Inoculating Loops and Needles are essential microbiology laboratory tools used for transferring, streaking, and culturing microorganisms under aseptic conditions.
What is Inoculating Loops and Needles?
- Inoculating loops and needles are small laboratory tools which can define as essential instruments for transferring microorganisms by sterile manner from one media to another.
- They are usually made of nichrome, platinum, or sometimes disposable plastic, and they designed for microbiological use in aseptic conditions.
- The loop part (wire forming circle at tip) is used mainly for streaking cultures on agar surface, while the straight needle is used for stabbing inoculation or for picking single colonies.
- It was developed long ago during early microbiological studies, after that time of Robert Koch and Louis Pasteur, when pure culture techniques started to prevail (though word prevail here used like prevent, but meaning same context).
- Inoculating loop’s concept was arose when scientists realized that direct contact transfer by instruments like pipette or stick was causing contamination, thus sterilizable metal loop was invented.
- For operation, the loop or needle is first sterilized by flame (mostly Bunsen burner flame) till red-hot, after cooling slightly it used to pick up the microbial sample.
- After transfer, again it is re-sterilized so that no residual organism remain and cross-contamination is avoided / minimized.
- These tools are very important for isolation, maintenance and sub-culturing of microorganisms which are used by microbiologists for research, teaching, diagnostic etc.
- They are also crucial for studying growth pattern, colony morphology and motility in semi-solid medium.
- The invention and continuous improvement of inoculating loops & needles have made sterile technique more precise and safer; it’s now used universally in labs of microbiology.
- Some modern loops are pre-sterilized disposable plastic one, used mostly in clinical labs for convenience; but traditional metal ones still preferred for repeated use.
- In short, this simple instrument play a vital role in microbiology since early historical period of bacteriology, and it continues to be indispensable tool for inoculation works.
Definition of Inoculating Loops and Needles
Inoculating loops and needles are handheld tools used in microbiology laboratories to transfer and manipulate microorganisms. Inoculating loops are wire loops with a small diameter used to collect and streak microbial cultures on solid agar-based media. Inoculating needles are sharp needles used to transfer solid media between Petri plates or other solid substrates, particularly for extracting samples from small colonies. These tools facilitate the controlled transfer of microorganisms for further analysis and investigation.
Principle of Inoculating Loops and Needles
- Inoculating loops and needles are mechanisms of a scientific practice which involves a sterile transfer of a very small amount of a microbial sample from one source to another without contamination.
- Once the tool (either a loop or a needle) is being sterilized by a flame to the point where the wire becomes red-hot, it means that microbes are killed and then it is cooled briefly before use.
- Next, after the loop (or the needle) is cooled, it is utilized to pick up a small portion of culture or colony, and after that, the loop (or the needle) is inoculated into another growth medium (agar plate, slant, or broth) so that the microbes can proliferate under controlled conditions.
- The size of the loop (or the tip of the needle) roughly determines the volume or the amount of inoculum that goes into the medium; this is very important to be done because the number of the cells that are introduced can have an impact on the isolation of single colonies.
- The method is performed under aseptic conditions where the surrounding surfaces, instruments, and media are sterilized and the instrument is handled in such a manner that it does not introduce unwanted microbes or cross-contamination.
- After the transfer, the instrument is again sterilized (flamed) so that it is free of any residual microbes which could contaminate subsequent transfers. This cycle of sterilize-cool-transfer-sterilize is the core of the method.
- If a needle is used (for example in stab inoculation of semi-solid medium), the point is similar that the penetration of the deeper media is done rather than surface streaking; the same sterilization and transfer rules apply, and the method locates the microbes at the desired depth.
- The primary goal of the principle, therefore, is to make a controlled and accurate transfer of a specimen of microorganism while at the same time prevent contamination, and also to make sure that the growth is the result of the intended specimen only.
