What is Loa loa?
- Loa loa, commonly referred to as the African eye worm, is a nematode that resides in the blood and is parasitic to humans. This nematode is particularly noticeable when it traverses the conjunctiva of the eye. Therefore, it is essential to understand its lifecycle, transmission, and the disease it causes, known as loa loa filariasis or loiasis.
- Loa loa is a filarial nematode, which means it is transmitted by arthropods. Specifically, it is one of three parasitic filarial nematodes responsible for subcutaneous filariasis in humans. Besides Loa loa, the other two are Mansonella streptocerca and Onchocerca volvulus, the latter being the causative agent of river blindness.
- The lifecycle and transmission of Loa loa are intricate. Adult worms of this species inhabit the subcutaneous layer of human skin, which is the fat layer. Here, the female worm lays microfilariae, which then enter the host’s bloodstream.
- Within 5 to 6 months, these microfilariae mature and can survive for up to 17 years. Then, when a human is bitten by horseflies of the genus Chrysops, specifically the species C. dimidiata and C. silacea, the microfilariae are taken up. These larvae are unique in their behavior, traveling in the peripheral blood during daylight and migrating to the lungs at nighttime.
- The disease caused by Loa loa, loiasis, manifests as a skin and eye condition. Humans contract this ailment through the bite of vectors such as the deer fly or mango fly (Chrysops spp.). The adult Loa loa worm moves through the subcutaneous tissues, occasionally entering the subconjunctival tissues of the eye.
- Although it doesn’t typically impair vision, its movement can be painful, especially when it crosses the eyeball or the bridge of the nose. Furthermore, the disease can lead to red, itchy swellings beneath the skin, termed “Calabar swellings”. Treatment for loiasis involves the administration of the drug diethylcarbamazine (DEC). In certain cases, surgical methods might be necessary to extract adult worms from the conjunctiva.
- Historically, the Loa loa infection was first documented by Mongin in 1770 when he removed an adult worm from an African slave’s eye. However, it wasn’t until 1781 that Guyot provided a detailed description of its clinical manifestations.
- While many individuals infected with Loa loa remain asymptomatic, some treatments, including diethylcarbamazine and ivermectin, can lead to severe reactions. This poses challenges for global elimination programs targeting lymphatic filariasis and onchocerciasis, especially in populations co-infected with multiple filariae.
Epidemiology of Loiasis
- Geographical Distribution Loiasis is an endemic disease confined to the African continent, with its prevalence primarily concentrated in the rainforest regions of Central and West Africa. The distribution of loiasis closely follows the habitat of the Chrysops vector flies, which are instrumental in the disease’s transmission. These flies have a predilection for residing in the forest canopy and are known to lay their eggs in swampy areas and along riverbanks.
- Vector Habitat and Behavior The Chrysops flies, which serve as vectors for Loa loa, exhibit specific behaviors that influence the epidemiology of loiasis. They are attracted to movement, dark colors, and wood smoke. Rainforests with lower canopies and minimal undergrowth provide an ideal environment for these flies, thereby increasing the risk of transmission in such areas.
- Human Infection Rates Infection rates of loiasis vary within the endemic regions, with a higher incidence observed in adults compared to children. This trend suggests that the likelihood of infection increases with age, potentially due to cumulative exposure to the Chrysops flies. Additionally, there is a tendency for higher infection rates among males in these areas.
- Isolated Cases and Non-Human Primates While loiasis is predominantly found in specific African regions, isolated cases have been reported in countries such as Uganda, Malawi, Zambia, Ethiopia, and from Ghana to Guinea. Non-human primates are known to harbor a form of Loa; however, there is no evidence to suggest that they act as reservoir hosts for the human strain of Loa loa, nor has zoonotic transmission been demonstrated.
- Implications for Public Health The epidemiology of loiasis has significant implications for public health in the affected regions. Understanding the distribution of the disease and the behavior of its vector is crucial for developing effective control and prevention strategies. The higher infection rates in adults, particularly males, may necessitate targeted interventions to reduce the burden of disease.
Morphology of Loa loa
- General Morphological Structure The Loa loa worm exhibits a simple anatomical structure that is characteristic of nematodes. This structure is composed of three primary segments: the head, which notably lacks lips, a cylindrical body, and a blunt tail. The absence of lips on the head is a distinctive morphological feature of the Loa loa species.
- Cuticular Composition The external layer of the Loa loa worm is a cuticle, which is composed of three main layers. These layers are primarily made up of collagen and other compounds that serve a protective function. The cuticle’s composition is essential for the survival of the nematodes, particularly as it provides protection while they reside within the digestive system of their host.
