Dr. K. Ikeda, a Japanese scientist, extracted glutamic acid from kelp, a marine alga, by means of acid hydrolysis and separation in 1908. In addition, he observed that when glutamic acid was neutralised with caustic soda, it acquired a totally new, exquisite flavour.
This marked the beginning of using monosodium glutamate (MSG) as a flavour enhancer. In 1957, Dr. S. Ukada and Dr. Kinoshita isolated an unique soil-dwelling gram-positive bacterium, Corynebacterium glutamicum, which represented a breakthrough in the manufacture of MSG. The successful commercialization of monosodium glutamate (MSG) with this bacterium, and later with other bacteria such as E. coli, offered a significant boost to amino acid synthesis.
Glutamic Acid Production
- Through the Embden Meyerhof-Parnas (EMP) pathway and the pentose-phosphate pathway, glutamic acid-producing bacteria break down glucose into C3 and C2 fragments, which are then funnelled into the tricarboxylic acid (TCA) cycle.
- The reactions of the EMP pathway are more prevalent when glutamic acid is produced.
- The most important precursor of glutamic acid is α-ketoglutarate, which is produced in the TCA cycle from citrate, isocitrate, and α-ketoglutaric acid. -ketoglutarate is subsequently transformed to L-glutamic acid via reductive amination with free NH4+ ions.
- The final step is catalysed by glutamate dehydrogenase dependent on NADP.
- The NADPH2 necessary at this stage of the reaction is supplied by isocitrate dehydrogenase’s subsequent oxidative decarboxylation.
- The NADPH2 is then regenerated by α-ketoglutarate’s reductive amination.
Effect of Permeability on Glutamic Acid Production
The production and excretion of glutamic acid depend on the permeability of the cell membrane. It is possible to increase the permeability of glutamic acid-producing bacteria in one of the following ways:
- Because of biotin deficiency.
- Due to the administration of penicillin.
- By adding saturated fatty acids or derivatives of fatty acids.
- Due to the lack of oleic acid in oleic acid auxotrophs.
- Due to the lack of glycerol in glycerol auxotrophs.
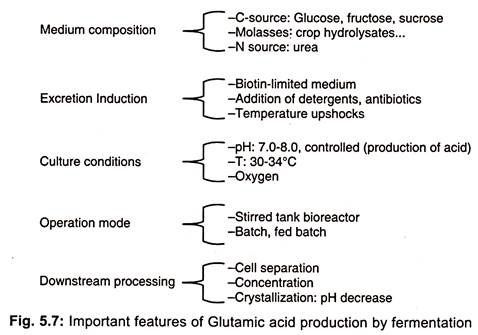
Factors affect the glutamic acid fermentation
1. Carbon Source
- As a carbon source, a wide variety of carbohydrates are utilised in the fermentation process. Glucose and sucrose are commonly employed.
- However, starch hydrolysates, fructose, maltose, ribose, and xylose are less frequently employed. Additionally, sucrose, sugarcane molasses, and sugar beet molasses may be utilised.
- Both molasses have a high biotin concentration (0.4-1.2 mg kg-1 in cane molasses and 0.02-0.08 mg kg-1 in beet molasses).
- The fermentation medium must contain penicillin or fatty acid derivatives (such as Tween-66).
- When these compounds are added to the medium preparation, the L-glutamic acid permeability of the cells is increased. Molasses or starch hydrolysate are typically utilised in industrial operations.
2. Nitrogen Source
- As nitrogen sources, ammonium sulphate, ammonium chloride, ammonium phosphate, aqueous ammonia, ammonia gas, and urea have been utilised.
- A high concentration of ammonium ions hinders the growth of the microorganism and the production of L-glutamic acid, despite their necessity.
- Consequently, a proper amount of ammonia is injected as fermentation continues. These salts also aid in pH regulation.
3. Growth Factors
- Biotin is the most critical growth factor. Its ideal concentration is determined by the carbon source employed.
- Its need in media containing 10% glucose is 5 mg liter-1. In media with a lower content of glucose, it is significantly reduced.
- Some strains require L-cystine as a growth supplement.
4. Oxygen Supply
- The oxygen level should be neither too low nor too high. At a kb value of 3.5 x 10-6 mole 02 atm-1 min-1 ml-1, optimal L-glutamic acid yields are attained.
- Under oxygen insufficiency, lactate and succinate are excreted, whereas abundant oxygen under ammonium ions lack inhibits development and produces α-ketoglutarate. In both instances, yields of glutamic acid are modest.
5. pH
- The addition of ammonium salts regulates the optimal pH range for growth and glutamic acid production, which is 7.0-8.0.
Glutamic acid Fermentation
L-glutamic acid can be manufactured using the following methods:
- In a two-stage fermentation process, one bacterium produces -ketoglutaric acid, which is then transformed into L-glutamic acid by another microorganism.
- utilising a single-stage fermentation method and a single microbe.
1. Inoculum Production
- Preparing a medium with the following composition for inoculum production.
- A appropriate strain of C. glutamicum is selected from a stock culture and injected into the medium described above.
- The culture is incubated at 35°C for up to 16 hours. Approximately 6% by volume of inoculum is fed to the production fermenter once adequate growth has occurred.
2. Preparation of Medium
With the following mixture, a manufacturing medium is prepared:
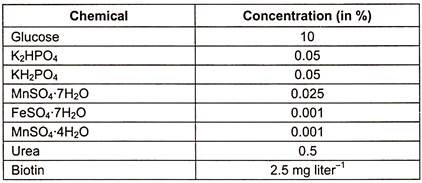
The medium containing the aforementioned composition is sterilised and used to produce L-glutamic acid.
3. Fermentation Process
- The fermentation is conducted for roughly 40 to 48 hours at 30 degrees Celsius. Adjusting the pH to 7.0-8.0.
- Urea is supplied intermittently throughout the fermentation process. Approximately fifty percent of the available carbohydrates are metabolised into L-glutamic acid.
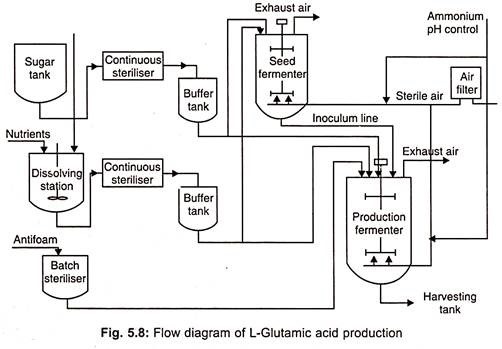
4. Harvest and Recovery
- L-glutamic acid is harvested and recovered utilising the same method as L-lysine.
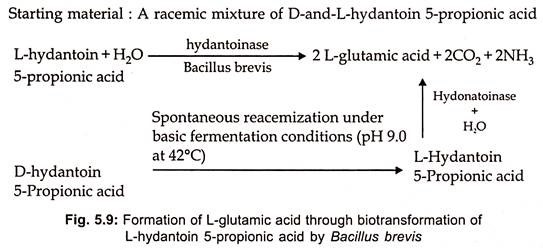
- With the aid of hydantoinase, a racemic mixture of D- and L-hydantoin-5-propionic acid can be biotransformed into glutamic acid.
- In the presence of hydantoin racemase, D-hydantoin is converted into L-hydantoin-5-propionic acid simultaneously.