Parts of Inoculating Loops and Needles
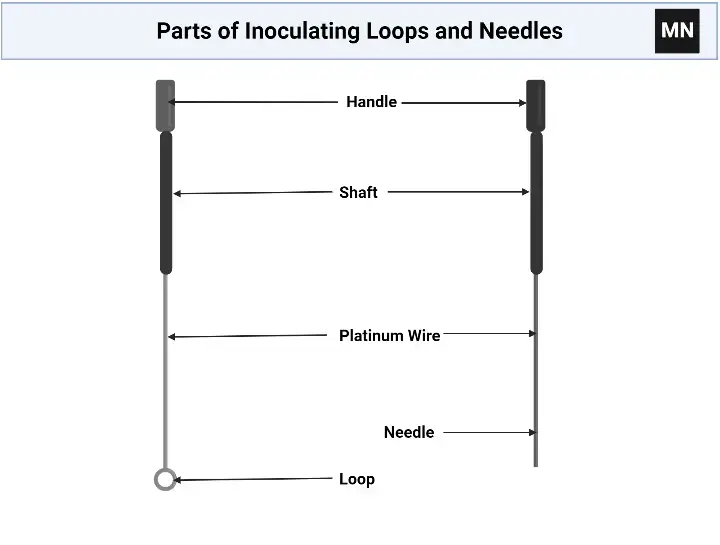
- Handle is provided as a rod or tube (metal or plastic) and is usually grasped by the user, it is sometimes insulated, and marked for size.
- Shaft is formed as the slender extension from handle to working end, and it is often straight or slightly bent for reach, this part is made of wire or metal.
- Loop is the rounded wire portion (small or large) that is used to pick up culture, the size is chosen depending on colony size and was sterilized by flame.
- Needle is the pointed end (for stab inoculation) which is formed by straightening and sharpening of wire, and it is usually heated for sterilization.
- Wire (nichrome/ platinum- nichrome) is selected for heat resistance, and it is attached to the shaft or handle, sometimes soldered or welded.
- Tip is shaped (loop rounded, or needle sharp) and is checked for continuity and integrity before use, because a bent tip will disturb colonies.
- Insulation / Grip is added on some handles (plastic or wooden), to protect hand from heat, and sometimes removed in metal types.
- Loop size marking is printed or inferred by colour/shape, and it is used to standardize inoculum volume (0.01 µl, 1 µl etc.), thus reproducibility is aimed.
- Connection point (where wire meets handle) is secured by crimping or welding, weak joints are avoided, otherwise loop/needle will detach.
- Sterilization surface (the part that is exposed to flame) is designated, users are instructed to heat only working end, but careless heating may warp the handle or melt plastic, so caution is advised.
Types of Inoculating Loops and Needles
A. Based on Calibration of Loops –
- Calibrated inoculation loop – used when quantitative culture is required; size usually 1 µL or 10 µL; calibration is licensed and accurate; ensures uniform inoculum delivery every time.
- Non-calibrated inoculation loop – have internal diameter (Ø) around 2 mm, 3 mm, 4 mm or 5 mm; prepared sterile and ready to use; very easy to handle and disposable type, mostly gamma-radiation sterilized before packaging.
B. Based on Reusability of Needles –
- Reusable inoculation needle – made by fixing nichrome or platinum wire with metallic handle; strong, can be flamed repeatedly; often used for stab cultures and routine transfers.
- Disposable inoculation needle – composed from plastic resin (rigid type); end is slightly blunted to avoid puncturing too deep; ideal when open flame cannot be used like under anaerobic chamber or biosafety hood; prevent infection risk, aerosol, and cross contamination; color-coded for quick identify.
C. Based on Material of Loop/Needle –
- Metallic type – generally nichrome or platinum-iridium alloy; durable and can tolerate repeated flaming.
- Plastic type – made from medical-grade plastic (polystyrene or polypropylene), sterile, single-use, and gamma-irradiated; no need of sterilization between uses.
D. Based on Structure/Design –
- Loop type – rounded wire at end, used for streaking on agar surface.
- Needle type – straight pointed end, used for deep inoculation or picking single colonies.
E. Based on Usage Environment –
- Flame-sterilized loops/needles – used in open bench microbiology where flame source (like Bunsen burner) available.
- Flameless/closed system loops – mainly disposable sterile plastics, used inside biosafety cabinet or anaerobic work zone to prevail (prevent) flame hazards and contamination etc.
Operating Procedure of Inoculating Loops and Needles
- Before operation, loop/needle must be checked for cleanliness and intact tip, damaged wire should not used.
- The instrument is held firmly by handle, usually in right hand while other hand handles tube or plate.
- Sterilization of loop or needle is done by passing it slowly through Bunsen burner flame, from handle toward tip until it glows red hot.
- Heating should be done evenly so that whole wire portion get sterilized, but over heating may deform loop shape.
- After flaming, it’s allowed to cool for few seconds (5–10 sec), otherwise hot loop can kill organism when it touch culture.
- During cooling, loop should not touch any surface, lid, or air dust, else contamination will occur again.
- The sterile loop/needle then is inserted into culture tube or plate carefully, mouth of tube is flamed shortly before and after inserting loop.
