What is Influenza Virus?
- Influenza viruses are members of the family Orthomyxoviridae, which consists of enclosed viruses with negative, single-strand RNA regions in their genomes.
- Humans have suffered from influenza for millennia. It is hypothesized that humans could get influenza at the onset of domesticating animals.
- The growth of agriculture and the construction of permanent communities produced a large number of potential epidemic hosts.
- Mid-17th century Italians used the term “flu” to designate an illness caused by miasma. Until 1933, the human influenza virus had not been isolated.
Classification of Influenza Virus
Influenza viruses are often classified into three species: influenza A, influenza B, and influenza C. Only types A and B are relevant to human health. Despite the fact that both viruses are capable of infecting humans, genus A often causes widespread disease and epidemics.
- Influenza A virus: In 1933, it was successfully isolated. Influenza Viruses are frequently transmitted by animals such as birds and pigs and are the causative agent of zoonotic diseases. It is known that only influenza A viruses have triggered pandemics. Influenza An antigenic shift is an abrupt change in antigenicity caused by genetic recombination between viral strains that results in a new virus subtype. Influenza A viruses are subclassified and designated by combining the H and N numbers, such as A(H1N1), A(H3N2), and A(H3N2), based on the surface proteins hemagglutinin (H) and neuraminidase (N) (H3N2).
- Influenza B virus: In 1940, influenza B virus type B was discovered. It is exclusive to humans and typically causes less severe symptoms than type A virus. Although it can cause seasonal disease and epidemics in humans, the influenza B virus has never triggered a pandemic. B/Yamagata and B/Victoria are the two subtypes of influenza B viruses.
- Influenza C virus: Infections of type C often produce moderate illness and are not believed to trigger influenza epidemics in humans.
Influenza A Virus
- Influenza A virus is a type of virus that causes respiratory illness in humans and animals. It is a highly contagious virus that spreads easily from person to person through droplets in the air when an infected person coughs, sneezes, or talks. The virus can also spread by touching a surface contaminated with the virus and then touching one’s mouth, nose, or eyes.
- Influenza A virus can cause a range of symptoms, including fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills, and fatigue. In some cases, it can lead to severe complications such as pneumonia, respiratory failure, and even death, especially in high-risk groups such as young children, older adults, and people with weakened immune systems.
- The virus is constantly evolving, which is why it is important to get vaccinated against the flu every year. The vaccine is formulated to protect against the strains of influenza A virus that are expected to be most prevalent in a given flu season. In addition to vaccination, preventive measures such as frequent hand washing, covering one’s mouth and nose when coughing or sneezing, and avoiding close contact with people who are sick can help reduce the spread of the virus.
- Treatment for influenza A virus typically involves rest, hydration, and over-the-counter medications to manage symptoms such as fever and cough. Antiviral medications may also be prescribed in some cases to reduce the severity and duration of the illness, especially in high-risk individuals. It is important to seek medical attention if symptoms are severe or if complications are suspected.
Structure of Influenza A Virus
- Influenza A viruses are negative-sense, single-stranded, segmented RNA virus. The various subtypes are designated by a H number (for the hemagglutinin type) and a N number (for the type of neuraminidase).
- There are 18 known H antigens (H1 to H18) and 11 known N antigens (N1 to N11). In 2012, H17N10 was isolated from fruit bats, and in 2013, H18N11 was identified in a Peruvian bat.
- Influenza type A viruses are structurally similar to influenza viruses types B, C, and D. The virus particle (also known as the virion) is 80–120 nanometers in diameter, with the smallest virions adopting an elliptical shape.
- The length of each particle varies considerably, as influenza is pleomorphic, and can exceed tens of micrometers, producing filamentous virions. All of them are composed of a viral envelope containing two major types of proteins and a central core .
- Hemagglutinin (HA) and neuraminidase are the two major proteins found on the surface of viral particles (NA). HA is a protein that facilitates virion binding to target cells and viral genome entry into the target cell.
- NA is involved in the release from the abundant non-productive attachment sites present in mucus and the release of progeny virions from infected cells. These proteins are typically the targets of antiviral drugs.
