Intracellular stimuli, such as DNA damage, predominantly trigger apoptosis via the intrinsic route. The intrinsic apoptosis pathway, which is comprised of conserved signalling proteins, is physically connected with mitochondria and sensitive to mitochondrial oxidative stress in vertebrates. Members of the Bcl family linked to the mitochondrial membrane have an effect on the process, including the pro- or anti-apoptotic regulatory proteins Bax and Bcl-2 gene.
What is Intrinsic Apoptosis Pathway?
- Initiators of the intrinsic apoptosis pathway include chemotherapy and/or radiotherapy. It is triggered by a variety of external and endogenous events, including DNA damage, ischemia, and oxidative stress. In addition, it is essential for development and the elimination of damaged cells.
- The functional consequence of pro-apoptotic signalling in the intrinsic pathway is disruption of the mitochondrial membrane and release of cytochrome c into the cytoplasm, where it forms a complex or apoptosome with apoptotic protease activating factor 1 (APAF1) and the inactive form of caspase-9.
- This complex hydrolyzes adenosine triphosphate in order to cleave caspase-9 and activate it. The initiator caspase-9 then cleaves and activates the executor caspases-3/6/7, resulting in apoptosis of the cell.
- It is completely distinct from extracellular signals, which are often generated by cytotoxic immune cells and induce apoptosis mostly via the extrinsic pathway.
Process and Regulation of Intrinsic Apoptosis Pathway
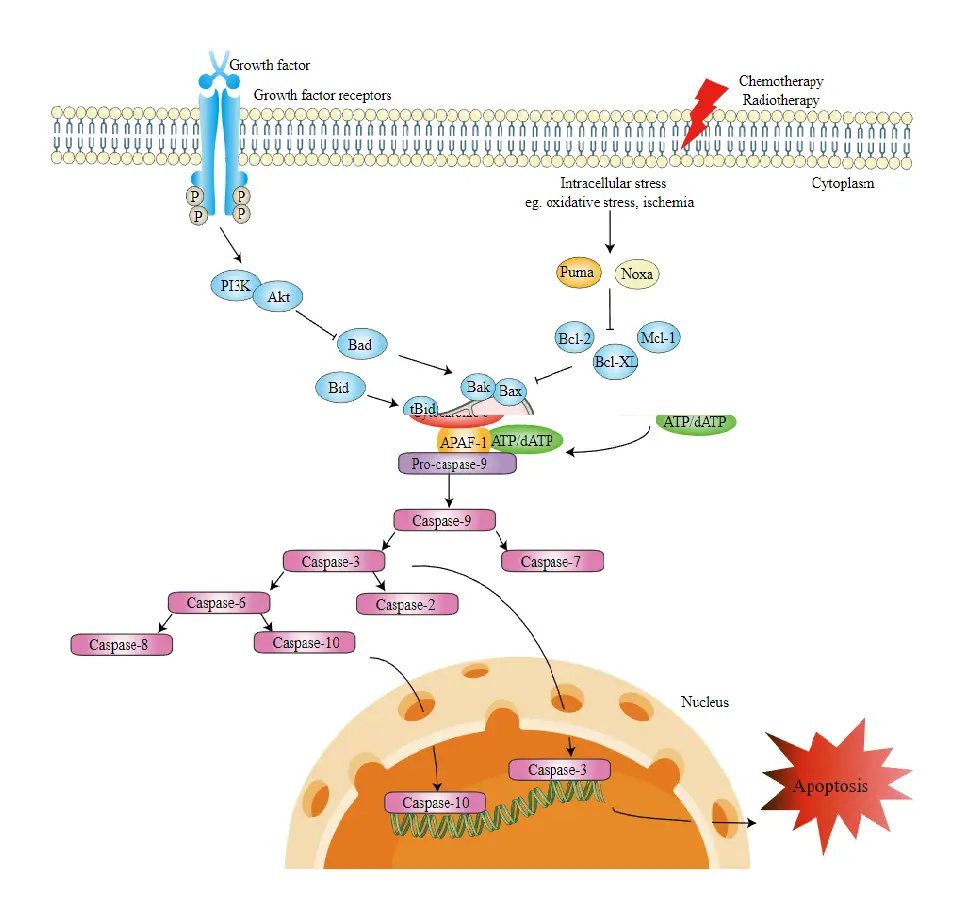
- The intrinsic apoptosis pathway promotes apoptosis by activating caspase-3 directly or by cleaving bid (BH3 interacting domain death agonist), resulting in mitochondrial malfunction, release of cytochrome c, and activation of caspases-9 and caspases-3. In numerous organs, Caspase-3 enhances apoptotic characteristics such as DNA fragmentation and cell death.