- Inoculum is gently collected by touching colony or broth surface, excessive pressure must be avoided, so agar surface don’t get damaged.
- For streak culture, loop is dragged lightly over surface of agar in zigzag manner, and for stab culture, needle is inserted straight into medium center.
- After inoculation, loop or needle again sterilized by flaming till red hot, to destroy any remaining cells.
- Tube mouth flamed again and closed properly, after that instrument placed on stand or hung to cool down.
- All used loops, needles or disposable ones are discarded in proper biohazard container, especially when working with pathogenic bacteria.
- Work area should be cleaned and wiped with 70% alcohol to prevail (prevent) contamination and infection spreading.
- Hands must be washed thoroughly after completion of procedure, and instruments stored safely for next use.
Applications of Inoculating Loops and Needles
- Inoculation of culture media is done by loops/needles for transferring microorganisms from one medium to another under aseptic condition.
- They are used for streaking technique on solid agar plates to obtain isolated colonies from a mixed microbial population.
- Stab inoculation with needle performed for testing motility or oxygen requirement of bacteria inside semi-solid media.
- Used in slant culture preparation where microorganisms are spread on surface of agar slant for maintenance or sub-culturing purpose.
- During broth transfer, loop picks up small inoculum and put into liquid medium, mixing occurs automatically after incubation.
- Calibrated loops are employed for quantitative analysis of bacterial load in urine or clinical samples (1 µL or 10 µL loop used).
- Needles are applied for picking single colonies or pure isolates without disturbing nearby growth.
- Helpful in microbial identification tests like oxidase, catalase, indole, etc., where isolated colonies must be taken precisely.
- Used in antimicrobial susceptibility testing (AST) for standard inoculum transfer onto Mueller–Hinton agar plate.
- For anaerobic or biosafety cabinet work, disposable plastic loops used instead of flame to prevail (prevent) aerosol formation.
- Utilized in teaching and research laboratories to demonstrate aseptic transfer and streak plate method for bacterial isolation.
- Applied during biochemical tests, enzyme assays, and other experimental works requiring pure culture transfer.
- Also used for maintaining stock cultures in laboratories where sterile inoculation without contamination is essential etc.
Advantages of Inoculating Loops and Needles
- Accurate transfer of microorganisms is achieved without damaging the culture or media surface.
- Aseptic handling of samples can done easily by sterilizing loop/needle before and after use.
- They allow precise inoculation, even with very small volume of culture (like 1 µL or 10 µL calibrated loops).
- Reusability of metallic loops/needles makes them economical and sturdy for long-term laboratory usage.
- Disposable types reduce the risk of contamination, cross-infection, and flame hazard in biosafety cabinet work.
- They are easy to sterilize by simple flaming, no complex apparatus needed for cleaning.
- Loops provide uniform spreading of inoculum on agar surface, giving well-isolated colonies for study.
- Needles permit deep inoculation into agar for observing motility or anaerobic growth characteristics.
- Instruments are lightweight and simple, can be used by beginners as well as experienced microbiologists.
- Less culture loss occurs because only tiny sample amount required, making tests more efficient.
- Can be used for multiple techniques – streaking, stabbing, transferring, maintaining pure cultures etc.
- Plastic disposable loops already sterile and ready to use, so they save time and avoid flame dependency.
- Provide high precision and control over inoculum size, improving reliability of quantitative culture results.
- Because of their compact size and durability, they are convenient to carry, store, and operate even in small workspace.
- Overall, loops and needles are reliable, simple and effective tools that prevail (prevent) contamination during microbial transfer.
Limitations of Inoculating Loops and Needles
- Sterilization by flame sometimes deform loop shape or damage thin wire after repeated heating.
- Cross-contamination risk is high if loop/needle not cooled properly before touching culture.
- Disposable plastic loops are single-use only, which increase laboratory waste and cost over time.
- Uneven cooling time may kill or injure microorganisms, leading to poor inoculation results.
- In anaerobic chamber, metallic loops cannot be used easily due to absence of open flame.
- Manual operation requires steady hand and skill, untrained person may tear agar or spill sample.
- Volume transfer by non-calibrated loops not accurate, so quantitative cultures sometimes unreliable.
- Heat sterilization may produce aerosols, which prevail (prevent) safe handling of pathogenic bacteria.
- Plastic loops though convenient, are not reusable, and may bend or break during streaking.
- During heavy workload, flame sterilization process becomes time consuming and tedious.
- Metal fatigue develops in wire loop after continuous bending and heating, making them fragile.