- Additionally, they are the antigen proteins to which a host’s antibodies can bind and elicit an immune response. Viruses of influenza type A are classified into subtypes according to the type of these two proteins on the surface of the viral envelope. There are 16 subtypes of HA and 9 subtypes of NA, but only H 1, 2, 3, and N 1 and 2 are prevalent in humans.
- Using electron microscopy, Influenza A and B viruses are almost indistinguishable. They are typically spherical with a diameter of approximately 100 nm, but occasionally filamentous with a mean length greater than 300 nm.
- The outermost covering of influenza virus particles is the envelope derived from the host cell. The envelope is studded with projecting glycoprotein spikes consisting of hemagglutinin (HA) and neuraminidase (NA).
- Both HA and NA determine the influenza virus subtypes. The HA to NA ratio is approximately 4:1. M2 matrix ion channels traverse the lipid envelope less frequently. The matrix protein M1 is located beneath the envelope and encases the virion’s core.
- Eight bits of single-stranded RNA make up the viral genome within the virion (only type A and B). A helical ribonucleoprotein (RNP) complex is formed from RNA that is coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (PB1, PB2, and PA). Nuclear export protein (NEP) is also present within the virion’s interior.
| Target | Protein | Description |
|---|---|---|
| HA | Hemagglutinin | During viral replication, the HA protein is converted into HA1 and HA2 by serine proteases, which confers infectivity to the virus. The HA2 is involved in the fusing of the viral envelope with the membrane of the host cell, whereas the HA1 comprises receptor-binding and antigenic sites. It is also an antigen that the immune system may identify. |
| NA | Neuraminidase | It is able to degrade receptors by cleaving terminal sialic acid residues from cell-surface glycoproteins and gangliosides in order to release virus progeny from the host cell. In addition, the NA eliminates sialic acid residues from the virus envelope, preventing the aggregation of viral particles in order to increase infectiousness. |
| M1 | matrix protein M1 | M1 interacts with both viral RNA and nucleoprotein within the RNP complex, bringing them together. It also connects with NEP, promoting the export of M1-RNP into the cytoplasm via nucleoporins. |
| M2 | matrix ion channels M2 | It is the target of anti-influenza medicines in the amantadine class, which decrease ion channel activity and prevent viral unshelling. As a surface protein, it can be a vaccine component. |
| NEP/NS | Nuclear export protein | NEP is involved in the translocation of M1-RNP into the cytoplasm alongside M1. |
| NP | Nucleoprotein | Along with nucleoprotein, viral RNA is packed into a helical ribonucleoprotein complex. |
| RNP | Ribonucleoprotein | Coated with nucleoprotein and heterotrimeric RNA-dependent RNA polymerase (PB1, PB2, and PA), RNA forms a helical ribonucleoprotein (RNP) complex. |
Genome of Influenza A Virus
- The viral genome and other viral proteins that package and protect the genetic material are located in the core of a virion. Unlike the genomes of most organisms (including humans, animals, plants, and bacteria), which are composed of double-stranded DNA, the genomes of many viruses are composed of RNA, a single-stranded nucleic acid.
- The influenza type A virus genome, however, is not a single piece of RNA; rather, it consists of segmented pieces of negative-sense RNA, each containing one or two genes that code for a gene product (protein).
- The term negative-sense RNA simply indicates that the RNA genome cannot be directly translated into protein; it must first be transcribed to positive-sense RNA before being translated into protein products. The genome’s segmented structure enables the exchange of entire genes between different viral strains.
- The complete genome of the Influenza A virus consists of 13,588 bases and eight RNA segments that, depending on the strain, code for at least 10 and as many as 14 proteins. Relevance or presence of alternative gene products can vary based on the following factors:
- Segment 1 encodes RNA polymerase subunit (PB2).
- Using distinct reading frames from the same RNA segment, Segment 2 encodes the RNA polymerase subunit (PB1) and the protein PB1-F2, which induces cell death.