- Size and Sexual Dimorphism Adult Loa loa worms exhibit sexual dimorphism, meaning that there are distinct differences in size between males and females. Male adults typically measure between 20 to 34 millimeters in length and 350 to 430 micrometers in width. In contrast, female adults are larger, ranging from 20 to 70 millimeters in length and approximately 425 micrometers in width. The variation in size between the sexes is a significant aspect of their morphology.
- Color Variation The Loa loa worms display variability in color, although specific coloration details are not provided in the content. This variation may be related to factors such as age, sex, or environmental conditions.
- Juvenile Morphology Juvenile Loa loa worms, also known as larvae, bear a resemblance to adult worms but are considerably smaller in size. This similarity in appearance across life stages is typical of nematodes and is important for the identification of the species at different stages of its lifecycle.
- Microfilariae Characteristics The reproductive process of adult Loa loa worms results in the production of wormlike eggs known as microfilariae. These microfilariae measure 250 to 300 micrometers in length and 6 to 8 micrometers in width. They are morphologically distinct from other filariae due to their sheathed structure and the presence of body nuclei that extend to the tail’s tip. These characteristics are crucial for the identification and differentiation of Loa loa microfilariae from other filarial species.
Loa loa Life Cycle
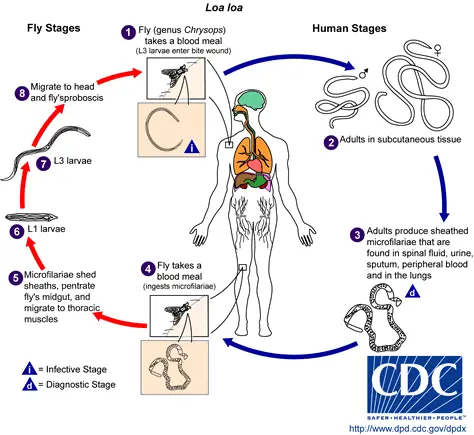
The life cycle of Loa loa is a complex process involving two hosts: humans as the definitive host and Chrysops flies as the intermediate host. The cycle begins with the transmission of the parasite through the bite of an infected female Chrysops fly.
- Human Infection: When a human is bitten by an infected Chrysops fly, the larva enters the skin through the puncture wound. These larvae then migrate to the subcutaneous tissue, where they develop into adult worms over a period of 6 to 12 months. Occasionally, adult worms may migrate into the subconjunctival tissue of the eye.
- Maturation and Reproduction: Within the human host, the adult worms reach sexual maturity. The fertilized female worm produces microfilarial larvae, which circulate in the peripheral blood during the daytime and are also found in the subcutaneous tissue.
- Transmission to the Vector: The life cycle continues when a female Chrysops fly bites an infected human to take a blood meal. During this process, the fly ingests microfilariae, which then enter the fly’s stomach.
- Development within the Vector: Inside the fly, the microfilarial larvae lose their protective sheath and penetrate the stomach wall, invading the thoracic muscles. Here, they undergo morphological changes to form the infective third-stage larvae. This development within the fly is completed in approximately 10 days.
- Infective Stage and Transmission: The mature infective larvae migrate to the mouthparts of the Chrysops fly. When this fly bites a new human host for a blood meal, the infective larvae are transmitted, and the cycle begins anew.
- Vector Specificity: The vectors for Loa loa are two species of Chrysops deerflies, specifically C. silacea and C. dimidiata. These flies are the main vectors for the transmission of filariasis caused by Loa loa.
- Adult Worms in Humans: After entering the human host, the larvae mature into adult worms, with females measuring about 40 to 70 mm in length and males being slightly smaller. The adult worms reside primarily in the subcutaneous tissue.
- Microfilariae Characteristics: The adult female worm produces a large number of microfilariae, which have a length of about 250 to 300 μm and a width of 6 to 8 μm. These microfilariae are present in various bodily fluids and have a diurnal periodicity, circulating in the bloodstream during the day and residing in the lungs during the noncirculation phase.
- Completion of the Vector’s Life Cycle: Inside the vector fly, the microfilariae shed their sheaths and migrate to the thoracic muscles, where they develop into first-stage larvae and then into third-stage infective larvae. These larvae then move to the fly’s proboscis, ready to infect another human host, thus perpetuating the life cycle of Loa loa.
Transmission of Loa loa
The transmission of Loa loa begins when infective larvae, known as L3, are transmitted to humans through the bite of deer fly vectors, specifically Chrysops silacea and C. dimidiata. These flies are hematophagous, meaning they feed on blood, and are diurnal, preferring to bite during daylight hours.