- Improper cooling or flaming can char the medium surface, altering bacterial growth pattern.
- Handling near burner poses burn hazard and risk of spreading flame to flammable reagents.
- Sometimes loop fails to pick single colony cleanly, so mixed culture obtained instead of pure one.
- Due to human dependency, consistency of inoculum transfer varies from one operator to another etc.
Precautions
- Loop or needle must be sterilized properly before and after every use to avoid contamination.
- While flaming, wire should be heated from handle end toward tip till it glows red-hot, uneven heating may cause breakage.
- After sterilization, loop must be cooled few seconds before touching culture, otherwise organisms will die instantly.
- During cooling, instrument should not touch bench surface or air dust, because that introduce unwanted microbes.
- Handle of loop must be held firmly but comfortably to maintain steady movement during inoculation.
- Work must done near Bunsen burner flame, where upward air current prevail (prevent) contamination from surrounding.
- Tube mouth should be flamed before and after inserting loop or needle, to maintain aseptic condition.
- Loop must not be overloaded with culture, as excess sample cause smearing and cross-spreading on agar surface.
- Avoid touching loop to inner wall of culture tube, which can carry unwanted material.
- Metallic loops should not be left in flame too long, since wire can melt or deform quickly.
- Disposable plastic loops are not reusable; they must be discarded immediately after one use in biohazard bin.
- Do not wave hot loop in air for cooling—it may cause aerosol or droplet spread of pathogens.
- Always keep flame area clean and uncluttered, to reduce fire hazard or accidental burns.
- After completion of inoculation, all cultures and instruments should be properly labeled and stored safely.
- Hands must be washed thoroughly with soap/alcohol before and after procedure to ensure personal safety etc.
FAQ
What are inoculating loops and needles used for?
Inoculating loops and needles are used in microbiology laboratories to transfer and inoculate microorganisms onto growth media for further study and analysis.
What are inoculating loops and needles made of?
Inoculating loops and needles are typically made of materials such as nichrome or platinum wire for the loop/needle portion, with handles made of aluminum, brass, or plastic.
How do you sterilize an inoculating loop or needle?
Inoculating loops and needles can be sterilized by passing the wire through a flame until it becomes red-hot, which helps eliminate any microorganisms present on the surface.
Can inoculating loops and needles be reused?
Yes, certain types of inoculating loops and needles, such as those with metallic handles and wire, can be reused after proper sterilization. However, disposable options are also available for single-use purposes.
How do you prevent contamination when using inoculating loops and needles?
To prevent contamination, it is important to maintain a sterile working environment, follow proper aseptic techniques, flame the loop or needle before and after use, and avoid touching the heated portion with bare hands.
What is the difference between an inoculating loop and an inoculating needle?
An inoculating loop has a small loop at the end of the wire, while an inoculating needle has a straight wire. The choice between the two depends on the specific technique and purpose of the inoculation.
Are there different sizes of inoculating loops and needles available?
Yes, inoculating loops and needles come in various sizes to accommodate different sample volumes and experimental requirements. Common sizes range from 2 mm to 5 mm in diameter.
Can inoculating loops and needles be used with liquid and solid media?
Yes, inoculating loops and needles are versatile and can be used for transferring samples to both liquid and solid growth media, depending on the technique and experiment.
Are there any risks associated with using inoculating loops and needles?
The primary risks associated with using inoculating loops and needles include burns from the heated wire and the potential for cross-contamination if proper sterilization and aseptic techniques are not followed.
Where can I purchase inoculating loops and needles?
Inoculating loops and needles can be purchased from scientific supply companies, laboratory equipment suppliers, or online sources specializing in microbiology and laboratory tools.
- Microbiology textbooks: Textbooks on microbiology, such as “Microbiology: An Introduction” by Gerard J. Tortora, Berdell R. Funke, and Christine L. Case, often cover topics related to laboratory techniques and equipment, including inoculating loops and needles.
- https://pharmaceuticalmicrobiologi.blogspot.com/2016/12/inoculation-loop.html
- https://www.slideshare.net/vidhyakalaivani29/inoculation-loop
- https://www.clinisciences.com/en/buy/cat-inoculating-loops-and-needles-for-5565.html
- https://www.fishersci.fi/fi/en/products/I9C8L41A/inoculating-loops-needles.html
- http://site.iugaza.edu.ps/aqabbas/files/general-micro.pdf
- https://www.fishersci.com/shop/products/fisherbrand-disposable-inoculating-loops-needles-6/p-3622181#?keyword=