Japanese Encephalitis (JE) Virus
- The Japanese encephalitis virus causes Japanese encephalitis (JE), an infection of the brain (JEV). While the majority of infections result in few or no symptoms, inflammation of the brain can occasionally occur.
- In such situations, possible symptoms include headache, vomiting, fever, confusion, and seizures. This occurs between 5 and 15 days following infection. JEV is typically transmitted by mosquitoes, specifically the Culex species.
- Pigs and wild birds act as the virus’ reservoir. The disease is especially prevalent in rural areas. Testing of blood or cerebrospinal fluid is used for diagnosis.
- Generally, the safe and effective Japanese encephalitis vaccination is used for prevention. Among additional strategies is avoiding mosquito bites. Once infected, there is no specific therapy; only supportive care is available.
- This is often performed in a hospital. Up to fifty percent of those who recover from JE experience permanent complications.
- The disease is prevalent in East and Southeast Asia and the Western Pacific. Around three billion people reside in regions where the disease occurs.
- Around 68,000 symptomatic cases and 17,000 fatalities occur annually. Cases frequently occur in outbreaks. In 1871, the sickness was first identified in Japan.
Structure of Japanese Encephalitis (JE) Virus
- Japanese encephalitis (JE) virus belongs to the Flaviviridae family. The virus has spherical particles that are approximately 50 nm in diameter.
- These particles contain an electron-dense core that is around 30 nm in diameter, surrounded by a lipid bilayer.
- Mature JE virions have a buoyant density of 1.19 to 1.23 g/mL and can be found sedimenting between 170 and 210S.
- The virus has a small lipoprotein envelope that surrounds a nucleocapsid composed of core protein. The nucleocapsid also encloses a single-stranded RNA genome with positive polarity, which is approximately 11kb long.
Genome of Japanese Encephalitis (JE) Virus
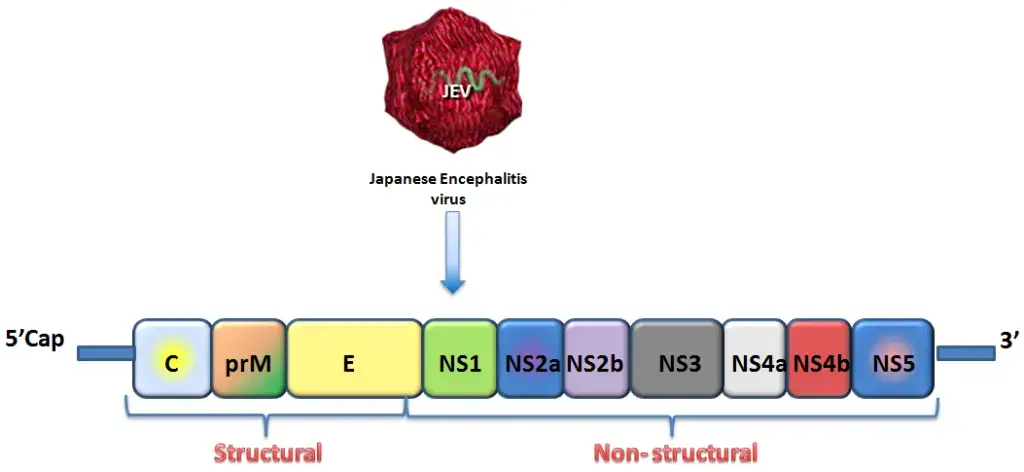
- The Flaviviridae family of viruses includes the Japanese encephalitis virus, an RNA virus with a diameter of approximately ~ 40–50 nm and cubic symmetry. It is an infectious, single-stranded RNA-based virus with an envelope.
- The genome is around ~ 11 kb in size, has a positive sense strand, a 5′ cap, but no 3′ poly tail. It has a lipid envelope surrounding the nucleocapsid that it contains.
- A single open reading frame (ORF) in the genomic RNA codes for a 3400 amino acid polyprotein. Viral and host proteases split this polyprotein into 10 proteins.