- The B-cell lymphoma 2 (Bcl-2) protein family controls activation of the intrinsic pathway with great precision. It was initially discovered as one of the genes implicated in cell death by activating pro-apoptotic or suppressing anti-apoptotic apoptosis in follicular lymphoma.
- One subgroup of proteins, including Bid, Bad, Bim, Bmf, Puma, and Noxa, have pro-apoptotic activity and include a single Bcl-2 homology 3 domain (BH3-only proteins). Two other categories of proteins include numerous BH domains.
- The first subset consists of pro-apoptotic proteins such as Bcl-2 associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak), and Bcl-2 family apoptosis regulator (Bok), whereas the second subset consists of anti-apoptotic proteins such as Bcl-2, Bcl-XL, and Mcl-1.
- Bcl family members attached to the mitochondrial membrane impact the mitochondrial pathway, including pro-apoptotic regulatory proteins Bax and anti-apoptotic regulatory proteins Bcl-2.
- The pro-apoptotic chemicals induce the permeabilization of the outer mitochondrial membrane, resulting in the efflux of cytochrome c, which binds Apaf-1 and caspase-9 in the cytosol to form the apoptosome complex.
- This activates the effector caspases by stimulating caspase-9. Additionally, the mitochondrion releases a protein known as Smac/DIABLO into the cytosol. Smac/DIABLO indirectly induces apoptosis by inhibiting the activities of inhibitor of apoptosis proteins, a group of anti-apoptotic proteins (IAPs).
- The anti-apoptotic proteins Bcl-2 and Bcl-XL block the release of cytochrome c from mitochondria, whereas the pro-apoptotic proteins Bax, Bak, and Bid enhance its release.
- Cytochrome c and deoxyadenosine triphosphate (dATP) link to APAF-1 to create a multimeric complex that attracts and activates pro-caspase-9, an apoptosis-mediating executioner protease, culminating in cell apoptosis.
- Caspases-2, caspase-8, caspase-9, and caspase-10 are implicated in the initiation of apoptosis throughout this process.
- Caspases-3, -6, and -7 all play a role in apoptosis. Caspases 3 and 7 regulate DNA repair inhibition and initiate DNA degradation. In addition, caspase-6 governs lamina and cytoskeleton breakdown.
Intrinsic Apoptosis Pathway in Pathophysiology
- The vast majority of chemotherapeutic and targeted cancer treatments eradicate tumour cells by generating pro-death signals that trigger the intrinsic apoptotic mechanism of programmed cell death.
- The point of no return in the apoptotic cascade is mitochondrial outer membrane permeabilization (MOMP); mitochondrial permeabilization leads to the creation of an apoptosome, which enables caspase activation and subsequently activates the other hallmarks of apoptotic cell death.
- A delicate balance between pro-apoptotic and anti-apoptotic BCL-2 family molecules controls the cellular decision to activate MOMP.
- Inability of tumour cells to undergo apoptosis because of abnormalities in the intrinsic apoptotic pathway is one of the causes of chemotherapy resistance (e.g., changes in p53). Many tumours continue to have unsatisfactory cure rates despite major advances in treatment.
- A fraction of tumours are now amenable to curative therapy as a result of the proliferation of cytotoxic chemotherapy, despite the fact that individual patients’ intrinsic treatment resistance is difficult to anticipate.
