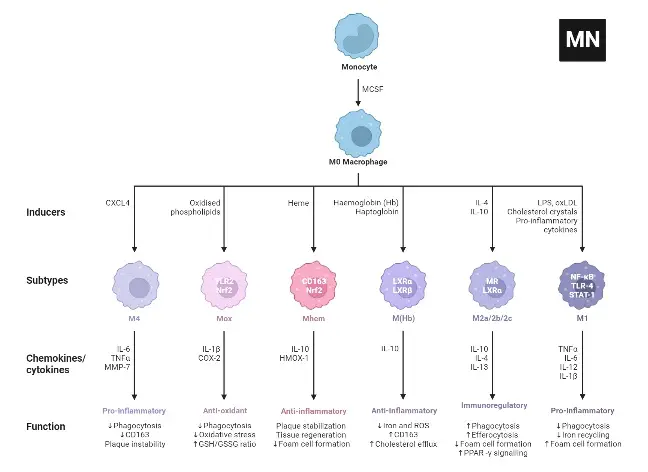
M2 macrophages are categorized into four subtypes: M2a, M2b, M2c, and M2d. These subtypes are differentiated based on their activation stimuli, cell surface markers, secreted cytokines, and biological functions. All M2 subtypes exhibit IL-10 expression, a key anti-inflammatory cytokine.
- M2a Macrophages
- Activation: M2a macrophages are activated by IL-4 or IL-13.
- Cell Surface Marker: IL-4 induces the expression of the mannose receptor (CD206).
- Function and Cytokine Profile: They are known for promoting cell proliferation, tissue healing, and endocytosis, with elevated levels of IL-10, TGF-b, CCL17, CCL18, and CCL22.
- M2b Macrophages
- Activation: This subtype is activated by immune complexes, Toll-like receptor (TLR) ligands, and IL-1b.
- Cytokine Secretion: M2b macrophages release both pro- and anti-inflammatory cytokines, including TNF-α, IL-1b, IL-6, and IL-10.
- Role: They primarily regulate the immune response and inflammation.
- M2c Macrophages
- Activation: M2c macrophages are activated by glucocorticoids, IL-10, and TGF-b.
- Distinguishing Features: They are characterized by high levels of anti-inflammatory IL-10, pro-fibrotic TGF-b, CCL16, CCL18, and Mer receptor tyrosine kinase (MerTK).
- Function: M2c macrophages are involved in the phagocytosis of apoptotic cells and exhibit pro-fibrotic activities.
- M2d Macrophages
- Activation: The M2d subtype is activated by TLR antagonists, IL-6, and adenosines.
- Induced Expression: Adenosines induce the expression of IL-10 and vascular endothelial growth factor (VEGF).
- Biological Impact: This subtype is implicated in angiogenesis and tumor progression.
Functions of Macrophages
- Phagocytosis and Innate Immunity
- Role in Homeostasis: Macrophages are pivotal in maintaining homeostasis through the removal of dead cells and cellular debris.
- Mechanism: They employ phagocytosis, a key mechanism in innate immunity, to engulf and digest cellular debris and pathogens.
- Significance: This function is crucial for clearing infections and maintaining tissue integrity.
- Antigen Presentation and Immune Response Initiation
- Interaction with Immune Cells: Macrophages present antigens to T cells, thereby initiating adaptive immune responses.
- Secretion of Chemokines: They secrete a variety of chemokines and substances that activate adaptive immune cells.
- Role in Immunity: This antigen-presenting capability is essential for the body’s defense against a wide array of pathogens.
- Muscle Repair and Regeneration
- Involvement in Muscle Health: Macrophages play a significant role in muscle repair, growth, and regeneration.
- Post-Inflammatory Action: They are particularly active in repairing and regenerating tissues following inflammation.
- Therapeutic Implications: Understanding this function is vital for developing treatments for muscle-related injuries and diseases.
- Wound Healing and Tissue Repair
- M2 Macrophages in Healing: M2 macrophages, also known as wound healing macrophages, are instrumental in limiting inflammation.
- Facilitation of Tissue Repair: They enable tissue repair and regeneration by modulating the inflammatory response.
- Clinical Relevance: This aspect is crucial in wound management and recovery processes.
- Iron Homeostasis
- Scavenging Role: As scavengers, macrophages continuously remove dead erythrocytes from the blood.