- RNA polymerase subunit (PA) and the PA-X protein, which plays a role in host transcription shutoff, are encoded by Segment 3.
- Segment 4 encodes for HA (hemagglutinin). Approximately 500 molecules of hemagglutinin are required to produce one virion. The extent and severity of a viral infection in a host organism are determined by HA.
- NP is encoded by Segment 5, which is a nucleoprotein.
- Segment 6 encodes NA (neuraminidase). Approximately 100 molecules of neuraminidase are required to produce a virion.
- Segment 7 encodes two matrix proteins (M1 and M2) using distinct reading frames within the same RNA segment. Approximately 3,000 matrix protein molecules are required to construct a virion.
- Using distinct reading frames from the same RNA segment, Segment 8 codes for two distinct non-structural proteins (NS1 and NEP).
- RNA segments of the viral genome possess complementary base sequences at their terminal ends, allowing them to form hydrogen bonds. Transcription of the viral (-) sense genome (vRNA) can only occur after the PB2 protein binds to host capped RNAs, allowing the PA subunit to cleave several nucleotides after the cap.
- This host-derived cap and the nucleotides that accompany it serve as the primer for the initiation of viral transcription. Transcription continues along the vRNA until a stretch of multiple uracil bases is encountered, initiating a’stuttering’ in which the nascent viral mRNA is poly-adenylated, resulting in a mature transcript for nuclear export and translation by host machinery.
- RNA synthesis occurs in the nucleus of the cell, whereas protein synthesis occurs in the cytoplasm. Once the viral proteins are assembled into virions, the virions leave the nucleus and migrate towards the cell membrane.
- The host cell membrane has patches of viral transmembrane proteins (HA, NA, and M2) and an underlying layer of the M1 protein that assist the assembled virions in budding through the membrane and releasing finished enveloped viruses into the extracellular fluid.
- It is estimated that the subtypes of influenza A virus diverged 2,000 years ago. Influenza viruses A and B are believed to have diverged from a common ancestor approximately 4,000 years ago, whereas the ancestor of influenza viruses A and B and the ancestor of influenza virus C are believed to have diverged approximately 8,000 years ago.
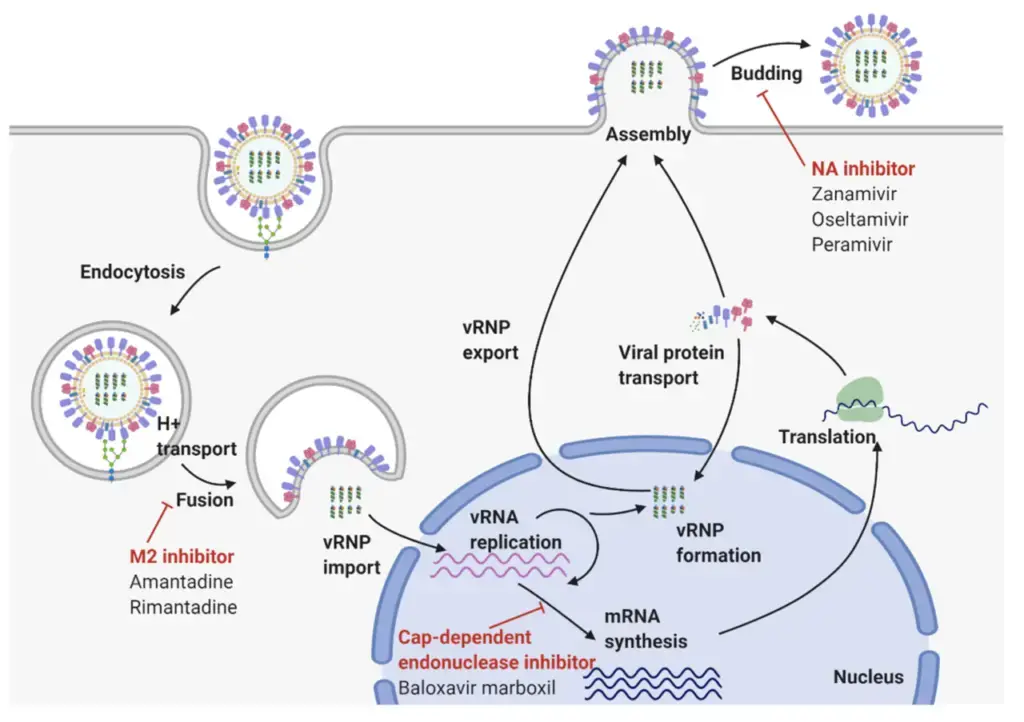
Epidemiology of Influenza A Virus
- Influenza A virus is a highly diverse virus that can infect multiple species, including humans, birds, swine, horses, seals, mink, and whales. The virus is classified into subtypes based on the antigenic difference of the major membrane glycoproteins HA and NA, with 18 HA subtypes and 11 NA subtypes currently identified. Different combinations of HA and NA subtypes have been found in birds, animals, and humans.