- Three structural genes are involved in antigenicity because they are expressed on the virus and involved in the development of capsids: core (C), pre membrane (prM), and envelope (E). The E gene is the most significant and extensively researched of the three. Seven non structural genes are involved in virus replication: NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5.
- There has been a report of a new mutation in domain II of the JEV envelop gene that is present in North India. JEV has a high mutation rate because of RNA dependent RNA polymerase (RdRp), which is encoded by NS5.
- JEV grows on intracellular membranes and only replicates in the cytoplasm of infected cells, in a perinuclear site.
- The most significant type of viral encephalitis in Asia is Japanese encephalitis (JE), which is brought on by the Japanese encephalitis virus (JEV). In the last 50 years, the epidemiology of JE has evolved, and the areas impacted by JEV have grown to include India, China, Southeast Asia, and the Western Pacific.
- In JEV-endemic areas, there are about 50,000 cases and 10,000 fatalities reported per 3 billion people. The actual number is unknown, though, as diagnostic facilities are typically lacking in JEV-prone areas. Subclinical JEV infections predominate.
- The JEV serological complex, which has a high morbidity and fatality rate, includes JEV. The most significant biological amplifiers and reservoirs are pigs. JEV seldom, if ever, spreads directly from person to person unless it is transmitted intrauterinally.
- Transplantation of blood and organs is another method of transmission. Vertical transmission of JEV causes infection to spread from mother to fetus. Symptomatic infections typically manifest as non-specific febrile illnesses with diarrhea, rigors, headache, vomiting, decreased level of consciousness, aseptic meningitis, or encephalitis as well as other symptoms.
- After JEV exposure, the incubation period lasts somewhere between 6 and 16 days. Clinical symptoms like a high temperature and nausea appear in one in every 200 or 800 affected individuals. Patients with symptoms account for 25% of deaths, and a third of those who survive have brain damage.
Epidemiology of Japanese Encephalitis (JE) Virus
- Japanese encephalitis (JE) is the primary cause of viral encephalitis in Asia, with up to 70,000 cases reported each year. Case-fatality rates range from 0.3% to 60%, depending on population and age. Moreover, there have been sporadic outbreaks in U.S. possessions in the Western Pacific.
- People of rural communities in endemic areas are at the greatest risk; Japanese encephalitis rarely occurs in urban settings.
- China, South Korea, Singapore, Japan, Taiwan, and Thailand are examples of nations that have experienced severe epidemics in the past, but have primarily controlled the disease with vaccine. Vietnam, Cambodia, Myanmar, India, Nepal, and Malaysia are also countries that still experience periodic epidemics.
- There have been reports of Japanese encephalitis in the Torres Strait Islands, and in 1998, there were two fatal cases recorded in northern Australia. In 2013, Kachin State, Myanmar, had recorded instances. In 2016, there were 116 reported deaths in the Malkangiri district of Odisha, India.
- The population has limited immunity to the disease, and the presence of large numbers of farmed and feral pigs could serve as reservoirs for the virus. In February 2022, Japanese encephalitis was confirmed in piggeries in Victoria, Queensland, and New South Wales.
- Cases were reported in South Australia on March 4th. By October 2022, the epidemic in eastern mainland Australia had caused 42 symptomatic instances of the disease in humans, resulting in seven deaths.
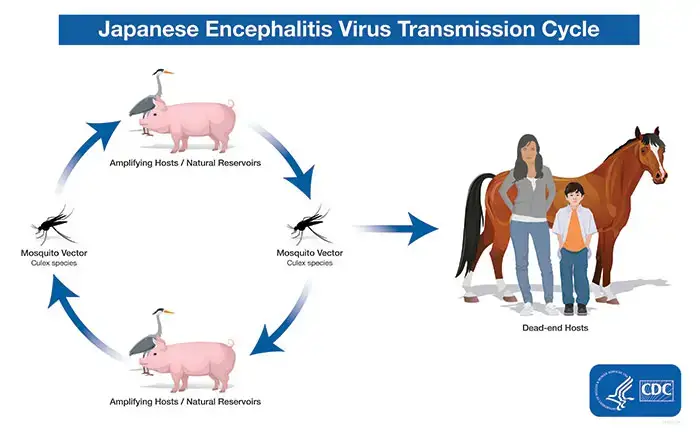
- The disease presents as deadly encephalitis in human, bovine, and equine hosts, rendering them ineffective hosts. Pigs serve as amplification hosts and play a crucial part in the epidemiology of the disease.