- Vector Habitat The Chrysops vectors thrive in rainforest-like environments, which are prevalent in western and central Africa. These habitats provide the necessary conditions for the flies to breed and sustain the transmission cycle of Loa loa.
- Maturation in the Human Host Once the L3 larvae are introduced into the human host via a fly bite, they migrate to the subcutaneous tissues. Here, the larvae mature into adult worms, a stage referred to as L5. This maturation process is critical for the continuation of the life cycle.
- Reproduction of Adult Worms In the presence of both male and female adult worms within the human host, mating occurs, leading to the production of microfilariae. These offspring are the next generation of parasites that can perpetuate the transmission cycle.
- Microfilariae and Diurnal Periodicity The microfilariae exhibit diurnal periodicity, meaning they are present in the peripheral blood of the human host during the day. This behavioral pattern aligns with the day-biting tendencies of the Chrysops spp. flies, facilitating the next stage of transmission.
- Ingestion by the Vector The cycle of infection is poised to continue when a non-infected mango or deer fly takes a blood meal from a human host who has microfilariae in their bloodstream. The synchronization of the microfilariae’s presence in the blood during the day with the feeding habits of the flies is a crucial aspect of the transmission process.
Reservoir of Loa loa
- Primary Reservoir Humans serve as the primary reservoir for Loa loa. This means that humans are the main source of the parasite, playing a crucial role in its transmission and lifecycle.
- Potential Secondary Reservoirs Besides humans, there are other potential reservoirs that have been identified through various fly-biting habit studies. These include:
- Hippopotamus
- Wild ruminants, such as buffalo
- Rodents
- Lizards
- Simian Loiasis A variant of loiasis exists in monkeys and apes, known as simian loiasis. This form of the disease is transmitted by a different vector, Chrysops langi. However, it’s important to note that there is no crossover between the human and simian types of the disease. This means that the simian type does not infect humans and vice versa.
- Vector for Simian Loiasis Chrysops langi is identified as the vector responsible for transmitting simian loiasis. Unlike the vectors for human loiasis, this fly is adapted to hunting within the forest. As of the provided information, Chrysops langi has not been associated with human infection.
Clinical manifestation of loa loa filariasis
Loa loa filariasis, commonly referred to as loasis, is a disease caused by the adult worm of Loa loa. The clinical manifestations of this disease can vary, with some individuals remaining asymptomatic while others exhibit noticeable symptoms.
- Incubation Period
- The incubation period for Loa loa filariasis typically averages between 3 to 4 years. This is the time between the initial infection and the onset of symptoms.
- Asymptomatic Cases
- Many individuals infected with Loa loa remain asymptomatic, meaning they do not show any noticeable symptoms of the disease.
- Skin Lesions
- One of the primary symptomatic manifestations of Loa loa filariasis is skin lesions.
- These lesions, known as Calabar swellings or fugitive swellings, arise due to the migration of the adult worm through the subcutaneous tissues.
- Calabar swellings are believed to be an allergic reaction to the toxins released by the filarial worm. They typically disappear within 2-3 days.
- Prior to the onset of the swelling, individuals may experience localized pain and itching that can last for several hours.
- The swellings are non-erythematous, measuring between 3 to 10 cm in diameter, and can last from a few days to weeks.
- Commonly affected areas include the wrist and knee joints. It’s important to note that the worms are not typically present within the swellings but are located just below the skin’s surface.
- Ocular Lesions
- Loa loa filariasis can also manifest in the form of ocular lesions.
- Conjunctival granuloma: This is caused by the migration of adult worms in the sub-conjunctival tissues. These granulomas appear as small nodules, measuring about 2mm in diameter, and are found in the deeper layers of the conjunctiva near the sclera.
- Oedema of the eyelid: This painless condition is often accompanied by itching. It does not typically present with fever or other systemic symptoms.
- Proptosis: Also known as “bug eye” or “bulge eye,” this condition results from the edema of the orbital cellular tissue. It is painless, rapidly onset, and frequently associated with itching.
- Complications
- Loa loa filariasis can lead to several complications, including:
- Recurrent fugitive swellings
- Endomyocardial fibrosis
- Retinopathy
- Encephalopathy
- Neuropathy
- Arthritis
- A particularly severe and potentially fatal complication is Loa loa meningoencephalopathy.
- Loa loa filariasis can lead to several complications, including:
Pathogenesis and pathology of loa loa
The pathogenesis and pathology of Loa loa infection involve the biological processes and tissue changes associated with the disease. Understanding these aspects is crucial for diagnosing and treating the condition effectively.