- In humans, four HA (H1, H2, H3, H5) and two NA (N1, N2) subtypes have been recovered. Birds are the primary reservoir for influenza A virus, and pandemics often occur when the virus undergoes an antigenic shift, resulting from the acquisition of a complete new RNA segment 4 and/or 6, either as a result of reassortment or infection of humans with an animal virus.
- Influenza A virus causes annual outbreaks of variable intensity worldwide, with an estimated 3-5 million cases of severe illness and 250,000-500,000 deaths annually. The economic impact of these outbreaks is significant due to the associated morbidity. Major pandemics have occurred in 1918, 1957, 1968, and 2009, with the emergence of H1N1 Spanish influenza, H2N2, H3N2, and H1N1 2009pdm from swine, respectively.
- The great pandemic of 1918-1919 was particularly severe, killing 20-40 million people as it spread over a few years. Influenza A virus was first isolated from a patient’s throat washing in 1933 by Smith Andrews and Laidlaw. Two influenza A subtypes, H3N2 and H1N1, have been circulating concurrently, with epidemics occurring regularly in winter months between pandemics associated with genetic drift in the HA antigen.
Replication Cycle of Influenza A Virus

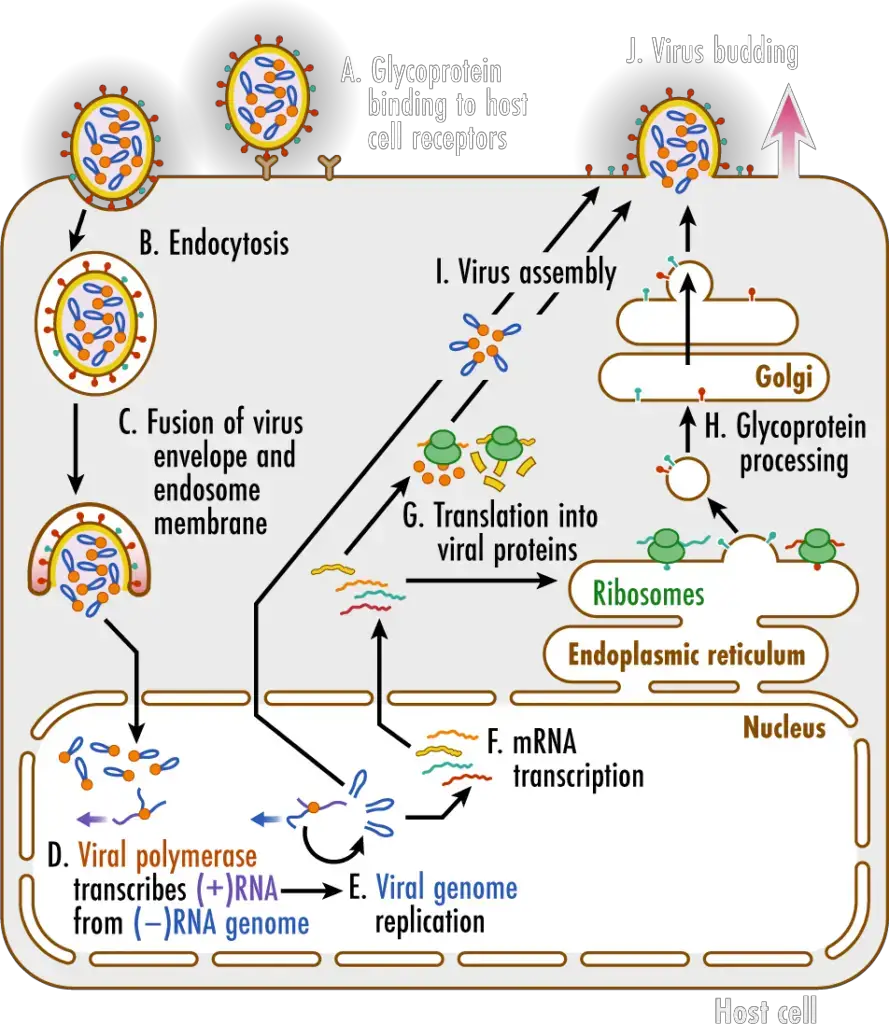
- Glycoproteins on the surface of influenza viruses A virus binds to specific receptors on the host cell’s surface (A). This initiates a process known as endocytosis (B), which transports the virus into the cell in a vesicle known as an endosome. Acidic chemicals accumulate within the endosome, decreasing the pH and causing the endosome membrane to merge with the virus’s envelope. This releases the virus’ (–)RNA genomic segments and viral polymerase into the cytosol (C).
- The viral genome enters the nucleus of the host cell, where the viral polymerase transcribes the (–)RNA genome segments into complementary (+)RNA templates (D). These templates are utilized to produce copies of all the virus’s (–)RNA genomic segments (E).
- The (–)RNA segments of the virus are also transcribed into messenger RNAs (F), which are translated into proteins by ribosomes in the cytosol and endoplasmic reticulum (ER) (G). The ER-Golgi network (H) processes influenza virus glycoproteins, which are then delivered to the cell membrane.