- Infections in pigs generally asymptomatic, except in pregnant sows, when abortion and congenital malformations are common. Culex tritaeniorhynchus, which prefers to feed on cattle rather than people, is the most significant vector.
- The natural hosts of the Japanese encephalitis virus are birds, not people; hence, many believe the virus will never be eradicated. In November 2011, the virus was detected in Culex bitaenionchus in South Korea.
- Current whole genome microarray study on neurons infected with the Japanese encephalitis virus has demonstrated that neurons play a crucial role in their own resistance against Japanese encephalitis infection.
- Despite the fact that this contradicts the long-held belief that neurons are immunologically quiescent, a better understanding of the proinflammatory effects responsible for immune-mediated control of viral infection and neuronal injury during Japanese encephalitis infection is necessary for the development of strategies to reduce the severity of CNS disease.
Transmission of disease
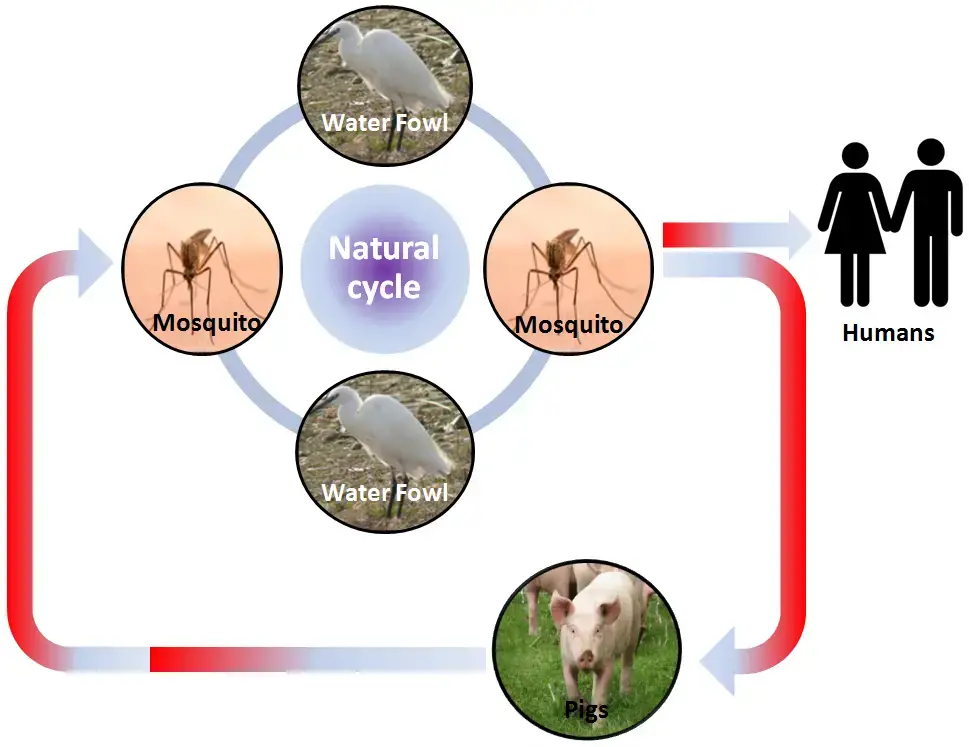
- The JE virus traverses zoonotic cycles including mosquitoes, other vertebrate species, and humans as terminal hosts. Principal vectors include Culex tritaeniorhyncus and Culex gelidus, according to reports.
- These vectors reproduce in rice fields, irrigation canals, pools of stagnant water, puddles, open sewage systems, fish ponds, etc.