- Iron Storage: They store iron released during this process in the form of ferritin.
- Importance in Iron Regulation: This function is integral to the body’s iron homeostasis, impacting various physiological processes.
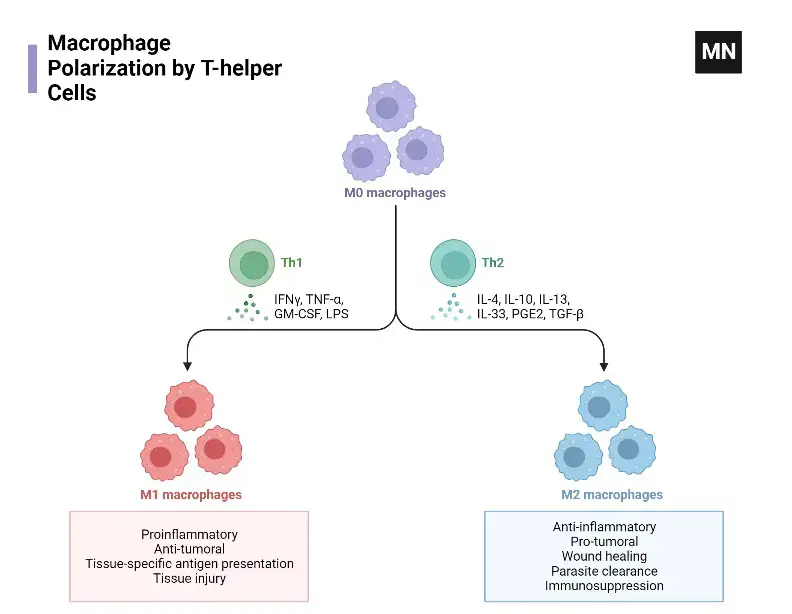
What is Macrophage polarization?
- Definition and Significance
- Concept: Macrophage polarization is the process where macrophages acquire diverse functions and phenotypes based on activation states.
- Adaptability: This process is dynamic, allowing macrophages to adapt to different pathogens, tissue environments, and inflammatory conditions.
- Relevance: Understanding polarization is crucial for comprehending macrophage roles in immune responses and disease processes.
- Regulatory Mechanisms
- Epigenetic and Cell Survival Factors: These mechanisms are fundamental in determining the polarization state of macrophages.
- External Stimuli Influence: Pathogens, PAMPs, and allergens significantly impact macrophage polarization.
- Tissue Environment Role: DAMPs in the tissue environment also contribute to the polarization process.
- Classification of Polarization Subtypes
- Major Categories: The primary subtypes include M0 (naïve), M1 (classically activated), and M2 (alternatively activated) macrophages.
- M1 Activation: M1 macrophages are activated by bacterial components and Th1 cytokines, exhibiting pro-inflammatory functions.
- M2 Activation and Subtypes: M2 macrophages, induced by Th2 cytokines, are further divided into M2a, M2b, and M2c, each with specific activation stimuli and roles in tissue remodeling and anti-inflammatory responses.
- Metabolic Processes and Functions
- M1 Metabolism: M1 macrophages utilize glycolysis, characterized by increased lactate production and reduced oxygen consumption.
- M2 Metabolism: M2 macrophages rely on FAO and OXPHOS for energy, exhibiting anti-inflammatory functions.
- Succinate’s Role: This metabolite regulates polarization via SUCNR1, influencing the balance between M1 and M2 phenotypes.
- Resolution-Phase Macrophages (rMs)
- Unique Phenotype: rMs express markers of both M1 and M2 macrophages.
- Role in Post-Resolution Events: They are crucial in lymphocyte repopulation and restoring tissue homeostasis after inflammation.