- Viral genomes and proteins are assembled into new viruses (I), which then exit the cell via a process known as budding (J). This procedure envelops the virus in a portion of the cell membrane containing viral proteins, which creates the envelope for the virus.
Pathogenesis of Influenza A Virus
- The influenza virus has the ability to spread from one individual to another through airborne droplets or contact with infected hands or surfaces. The virus can enter a person’s body when they inhale tiny droplets containing the virus. The upper and lower respiratory tracts possess a substance known as sialic acid, which the virus’s HA portion can bind to. When virus particles manage to evade the body’s cough reflex and specific immunoglobulin A (IgA) antibodies or inactivation by nonspecific inhibitors in the mucous secretions, a few cells in the respiratory epithelium get infected. Subsequently, the virus reproduces in the nucleus and produces offspring virions that quickly spread to nearby cells.
- The viscosity of the mucous film in the respiratory tract is lowered by the viral NA, which exposes the cellular surface receptors and promotes the distribution of fluid containing the virus to lower parts of the tract. Within a brief period, many cells in the respiratory tract are infected and eventually destroyed, leading to additional flu-like symptoms such as fever, chills, muscle ache, headache, and fatigue. The duration of the incubation period from virus exposure to the onset of symptoms varies from one to four days, depending on the viral dose size and the immune system’s status.
- Viral shedding, which occurs the day before symptom onset, peaks within 24 hours, remains elevated for 1-2 days, and then declines over the next five days. Approximately one day after viral shedding begins, interferon is detectable in respiratory secretions. If the virus migrates to the lower respiratory tract, it can cause severe shedding of bronchial or alveolar epithelium, which can extend down to a single-cell basal layer or to the basement membrane. Influenza infection harms the respiratory tract epithelium, reducing its ability to resist secondary bacterial invaders, especially staphylococci, streptococci, and Haemophilus influenza.