- Microfilariae and Pathogenicity
- Microfilariae, the larval stage of Loa loa, are generally not considered pathogenic. This means they do not typically cause disease or significant tissue damage in the human host.
- Adult Worms and Subcutaneous Tissue
- In contrast to microfilariae, adult Loa loa worms, which reside in the subcutaneous tissue, are pathogenic.
- The subcutaneous tissue is the layer of tissue beneath the skin, and it is here that the adult worms can cause disease.
- Inflammatory Response
- The migration of adult worms through the subcutaneous tissue provokes an intense inflammatory reaction.
- This inflammatory response is the body’s immune system reacting to the presence of the worms.
- Calabar Swellings
- Calabar swellings are the typical pathological feature of Loa loa filariasis.
- These swellings are localized, transient, non-pitting edemas that occur due to an allergic response to the migrating adult worms.
- The allergic response is characterized by the release of various immune mediators that lead to the swelling and associated symptoms.
- Allergic Reaction Mechanism
- The allergic reaction to the adult worms is a complex immune response that involves the activation of certain cells and the release of antibodies.
- This response can cause various symptoms, including itching, pain, and localized swelling.
Vector of Loa loa
Loa loa is transmitted to humans primarily through the bite of tabanid flies, specifically from the genus Chrysops. The most prominent vectors responsible for the transmission are Chrysops silacea and Chrysops dimidiata. These flies are endemic to Africa and are commonly referred to as deer flies or mango flies.
- Physical Characteristics of Chrysops spp.: Chrysops spp. flies are relatively small, measuring between 5–20 mm in length. They possess a large head with mouthparts that point downwards. Their wings can either be clear or have a speckled brown pattern. These flies are hematophagous, meaning they feed on blood.
- Habitat and Lifecycle: Chrysops spp. are typically found in forested and muddy environments, such as swamps, streams, reservoirs, and areas with rotting vegetation. Female flies require a blood meal to produce a second batch of eggs, which are laid near water sources. These eggs hatch within 5–7 days, releasing larvae that mature in water or soil. The larvae feed on organic materials, including decaying animal and plant matter. The entire lifecycle, from egg to adult, spans 1–3 years.
- Feeding Habits: The bite of the mango fly is notably painful. This pain arises from the fly’s unique feeding method. Instead of puncturing the skin like mosquitoes, mango flies create a laceration and lap up the blood that emerges. Due to their reproductive needs, female flies may take multiple blood meals from the same host, especially if they are interrupted during their initial feeding.
- Biting Locations and Preferences: While Chrysops silacea and C. dimidiata are attracted to rainforests, they predominantly bite outside of these forested areas, preferring open spaces. These flies are drawn to the smoke produced by wood fires. They also rely on visual cues and the detection of carbon dioxide plumes to locate their primary host, humans.
- Dietary Preferences: A study examining the biting habits of Chrysops spp. revealed that approximately 90% of their blood meals are derived from humans. The remaining 10% consists of blood from other animals, including hippopotamuses, wild ruminants, rodents, and lizards.
Diagnosis of Loa loa
Diagnosing Loa loa infection is crucial for effective treatment and management. The diagnosis can be based on clinical symptoms, microscopic examination, serologic testing, and other methods.
- Clinical Symptoms Individuals who have resided in endemic areas and present with symptoms such as transient localized swellings, urticaria, unexplained eosinophilia, or a worm traversing the conjunctiva or skin should be suspected of loiasis. Calabar swellings, which are localized allergic reactions to the worm, are a primary tool for visual diagnosis. Additionally, the rare observation of adult worms migrating across the eye can serve as a diagnostic indicator.
- Microscopic Examination Microscopic examination of blood samples is a practical diagnostic procedure. It’s essential to time the blood collection with the microfilariae’s known periodicity, which is between 10:00 a.m. and 2:00 p.m. The blood sample can be a thick smear, stained with Giemsa or haematoxylin and eosin. For increased sensitivity, concentration techniques like centrifugation or filtration through a nucleopore membrane can be employed.
- Serologic Testing While some reports suggest that no serologic diagnostics are available, recent developments have led to tests that are highly specific to Loa loa. The luciferase immunoprecipitation assay (LIPS) and its quick version (QLIPS) have shown high sensitivity and specificity. However, these tests are not yet widely available. It’s worth noting that a positive serologic test does not necessarily distinguish among infections due to antigenic cross-reactivity between filaria and other helminths.