Differences between M1 and M2 macrophages
| Aspects | M1 macrophages | M2 macrophages |
|---|---|---|
| 1. Phenotype | Express high levels of MHC-II, CD68, and CD80 and CD86 costimulatory molecules | Express higher levels of CD206, CD200R, CD163 and transcription factor called CMAF (musculoaponeurotic fibrosarcoma) and response gene to complement 32 (RGC-32) |
| 2. Upregulated genes | Suppressor of cytokine signaling 3 (SOCS3), iNOS or NOS2, Macrophage receptor with collagenous structure (Marco), Il12B, Il23a (Il23p19) and Ptgs2 (Cox2) | Arg1, MMR (Mrc1), resistin-like molecule α (FIZZ1) or Relma or Retnla, Ym1, Irf4, Cxcl12, Cxcl13, Ccl24 and Klf4 |
| 3. Action | Pro-inflammatory | Anti-inflammatory |
| 4. Cytokines and chemokines produced | IFN-γ, IL-8, TNF-α, IL-1β, RANTES (CCL5), CXCL10 | IL-13, IL-10, CCL17, CCL18, CCL22 |
| 5. Metabolic pathway | Glycolysis and glutaminolysis | FAO and OXPHOS |
| 6. HIF-1α expression | High | Low |
| 7. Inducers or stimuli | IFN-γ, PAMPs (i.e. LPS), GM-CSF | Glucocorticoids, IL-10, IL-4, IL-13 and M-CSF |
| 8. ROS and RNS production | High ROS and NO. production | Low ROS and NO. production |
| 9. Rate of acidification | Low | High |
| 10. Antimicrobial action | High | Low |
| 11. Glucose uptake | Mainly depends on HIF-1α and Akt/mTORC1 activation | Mainly depends on Akt/mTORC1 activation |
| 12. Macrophage galactase-type C-type lectins | Low | High |
| 13. Autophagy | Induce autophagy during tuberculosis (TB) infection | Decrease autophagy during TB infection |
Activation of Macrophages
- Macrophages can be activated by a variety of stimuli, including cytokines, bacterial products, and viral proteins.
- When macrophages encounter these stimuli, they undergo a process called activation, which leads to changes in their morphology, function, and gene expression.
- Activation of macrophages can be classified into two main types: classical activation and alternative activation.
- Classical activation is induced by molecules such as interferon-gamma (IFN-γ) and lipopolysaccharide (LPS), and leads to the production of pro-inflammatory cytokines and the enhancement of phagocytic activity.
- Alternative activation is induced by molecules such as interleukin-4 (IL-4) and IL-13, and leads to the production of anti-inflammatory cytokines and the promotion of tissue repair and regeneration.
- In addition to these two main types of activation, macrophages can also be activated by other stimuli such as oxidized lipids, immune complexes, and extracellular matrix components.
- The specific mode of activation of macrophages depends on the nature of the stimulus and the microenvironment in which they are located.
What are alveolar macrophages?
- Alveolar macrophages are a type of macrophage that resides in the air sacs (alveoli) of the lungs. These cells play a critical role in protecting the lungs from infection and other harmful substances.
- Alveolar macrophages are constantly exposed to a variety of airborne particles, including bacteria, viruses, and other foreign particles. When they encounter these substances, they engulf and digest them, helping to prevent infection and inflammation in the lungs.
- In addition to their role in innate immunity, alveolar macrophages also play a role in regulating the immune response in the lungs. They secrete cytokines and other signaling molecules that help to recruit and activate other immune cells, and can also promote the resolution of inflammation.
- Overall, alveolar macrophages are an important component of the immune system in the lungs, helping to maintain the delicate balance between protecting the lungs from infection and preventing excessive inflammation.
What are hemosiderin laden macrophages?
- Hemosiderin-laden macrophages are a type of macrophage that have ingested large amounts of iron, which is stored in the form of hemosiderin. Hemosiderin is a complex of iron and protein that is formed when red blood cells are broken down and their iron is released into the bloodstream.
- Hemosiderin-laden macrophages are often found in tissues where there has been bleeding or other forms of tissue damage that result in the release of red blood cells. These macrophages play an important role in clearing the excess iron from the tissues and preventing iron toxicity.
- In some diseases, such as hemochromatosis, iron can accumulate in the body to toxic levels. In these cases, hemosiderin-laden macrophages can be seen in various tissues throughout the body, as they work to remove excess iron from the circulation and store it safely. The presence of these macrophages can be seen on microscopic examination of tissues, and can help to diagnose and monitor the progression of these diseases.
What is m2 macrophages?
- M2 macrophages are a subset of macrophages that are characterized by their anti-inflammatory and tissue repair functions. These macrophages are activated by cytokines such as interleukin-4 (IL-4) and IL-13, and are involved in tissue remodeling, wound healing, and regulation of the immune response.