- These infected mosquitoes (3%) bite domestic animals and birds, but occasionally they may bite a healthy host (human), allowing the virus’ transfer to humans.
- Hosts that act as reservoirs and amplifying hosts are pigs and birds. Homo sapiens is an accidental host of the JEV.
- After an infected mosquito bites a human, the earliest viral replication occurs in local and regional lymph nodes. Viruses likely invade the central nervous system through the blood, producing infection and disease.
Replication of Japanese Encephalitis (JE) Virus
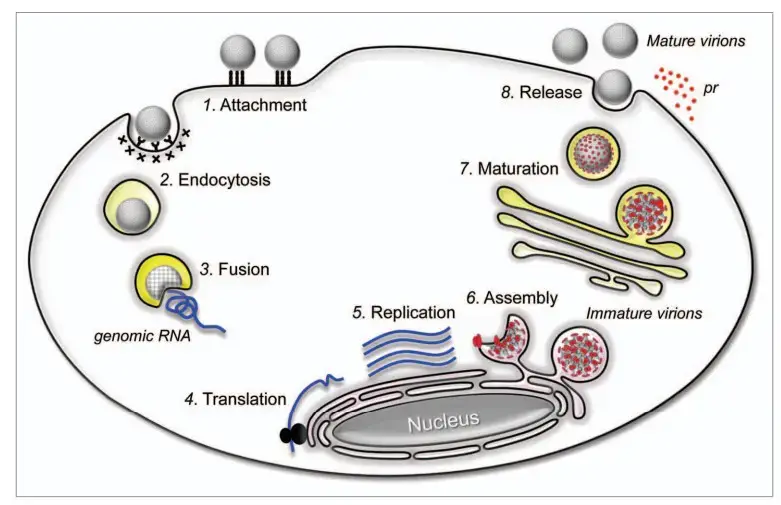
- The attachment of an infectious virion to a target cell is mediated by attachment and entrance receptor molecules on the plasma membrane (Step 1).
- This contact initiates receptor-mediated endocytosis within the cell to ingest the virion (Step 2).
- In endosomes, the low pH produces major conformational changes in the viral E glycoprotein, initiating the fusion of the viral membrane with the endosomal membrane of the host (Step 3).
- The viral genomic RNA is released into the cytoplasm at fusion, where it is first translated into the polyprotein precursor in conjunction with the rough ER (Step 4).
- The polyprotein undergoes processing to generate the mature viral proteins required for RNA replication and particle formation. The replication complex replicates genomic RNA within virus-induced, ER-derived vesicles (Step 5).
- A complex of newly generated genomic RNA and C proteins are released into the lumen of the ER, where they acquire the prM and E proteins on their membranes to form immature progeny virions (Step 6).
- The secretory channel subsequently transports the immature virions to the Golgi apparatus; in the trans-Golgi network, the cleavage of prM to M results in the maturity of the viral particles (Step 7).
- Exocytosis then releases mature virions from the cell into the extracellular milieu (Step 8).
Signs and symptoms of Japanese Encephalitis (JE) Virus
Japanese encephalitis (JE) is a viral infection that can cause inflammation of the brain. Symptoms of JE virus infection can range from mild to severe, and some people may not have any symptoms at all. Common signs and symptoms of JE virus infection include:
- Fever
- Headache
- Nausea and vomiting
- Fatigue and weakness
- Muscle pain and joint pain
- Encephalitis (inflammation of the brain)
- Seizures
- Stiff neck
- Confusion or disorientation
- Paralysis or muscle weakness
- Tremors or jerky movements
- Coma
Symptoms usually develop 5 to 15 days after being bitten by an infected mosquito. In some cases, JE virus infection can lead to severe complications such as brain damage, coma, and even death. It is important to seek medical attention immediately if you experience any of the symptoms associated with JE virus infection, especially if you have recently traveled to an area where JE is common.