- The wave of molecularly targeted medicines has concentrated on drug gable-activating mutations and is consequently limited to select subsets of patients.
- The intrinsic mitochondrial route of apoptosis is a prospective target for future therapeutics, and its successful targeting has the potential to transform the therapeutic landscape for the treatment of a variety of malignancies.
FAQ
What is the intrinsic pathway of apoptosis?
The intrinsic pathway of apoptosis is a cellular process that is initiated from within the cell due to stress signals, such as DNA damage or the presence of free radicals. This pathway is also known as the mitochondrial pathway since it involves the activation of proteins that are present in the mitochondria.
What are the main players in the intrinsic pathway of apoptosis?
The intrinsic pathway of apoptosis involves several proteins, including Bcl-2 family members, cytochrome c, apoptosome, and caspases. The Bcl-2 family members are important regulators of apoptosis and control the permeability of the mitochondrial membrane. Cytochrome c is a protein that is normally found in the mitochondria and is released into the cytosol during apoptosis. The apoptosome is a complex of proteins that is formed in the cytosol after the release of cytochrome c, and it activates the caspases.
What is the role of Bcl-2 family members in the intrinsic pathway of apoptosis?
Bcl-2 family members regulate the permeability of the mitochondrial membrane and can either promote or inhibit apoptosis. Some members of this family, such as Bcl-2 and Bcl-XL, inhibit apoptosis by preventing the release of cytochrome c from the mitochondria. Other members, such as Bax and Bak, promote apoptosis by increasing the permeability of the mitochondrial membrane.
How does cytochrome c contribute to the intrinsic pathway of apoptosis?
During apoptosis, cytochrome c is released from the mitochondria and enters the cytosol. Once in the cytosol, it binds to the apoptosome, which is a complex of proteins that activates caspases. The caspases then initiate a cascade of proteolytic reactions that ultimately lead to cell death.
What is the role of the apoptosome in the intrinsic pathway of apoptosis?
The apoptosome is a complex of proteins that is formed in the cytosol after the release of cytochrome c from the mitochondria. The apoptosome activates caspases, which are proteases that cleave specific proteins and initiate a cascade of proteolytic reactions that ultimately lead to cell death.
How does the intrinsic pathway of apoptosis differ from the extrinsic pathway?
The extrinsic pathway of apoptosis is initiated by extracellular signals that activate specific receptors on the cell surface. In contrast, the intrinsic pathway is initiated from within the cell due to stress signals, such as DNA damage or the presence of free radicals. Additionally, the intrinsic pathway involves the release of cytochrome c from the mitochondria and the activation of caspases through the apoptosome, while the extrinsic pathway involves the binding of specific ligands to death receptors on the cell surface, which in turn activate caspases.
What are some examples of diseases associated with defects in the intrinsic pathway of apoptosis?
Defects in the intrinsic pathway of apoptosis have been implicated in a variety of diseases, including cancer, neurodegenerative disorders, and autoimmune diseases. For example, mutations in Bcl-2 family members have been identified in many types of cancer, and dysregulation of this pathway has been implicated in the development and progression of cancer. In neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease, dysfunction of the intrinsic pathway of apoptosis may contribute to the death of neurons.
References
- https://www.creative-diagnostics.com/intrinsic-apoptosis-pathway.htm
- https://en.wikipedia.org/wiki/Apoptosis
- https://www.intechopen.com/chapters/38236
- https://www.sinobiological.com/research/signal-transduction/intrinsic-apoptosis
- https://www.researchgate.net/figure/The-extrinsic-and-intrinsic-apoptotic-pathways-The-extrinsic-pathway-is-initiated-by_fig1_320542263
- https://teachmephysiology.com/biochemistry/cell-growth-death/apoptosis/
- https://www.creative-diagnostics.com/intrinsic-apoptosis-pathway.htm
- https://www.semanticscholar.org/paper/The-Intrinsic-Pathway-of-Apoptosis-and-%3A-An-Update-Mokbel-Mokbel/e04558f6f8b82037be2b502992c2a64cc4b43a57
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/intrinsic-apoptosis