- M2 macrophages are involved in a variety of physiological and pathological processes, including tissue repair and regeneration, allergy and asthma, and cancer. They secrete anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta (TGF-β), and express high levels of scavenger receptors that allow them to clear cellular debris and apoptotic cells.
- M2 macrophages are important for promoting the resolution of inflammation and restoring tissue homeostasis after injury or infection. However, dysregulation of M2 macrophage activation has been implicated in the pathogenesis of diseases such as fibrosis and cancer, where their anti-inflammatory and tissue repair functions may become excessive and contribute to tissue damage and tumor progression.
What are m1 macrophages?
- M1 macrophages are a type of activated macrophage that play a key role in the early stages of the immune response to infections and other inflammatory stimuli. These macrophages are characterized by their pro-inflammatory phenotype, which is induced by signals such as lipopolysaccharide (LPS) and interferon-gamma (IFN-gamma).
- M1 macrophages produce a variety of pro-inflammatory cytokines and chemokines, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1beta), and IL-6, which help to recruit and activate other immune cells to the site of infection or inflammation. M1 macrophages also produce reactive oxygen and nitrogen species, which are important for killing bacteria and other pathogens.
- In addition to their role in the immune response to infections, M1 macrophages have been implicated in the pathogenesis of several inflammatory diseases, including atherosclerosis, rheumatoid arthritis, and multiple sclerosis. In these diseases, M1 macrophages contribute to tissue damage and inflammation through the production of pro-inflammatory cytokines and the activation of other immune cells.
- Overall, M1 macrophages play a critical role in the early stages of the immune response to infections and other inflammatory stimuli, but their prolonged activation can contribute to the pathogenesis of chronic inflammatory diseases.
What are tingible body macrophages?
- Tingible body macrophages, also known as histiocytes, are a type of macrophage that are commonly found in lymphoid tissues such as lymph nodes and the spleen. These macrophages have a distinctive appearance, with numerous phagocytosed cellular debris and apoptotic bodies within their cytoplasm, giving them a “tingible” appearance when viewed under a microscope.
- Tingible body macrophages play an important role in the immune response by removing and processing apoptotic cells and other cellular debris. This process, known as phagocytosis, is essential for maintaining tissue homeostasis and preventing the accumulation of potentially harmful cellular waste products.
- In lymphoid tissues, tingible body macrophages are particularly important for removing apoptotic lymphocytes and other immune cells that have fulfilled their function or become damaged during the immune response. This helps to prevent the accumulation of potentially autoreactive or harmful immune cells and contributes to the resolution of inflammation.
- The presence of tingible body macrophages in lymphoid tissues can be seen on microscopic examination, and their absence or abnormal appearance can be a sign of immune dysfunction or disease.
What are foamy macrophages?
- Foamy macrophages are a type of macrophage that have an unusual appearance due to the accumulation of lipid droplets within their cytoplasm. These lipid droplets give the macrophages a “foamy” or vacuolated appearance when viewed under a microscope.
- Foamy macrophages are commonly found in a variety of pathological conditions, including atherosclerosis, tuberculosis, and lipid storage diseases such as Niemann-Pick disease. The accumulation of lipid droplets within these macrophages is typically a sign of lipid overload, and can be caused by a variety of factors including impaired lipid metabolism, inflammation, and oxidative stress.
- In atherosclerosis, foamy macrophages are involved in the formation of lipid-rich plaques within arterial walls. These macrophages take up and accumulate low-density lipoprotein (LDL) cholesterol, leading to the formation of foam cells that contribute to the development of atherosclerotic plaques.
- In tuberculosis, foamy macrophages are involved in the containment of the infection. These macrophages engulf and sequester mycobacteria within lipid droplets, forming granulomas that can help to limit the spread of the infection.
- Overall, the presence of foamy macrophages is a sign of abnormal lipid metabolism and can be a marker of disease or pathological conditions.
What are tumor associated macrophages?
- Tumor-associated macrophages (TAMs) are a type of immune cell that infiltrate tumors and have been shown to play a key role in tumor growth and progression. These macrophages are recruited to the tumor microenvironment by chemokines and cytokines produced by cancer cells and other stromal cells.
- TAMs can have a variety of different phenotypes, depending on the signals they receive within the tumor microenvironment. In general, TAMs are thought to promote tumor growth and progression by promoting angiogenesis, suppressing the immune response, and remodeling the extracellular matrix to support tumor invasion and metastasis.