Pathogenesis and pathology of Japanese Encephalitis (JE) Virus
- The majority of human infections with encephalitic flaviviruses are asymptomatic or are accompanied by a slight fever. In a small proportion of infected persons, however, the moderate infection becomes life-threatening encephalitis.
- Thus, a crucial question in the pathophysiology of encephalitic flaviviral disease concerns the conditions that permit virus entry from the blood into the central nervous system (CNS). Hypertension, diabetes mellitus, and co-infection with other viruses may exacerbate the infection and aggravate the state of infected individuals by raising the risk of neurological consequences caused by virus penetration of the blood-brain barrier.
- The thalamus and brain neurons can be affected by a virus, according to histological analysis. Due to the development of adaptive immune, viral antigens are subsequently eliminated. Recovery from encephalitic infection may be facilitated by a robust virus-specific antibody response in the Brain.
- The majority of clinical infections with mosquito-borne encephalitic flaviviruses in humans occur without detectable viremia, consistent with the theory that humans are terminal hosts in the natural transmission cycle.
- Japanese encephalitis (JE) is currently the leading viral cause of Brain infection. JEV pathogenesis remains obscure. JE virus strains vary in their neurovirulence and peripheral pathogenicity.
- After an infected mosquito bite, the virus penetrates the reticulo-endothelial system and, after a brief period of viremia, invades the central nervous system.
- It spreads to the hypothalamus, hippocampus, substantia nigra, and medulla oblongata regions of the brain via vascular endothelial cells via endocytosis involving cholesterol and clathrin mediated pathways, also known as lipid rafts serving as virus entrance portals.
- The virus replicates within neurons and reaches maturity within the neuronal secretary system. Approximately 33% of JE-infected individuals die from neurocysticercosis (NCC), indicating that NCC may in some way predispose to JE infection.
- Brain congestion, edema, and hemorrhagic signs are present during the acute stages. There have also been reports of pathological changes in the neural tissues of lymphoid organs and immune cells, such as the spleen and kupffer cells, respectively.
Diagnosis of Japanese Encephalitis (JE) Virus
Japanese encephalitis (JE) is a viral disease that is spread by infected mosquitoes. The diagnosis of JE virus infection is usually based on a combination of clinical features, laboratory tests, and epidemiological information.
- Clinical Features: The clinical features of JE can range from mild flu-like symptoms to severe neurological illness. The most common symptoms include fever, headache, neck stiffness, seizures, and mental status changes.
- Laboratory Tests: Several laboratory tests are used to diagnose JE virus infection. These include:
- Polymerase Chain Reaction (PCR) test: A PCR test is used to detect the genetic material of the JE virus in blood or cerebrospinal fluid (CSF).
- Serology test: A serology test is used to detect antibodies against the JE virus in blood. This test is useful in detecting a past infection or in identifying people who have been vaccinated against JE.
- Viral culture: A viral culture can be used to isolate the JE virus from blood or CSF. This test is more difficult and time-consuming than PCR or serology tests.
- Epidemiological Information: JE virus is typically found in rural and agricultural areas in Asia. If a patient has traveled to or lives in an area where JE virus is endemic, this information can help support a diagnosis of JE virus infection.
In summary, a combination of clinical features, laboratory tests, and epidemiological information can help diagnose JE virus infection. If you think you or someone you know may have been infected with JE virus, it is important to seek medical attention immediately.
Transmission of Japanese Encephalitis Virus
- Being a flavivirus, the Japanese encephalitis (JE) virus is closely linked to the West Nile and St. Louis encephalitis viruses. JE virus is spread to humans by infected Culex mosquitoes, namely Culex tritaeniorhynchus.
- The virus is maintained through a cycle involving mosquitoes and vertebrate hosts, especially pigs and wading birds (also referred to as amplifying hosts or natural reservoirs). Humans are accidental or dead-end hosts because the JE virus concentration in their bloodstreams is typically insufficient to infect mosquitoes.
- Transmission of the JE virus occurs predominantly in rural agricultural settings, frequently in conjunction with rice cultivation and flooded irrigation. In certain regions of Asia, these characteristics might manifest close to urban centers.