- Antigen Detection An immunoassay for circulating filarial antigens can be a useful diagnostic approach, especially when microfilaremia is low and variable. This method helps identify the presence of the parasite in the blood.
- Other Diagnostic Methods In the past, a provocative injection of Dirofilaria immitis was used as a skin-test antigen for filariasis diagnosis. This would cause an artificial allergic reaction, leading to Calabar swelling. However, this method is not commonly used today. Blood tests to reveal microfilaremia are useful, but not in all cases, as some patients might not have microfilariae in their blood. Eosinophilia, an increase in eosinophil count in the blood, is almost always present in loiasis cases.
Treatment of Loa loa
Loiasis, caused by the Loa loa parasite, requires specific treatment approaches to effectively manage and eliminate the infection. Treatment can involve pharmaceutical interventions, and in some cases, surgical procedures.
- Pharmaceutical Interventions
- Diethylcarbamazine (DEC)
- DEC is the primary drug of choice for treating loiasis.Recommended dosage: 8–10 mg/kg/day, divided into three doses, for a duration of 21 days.It is effective against both microfilariae and somewhat against adult worms.Before initiating treatment, blood smears should be examined for microfilariae to prevent severe toxicity in those with significant microfilaremia.Coinfection with Onchocerca volvulus should be ruled out before using DEC, as severe adverse events may occur if DEC is administered to patients with onchocerciasis.Adverse effects of DEC include fever, angioedema, myalgia, malaise, and pruritus
- While ivermectin can reduce levels of microfilariae, it does not affect the adult worm.Severe adverse reactions have been reported when ivermectin is used in mass treatment programs, especially in individuals coinfected with Loa and having high-level microfilaremia.
- Albendazole can decrease levels of circulating microfilariae.
- Recommended dosage: 200 mg taken twice daily for 21 days.
- It offers a gradual reduction of the microfilarial load over several months.
- Diethylcarbamazine (DEC)
- Surgical Interventions
- In certain cases, surgical removal of adult worms might be necessary.
- A detailed surgical procedure involves the use of proparacaine and povidone-iodine drops, a wire eyelid speculum, and an injection of 0.5 ml 2% lidocaine with epinephrine. A small incision is made, and the worm is removed with forceps. Post-surgery, gatifloxacin drops and an eye-patch are applied.
- Precautions and Considerations
- Before initiating treatment, it’s crucial to determine the presence of microfilariae in the blood to prevent potential severe adverse events.
- Coinfections, especially with Onchocerca volvulus, should be ruled out to avoid complications.
- In patients with high microfilaria load, initial doses of DEC or ivermectin should be reduced to prevent encephalopathy. In such cases, albendazole can be administered initially.
Prevention of Loa loa
Prevention of Loa loa infection involves a combination of chemoprophylaxis, geo-mapping, personal protective measures, and vector control strategies. Implementing these measures can significantly reduce the risk of contracting the disease.
- Chemoprophylaxis – Diethylcarbamazine (DEC)
- DEC has been identified as an effective prophylactic agent against Loa loa infection.
- A study involving Peace Corps volunteers in Gabon, a region with high Loa loa prevalence, demonstrated the effectiveness of DEC. In this study, 6 out of 20 individuals in the placebo group contracted the disease, whereas none in the DEC-treated group did.
- The recommended prophylactic dose is 300 mg DEC taken orally once a week.
- The primary side effect noted was nausea.
- Geo-mapping for Risk Assessment
- Geo-mapping can be utilized to estimate Loa loa transmission by analyzing appropriate habitats and human settlement patterns.
- Predictor variables such as forest, land cover, rainfall, temperature, and soil type can be used to determine potential transmission zones.
- This approach can be particularly useful in the absence of point-of-care diagnostic tests.
- Personal Protective Measures
- Preventative strategies similar to those used for malaria can be employed to reduce the risk of loiasis.
- Using DEET-containing insect repellents, wearing permethrin-soaked clothing, and donning thick, long-sleeved and long-legged attire can decrease susceptibility to mango or deer fly bites.
- Since the vector is active during the day, mosquito nets may not offer additional protection against loiasis.
- Vector Control and Elimination
- Vector control measures have faced challenges due to difficulties in accessing breeding sites of the vector flies.
- Clearing forests around dwellings can potentially reduce the risk.
- The Chrysops vector has a limited flying range, making vector elimination efforts challenging. This is further complicated by the vector’s behavior of residing in forests but biting in open areas.
- Currently, there are no widespread vector elimination efforts.
- Vaccination
- As of now, no vaccine has been developed for loiasis, and there is limited information on the potential for such a development.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.