- TAMs have been shown to have complex interactions with cancer cells and other stromal cells within the tumor microenvironment. For example, TAMs can promote the survival and growth of cancer cells by producing growth factors and extracellular matrix proteins, while also suppressing the activity of cytotoxic T cells and other immune cells that would normally target and eliminate cancer cells.
- Targeting TAMs is an area of active research in cancer immunotherapy, with the goal of developing new treatments that can enhance the anti-tumor immune response and prevent TAMs from promoting tumor growth and progression.
m1 vs m2 macrophages
M1 and M2 macrophages are two different subsets of macrophages that are characterized by their distinct functions and gene expression profiles.
M1 macrophages are classically activated macrophages that are induced by cytokines such as interferon-gamma (IFN-γ) and lipopolysaccharide (LPS). M1 macrophages are involved in pro-inflammatory responses, and are important for host defense against intracellular pathogens such as viruses and bacteria. They produce pro-inflammatory cytokines such as interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α), and express high levels of inducible nitric oxide synthase (iNOS) which produces nitric oxide (NO), a potent antimicrobial molecule.
M2 macrophages, on the other hand, are alternatively activated macrophages that are induced by cytokines such as interleukin-4 (IL-4) and IL-13. M2 macrophages are involved in tissue repair and remodeling, and are important for regulating the immune response in the context of allergic reactions and parasitic infections. They produce anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta (TGF-β), and express high levels of scavenger receptors that allow them to clear cellular debris and apoptotic cells.
Overall, M1 and M2 macrophages represent two ends of a spectrum of macrophage activation states, with M1 macrophages involved in pro-inflammatory responses and M2 macrophages involved in anti-inflammatory and tissue repair functions. The balance between these two macrophage subsets is important for maintaining tissue homeostasis and protecting against infection and inflammation.
Dendritic cells vs Macrophages
- Dendritic cells and macrophages are both types of immune cells that play important roles in the immune system’s response to infections and other stimuli, but they have some key differences in their functions and behaviors.
- Dendritic cells are specialized antigen-presenting cells that are capable of activating T cells, which are key players in the adaptive immune response. Dendritic cells are found in tissues such as the skin, mucous membranes, and lymphoid organs, where they capture and process antigens from pathogens or other sources. Once activated, dendritic cells migrate to lymph nodes, where they present the antigens to T cells and initiate an adaptive immune response.
- Macrophages, on the other hand, are phagocytic cells that are involved in the innate immune response. They are found in many different tissues throughout the body, where they play roles in tissue homeostasis, clearance of dead cells and debris, and host defense against infections. Macrophages can be activated by a variety of stimuli, including microbial products, cytokines, and damaged tissue, and can assume different functional states depending on the signals they receive.
- While dendritic cells and macrophages share some common features, such as their ability to phagocytose and process antigens, they have different roles and functions within the immune system. Dendritic cells are specialized in activating T cells and initiating an adaptive immune response, while macrophages are involved in tissue homeostasis and host defense in the innate immune response.
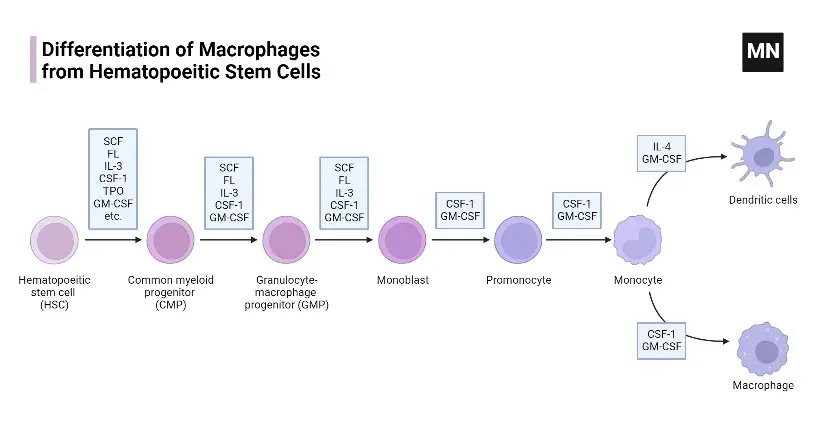
Differentiation of monocyte into a tissue macrophage involves a number of changes as follows
The process of monocyte differentiation into a tissue macrophage involves several notable changes and adaptations, which are as follows:
- Cell Enlargement: During the differentiation process, the monocyte undergoes a significant increase in size. The cell enlarges approximately 5 to 10 folds compared to its original monocyte form. This enlargement is necessary to accommodate the increased metabolic and functional demands of a mature tissue macrophage.