- In temperate regions of Asia, transmission of the JE virus is seasonal. Summer and fall are often peak seasons for human disease. Transmission can occur year-round in the subtropics and tropics, with a peak during the rainy season.
Treatment of Japanese Encephalitis (JE) Virus
Currently, there is no specific antiviral treatment available for Japanese encephalitis (JE) virus infection. Treatment is mainly supportive and focuses on relieving symptoms and preventing complications.
Symptomatic Treatment: Symptomatic treatment may include the following:
- Medications to reduce fever and pain: Acetaminophen is commonly used to reduce fever and pain.
- Fluids and electrolytes: Intravenous fluids and electrolytes may be given to prevent dehydration.
- Anticonvulsants: Anticonvulsant medications may be given to control seizures.
- Oxygen therapy: Oxygen therapy may be used if there is respiratory distress.
Preventive Measures: Preventive measures are the best way to avoid JE virus infection. These include:
- Vaccination: Vaccination is the most effective way to prevent JE virus infection. The JE vaccine is recommended for people who are at increased risk of infection, such as travelers to areas where JE virus is endemic.
- Mosquito control: Mosquito control measures, such as using insect repellents, wearing protective clothing, and using bed nets, can help reduce the risk of mosquito bites.
Prognosis: The prognosis of JE virus infection depends on the severity of the illness. Mild cases may resolve on their own without complications, while severe cases may lead to permanent neurological damage or death. It is important to seek medical attention immediately if you think you or someone you know may have been infected with JE virus.
Prevention and Control of Japanese Encephalitis (JE) Virus
Prevention and control of Japanese encephalitis (JE) virus infection primarily involve mosquito control measures and vaccination.
- Mosquito Control Measures: Mosquito control measures can help reduce the risk of JE virus transmission. These measures include:
- Using insect repellents that contain DEET or picaridin.
- Wearing long-sleeved shirts and pants to reduce skin exposure to mosquitoes.
- Using bed nets that are treated with insecticide.
- Eliminating standing water sources to reduce mosquito breeding sites.
- Vaccination: Vaccination is the most effective way to prevent JE virus infection. The JE vaccine is recommended for people who are at increased risk of infection, such as:
- Travelers to areas where JE virus is endemic.
- People living in or visiting rural areas where JE virus is common.
- Laboratory workers who handle the JE virus.
The JE vaccine is a two-dose series given at least 28 days apart. The vaccine provides protection for up to 10 years and may require booster doses for continued protection.
- Surveillance and Early Detection: Surveillance for JE virus activity can help identify areas at risk and guide mosquito control measures. Early detection of JE virus infection in humans can also help prevent further transmission.
- Personal Protection: Personal protection measures can help reduce the risk of mosquito bites and JE virus infection. These measures include:
- Using insect repellents that contain DEET or picaridin.
- Wearing long-sleeved shirts and pants to reduce skin exposure to mosquitoes.
- Using bed nets that are treated with insecticide.
- Avoiding outdoor activities during peak mosquito hours, such as dawn and dusk.
In summary, prevention and control of JE virus infection involve mosquito control measures, vaccination, surveillance, and personal protection measures. If you live in or plan to travel to an area where JE virus is endemic, it is important to take these measures to reduce your risk of infection.
FAQ
What is Japanese Encephalitis (JE) virus?
JE virus is a type of virus that is transmitted by mosquitoes and can cause inflammation of the brain.
Where is Japanese Encephalitis (JE) virus found?
JE virus is found in many countries in Asia and the western Pacific, including China, Japan, Korea, Taiwan, Vietnam, and Thailand.
How is Japanese Encephalitis (JE) virus spread?
JE virus is spread through the bite of an infected mosquito. It cannot be spread from person to person.
What are the symptoms of Japanese Encephalitis (JE) virus?
Symptoms of JE virus infection can include fever, headache, nausea and vomiting, fatigue, muscle pain, and neurological symptoms such as encephalitis, seizures, and paralysis.