- Increased Intracellular Organelles: As monocytes differentiate into tissue macrophages, their intracellular organelles undergo changes in both number and complexity. The macrophage develops a greater abundance of organelles such as mitochondria, endoplasmic reticulum, and Golgi apparatus. These organelles support the heightened metabolic activity required for the macrophage’s phagocytic and secretory functions.
- Enhanced Phagocytic Ability: One of the key characteristics of tissue macrophages is their increased phagocytic ability compared to monocytes. Through the process of differentiation, monocytes acquire specialized receptors and molecular machinery that enable them to efficiently engulf and internalize pathogens, cellular debris, and foreign substances. This enhanced phagocytic ability allows tissue macrophages to contribute to immune surveillance and clearance of potentially harmful materials.
- Increased Production of Hydrolytic Enzymes: As monocytes differentiate into tissue macrophages, they upregulate the production of hydrolytic enzymes. These enzymes, such as proteases and nucleases, play a crucial role in the degradation and breakdown of engulfed particles within the macrophage’s phagolysosomes. The increased production of hydrolytic enzymes enables tissue macrophages to effectively break down and eliminate internalized pathogens and cellular debris.
- Secretion of Soluble Factors: During differentiation, tissue macrophages begin to secrete a variety of soluble factors into their surrounding environment. These factors include cytokines, chemokines, growth factors, and other bioactive molecules. Soluble factors released by tissue macrophages serve multiple functions, including immune modulation, recruitment of other immune cells, tissue repair, and regulation of inflammatory responses. The secretion of these soluble factors allows tissue macrophages to communicate with other cells and coordinate immune and tissue homeostasis processes.
Overall, the differentiation of monocytes into tissue macrophages involves a series of changes that prepare the cells for their specialized roles in immune defense, tissue maintenance, and modulation of inflammatory responses. These changes include cell enlargement, increased intracellular organelles, enhanced phagocytic ability, elevated production of hydrolytic enzymes, and secretion of various soluble factors.
Examples of Macrophages
- Alveolar macrophages in the lung: These macrophages reside in the air sacs of the lungs and are responsible for clearing inhaled particles, pathogens, and cellular debris. They play a crucial role in maintaining lung health and defense against respiratory infections.
- Histiocytes in connective tissues: Histiocytes are macrophages found in connective tissues throughout the body. They participate in the immune response by engulfing foreign substances and debris, contributing to tissue repair, and interacting with other immune cells.
- Kupffer cells in the liver: Kupffer cells are specialized macrophages located in the liver sinusoids. They help remove pathogens, toxins, and cellular waste from the bloodstream, playing a vital role in maintaining liver function and detoxification processes.
- Mesangial cells in the kidney: Mesangial cells are a type of macrophage found in the glomeruli of the kidney. They regulate the filtration process and help eliminate immune complexes and cellular debris, contributing to kidney function and preventing inflammation.
- Microglial cells in the brain: Microglial cells are the resident macrophages of the central nervous system. They surveil the brain environment, phagocytose pathogens and cellular debris, and play a crucial role in neuroinflammation and the immune response within the brain.
- Osteoclasts in the bone: Osteoclasts are specialized macrophages involved in bone remodeling. They resorb bone tissue, allowing for the constant renewal and maintenance of bone structure.
Activation of Macrophages:
Macrophages require stimulation and activation to reach an “activated state” and exhibit enhanced immune functions. Various factors can activate macrophages, including:
- Cytokines: Gamma interferon (produced by helper T cells) is a potent activator of macrophages. Other cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-alpha), can also activate macrophages and promote their immune functions.
- Bacterial components: Macrophages can be activated by components of bacterial cell walls, such as lipopolysaccharides (endotoxins) and peptidoglycan. These substances are recognized by macrophage receptors, triggering their activation.
- Inflammatory mediators: Mediators of the inflammatory response, such as prostaglandins and leukotrienes, can activate macrophages and enhance their phagocytic and microbicidal capabilities.
Activated Macrophages:
Once activated, macrophages exhibit enhanced immune functions and become more potent in their roles. Some characteristics of activated macrophages include:
- Increased phagocytic ability: Activated macrophages have an enhanced capacity to engulf and eliminate microbes, cellular debris, and foreign substances through phagocytosis.
- Enhanced microbial killing: Activated macrophages release various cytotoxic proteins and reactive oxygen species, enabling them to effectively kill a broad range of pathogens, including virus-infected cells, tumor cells, and intracellular bacteria.
- Improved antigen presentation: Activated macrophages serve as more effective antigen-presenting cells (APCs). They process and present antigens to T cells, facilitating a more robust T-cell response and adaptive immune activation.
- Secretion of immune mediators: Activated macrophages secrete a range of cytokines, chemokines, and other immune mediators that help recruit and activate other immune cells, amplifying the immune response against pathogens or abnormal cells.
Overall, activated macrophages play a crucial role in the immune system by eliminating pathogens, promoting tissue repair, and coordinating immune responses to maintain health and combat infections and diseases.
FAQ
What are macrophages?
Macrophages are a type of white blood cell that play an important role in the immune system. They are large cells that can engulf and digest harmful substances such as bacteria, viruses, and dead cells. They are derived from stem cells in the bone marrow and can be found in all tissues of the body. Macrophages are important for both innate and adaptive immune responses and act as the first line of defense against invading pathogens. They also secrete cytokines and other molecules that help to recruit and activate other immune cells. Additionally, macrophages play a role in tissue repair and regeneration.
What do macrophages do?
Macrophages are a type of white blood cell that play an important role in the immune system. They are large, specialized cells that engulf and digest harmful substances such as bacteria, viruses, and dead cells.
Macrophages are derived from a type of stem cell in the bone marrow and can be found in all tissues of the body. They are important for both innate and adaptive immune responses and act as the first line of defense against invading pathogens.
In addition to phagocytosis (engulfing and digesting foreign substances), macrophages also secrete cytokines and other molecules that help to recruit and activate other immune cells. They also play a role in tissue repair and regeneration.
Overall, macrophages are crucial players in the immune system and play a critical role in maintaining health and fighting off infection and disease.
Where are macrophages found?
Macrophages can be found in all tissues of the body, including the liver, spleen, lymph nodes, lungs, skin, and brain. They are also present in the bloodstream and bone marrow. Macrophages are able to migrate to different tissues in response to infection or injury and can change their behavior and function depending on the needs of the immune system.
Which vesicular transport process occurs primarily in some white blood cells and macrophages?
The vesicular transport process that occurs primarily in some white blood cells and macrophages is phagocytosis. This is a type of endocytosis where cells engulf and internalize large particles, such as bacteria or dead cells, into a specialized membrane-bound compartment called a phagosome. The phagosome then fuses with lysosomes, which contain digestive enzymes, to form a phagolysosome. Within the phagolysosome, the engulfed particle is broken down and digested by the lysosomal enzymes. Phagocytosis is an important mechanism for immune cells to eliminate pathogens and debris from the body.
Where would you expect to find the stellate macrophages of the liver?
Stellate macrophages, also known as Kupffer cells, are a specialized type of macrophage that are found in the liver. Specifically, they are located within the walls of the liver sinusoids, which are the small blood vessels that run through the liver. Stellate macrophages are so named because of their characteristic star-shaped appearance, with numerous long processes radiating from the cell body. These cells are responsible for removing bacteria, viruses, and other foreign particles from the blood as it passes through the liver, and they also play a role in regulating immune responses within the liver tissue.
What is the role of alveolar macrophages in the lungs?
Alveolar macrophages are a specialized type of macrophage that reside in the alveoli of the lungs. Their main role is to protect the lungs from infection and injury by engulfing and digesting inhaled particles, such as bacteria, viruses, and debris.
Alveolar macrophages are an important component of the lung’s innate immune defense system, acting as the first line of defense against invading pathogens. They are also involved in the resolution of inflammation and tissue repair following injury or infection. In addition to phagocytosis, alveolar macrophages secrete various molecules that help to recruit and activate other immune cells in the lungs, such as T cells and neutrophils.
Overall, alveolar macrophages play a critical role in maintaining the health and function of the lungs and are essential for protecting the respiratory system from infection and disease.
What is the function of macrophages?