Who is at risk of Japanese Encephalitis (JE) virus infection?
People who live in or travel to areas where JE virus is present are at risk of infection. The risk is highest for people who spend a lot of time outdoors, especially during peak mosquito biting times.
Is there a vaccine for Japanese Encephalitis (JE) virus?
Yes, there is a vaccine available for JE virus. It is recommended for travelers to areas where JE is common, as well as for people who live in those areas.
How can Japanese Encephalitis (JE) virus infection be prevented?
Prevention methods include using mosquito repellent, wearing protective clothing, and staying indoors during peak mosquito biting times. Vaccination is also an effective prevention method.
How is Japanese Encephalitis (JE) virus diagnosed?
Diagnosis of JE virus infection is typically based on symptoms and a blood test to detect the presence of antibodies to the virus.
Is there a cure for Japanese Encephalitis (JE) virus infection?
There is no specific cure for JE virus infection, but treatment focuses on relieving symptoms and preventing complications.
Can Japanese Encephalitis (JE) virus be transmitted from animals to humans?
JE virus is primarily transmitted by mosquitoes, but there have been rare cases of transmission from animals such as pigs and birds to humans. However, the risk of transmission from animals to humans is very low.
References
- Tiwari, S., Singh, R. K., Tiwari, R., & Dhole, T. N. (2012). Japanese encephalitis: a review of the Indian perspective. The Brazilian Journal of Infectious Diseases, 16(6), 564–573. doi:10.1016/j.bjid.2012.10.004
- Bisen, Prakash & Raghuvanshi, Ruchika. (2013). Japanese Encephalitis History.
- Turtle, L., Solomon, T. Japanese encephalitis — the prospects for new treatments. Nat Rev Neurol 14, 298–313 (2018). https://doi.org/10.1038/nrneurol.2018.30
- K., S., Tiwari, S., Saxena, R., Mathur, A., & N. Nair, M. P. (2013). Japanese Encephalitis Virus: The Complex Biology of an Emerging Pathogen. InTech. doi: 10.5772/54111
- Yun SI, Lee YM. Japanese encephalitis: the virus and vaccines. Hum Vaccin Immunother. 2014;10(2):263-79. doi: 10.4161/hv.26902. Epub 2013 Oct 25. PMID: 24161909; PMCID: PMC4185882.
- Wangchuk S, Tamang TD, Darnal JB, Pelden S, Lhazeen K, Mynak ML, Letson GW, Khare S, Leader BT, Marfin AA, Hills SL. Japanese Encephalitis Virus as Cause of Acute Encephalitis, Bhutan. Emerg Infect Dis. 2020 Sep;26(9):2239-2242. doi: 10.3201/eid2609.200620. PMID: 32818416; PMCID: PMC7454061.
- https://www.agriculture.gov.au/biosecurity-trade/pests-diseases-weeds/animal/japanese-encephalitis
- https://www.business.qld.gov.au/industries/farms-fishing-forestry/agriculture/biosecurity/animals/diseases/guide/japanese-encephalitis
- https://www.woah.org/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/JAPANESE_ENCEPHALITIS.pdf
- https://www.platform-pmc.com/%E7%96%AB%E8%8B%97%E6%B3%A8%E5%B0%84-en/%E6%97%A5%E6%9C%AC%E8%85%A6%E7%82%8E%E6%98%AF%E4%BB%80%E9%BA%BC%EF%BC%9F/?lang=en
- https://www.news-medical.net/health/What-is-Japanese-Encephalitis.aspx
- https://microbeonline.com/japanese-encephalitis-je-virus-structure-life-cycle-pathogenesis-diagnosis/
- https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis
- https://www.cdc.gov/japaneseencephalitis/index.html
- https://www.nhs.uk/conditions/japanese-encephalitis/
- https://www.dpi.nsw.gov.au/biosecurity/animal/info-vets/japanese-encephalitis
- https://www.ava.com.au/policy-advocacy/disaster-response/emergency-animal-diseases/japanese-encephalitis/