The primary function of macrophages is to engulf and digest foreign substances such as bacteria, viruses, and dead cells. They also secrete cytokines and other molecules that help to recruit and activate other immune cells, and play a role in tissue repair and regeneration.
How do macrophages differ from other immune cells?
Macrophages are different from other immune cells such as T cells and B cells in that they are able to engulf and digest foreign substances, whereas T and B cells recognize and attack specific antigens.
How are macrophages activated?
Macrophages can be activated by a variety of stimuli, including cytokines, bacterial products, and viral proteins. Once activated, macrophages become more efficient at engulfing and digesting foreign substances.
What is the role of macrophages in cancer?
Macrophages can play both a pro-tumor and anti-tumor role in cancer, depending on their phenotype and the stage of the disease. In some cases, macrophages can promote tumor growth and metastasis, while in others they can help to eliminate cancer cells.
What is the link between macrophages and inflammation?
Macrophages play a key role in inflammation, as they are responsible for engulfing and digesting foreign substances that trigger the inflammatory response. They also secrete cytokines and other molecules that can either promote or resolve inflammation.
Can macrophages be targeted for therapeutic purposes?
Yes, macrophages are a promising target for the development of immunotherapies for cancer and other diseases. Researchers are exploring ways to modulate macrophage function and phenotype to promote anti-tumor activity and tissue repair.
How do macrophages interact with other immune cells?
Macrophages interact with other immune cells such as T cells, B cells, and natural killer cells to coordinate the immune response. They secrete cytokines and other molecules that can either promote or suppress the activity of other immune cells.
Can macrophages be used for diagnostic purposes?
Macrophages can be used as biomarkers for certain diseases, such as tuberculosis and rheumatoid arthritis. They can also be used in diagnostic imaging techniques, such as magnetic resonance imaging (MRI), to visualize inflammation and tissue damage.
References
- Toews, Galen B. (2009). Asthma and COPD || Macrophages. , (), 133–143. doi:10.1016/B978-0-12-374001-4.00011-0
- Dong, Zhongyun (2002). Encyclopedia of Cancer || Macrophages. , (), 77–88. doi:10.1016/b0-12-227555-1/00132-5
- Kumar, V. (2019). Macrophages: The Potent Immunoregulatory Innate Immune Cells. In (Ed.), Macrophage Activation – Biology and Disease. IntechOpen. https://doi.org/10.5772/intechopen.88013
- Shibata, Y., Abiko, Y., Takiguchi, H. (1991). Macrophage Membrane: Structure and Function. In: Harris, J.R. (eds) Megakaryocytes, Platelets, Macrophages, and Eosinophils. Blood Cell Biochemistry, vol 2. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-9531-8_8
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016 Mar 15;44(3):450-462. doi: 10.1016/j.immuni.2016.02.015. PMID: 26982353; PMCID: PMC4794754.
- Gordon, Siamon ; Martinez-Pomares, Luisa Berlin/Heidelberg: Springer Berlin Heidelberg Pflügers Archiv, 2017, Vol.469 (3-4), p.365-374
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013 Apr 25;496(7446):445-55. doi: 10.1038/nature12034. PMID: 23619691; PMCID: PMC3725458.
- Verschoor CP, Puchta A, Bowdish DM. The macrophage. Methods Mol Biol. 2012;844:139-56. doi: 10.1007/978-1-61779-527-5_10. PMID: 22262440.
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014 Jul 17;41(1):21-35. doi: 10.1016/j.immuni.2014.06.013. PMID: 25035951; PMCID: PMC4470379.
- https://www.rndsystems.com/resources/articles/macrophage-activation
- https://microbenotes.com/macrophages/
- https://www.technologynetworks.com/immunology/videos/macrophages-types-and-significance-344326
- https://www.immunology.org/public-information/bitesized-immunology/cells/macrophages
- https://biologyreader.com/macrophages.html
- https://en.wikipedia.org/wiki/Macrophage
- https://www.news-medical.net/life-sciences/What-is-a-Macrophage.aspx
- https://www.thermofisher.com/in/en/home/life-science/cell-analysis/cell-analysis-learning-center/immunology-at-work/macrophage-cell-overview.html
- https://www.kenhub.com/en/library/anatomy/macrophages
- https://askabiologist.asu.edu/macrophage
EXCELENTE MATERIAL. OBRIGADA