- An inflammatory cell response of the mucosal membrane occurs as a result of influenza infection, which mainly consists of monocytes and lymphocytes and a few neutrophils. Submucosal edema is present, and lung tissue may show hyaline membrane disease, alveolar emphysema, and necrosis of the alveolar walls. T-cell responses are critical in aspects of recovery and immunopathogenesis, but antibody production, including vaccine-induced antibody, can help prevent disease. Protection against reinfection is primarily linked to the creation of antibodies to HA, although antibodies to NA can also provide protection. The antibody response is specific to each strain of influenza, but the cell-mediated immune response is more general and can respond to influenza strains of the same type.
- Influenza virus spreads through airborne droplets when an infected person coughs, sneezes, or talks, as well as through contact with contaminated surfaces or objects.
- When a person inhales virus-containing microdroplets, the virus can bind to sialic acid present in the respiratory tract, particularly in the upper and lower respiratory tract.
- If virus particles manage to avoid being removed by the cough reflex or neutralized by preexisting specific antibodies or nonspecific inhibitors in the mucous secretions, they can infect a few cells of the respiratory epithelium.
- Once the virus enters the cell, it replicates in the nucleus and produces progeny virions that can spread to adjacent cells.
- The viral NA (neuraminidase) enzyme can lower the viscosity of the mucous film in the respiratory tract, which exposes the cellular surface receptors and helps to promote the spread of virus-containing fluid to lower portions of the tract.
- As more cells in the respiratory tract become infected and eventually die, the body’s immune system responds, leading to symptoms such as fever, chills, muscle aches, headache, and fatigue.
- The incubation period of influenza varies from 1 day to 4 days, depending on factors such as the size of the viral dose and the immune status of the person.
- Viral shedding, or the release of virus particles from the infected person, begins the day before symptom onset and peaks within 24 hours. Viral shedding remains elevated for 1-2 days and then declines over the next 5 days.
- Interferon, a protein that helps to activate the immune response, can be detected in respiratory secretions about 1 day after viral shedding begins.
- If the virus spreads to the lower respiratory tract, it can cause severe damage to the bronchial or alveolar epithelium, leading to shedding down to a single-cell basal layer or to the basement membrane.
- Influenza infection can also lower the respiratory tract epithelium’s resistance to secondary bacterial infections, such as those caused by staphylococci, streptococci, and Haemophilus influenza.
- The body’s immune response to influenza involves both antibodies and T-cells. Antibodies, particularly those targeting the HA (hemagglutinin) and NA proteins, can help to prevent infection and disease. However, the antibody response is specific to each strain of influenza, so a person needs to develop new antibodies if they encounter a new strain. T-cells, on the other hand, can react to influenza strains of the same type and may play a role in recovery and immunopathogenesis (the way that the immune system interacts with the virus).
Symptoms of Influenza A Virus
- Symptoms of Influenza A Virus include a sudden onset of fever, muscle aches, headache, malaise, nonproductive cough, sore throat, and rhinitis. In children, otitis media, nausea, and vomiting may also be commonly reported.
- These symptoms are similar to those of other respiratory infections, so laboratory tests are often needed to confirm a diagnosis of influenza A virus.
- Additionally, influenza virus infections can lead to various complications, such as primary influenza, viral pneumonia, exacerbation of underlying medical conditions, secondary bacterial pneumonia, sinusitis, or otitis media.
- In rare cases, influenza virus infections have also been associated with encephalopathy, transverse myelitis, myositis, myocarditis, pericarditis, and Reye’s syndrome.
Complications of Influenza A Virus
Complications of Influenza A Virus can be severe and potentially life-threatening. Here are some of the common complications associated with influenza A virus:
- Primary Influenza: Influenza A virus can cause primary influenza, which is a severe respiratory illness that can lead to hospitalization.
- Viral Pneumonia: Influenza A virus can also cause viral pneumonia, a lung infection that can be severe and may require hospitalization.
- Exacerbation of Underlying Medical Conditions: Influenza A virus can worsen underlying medical conditions such as pulmonary or cardiac disease.
- Secondary Bacterial Pneumonia: In some cases, influenza A virus can lead to secondary bacterial pneumonia, a potentially life-threatening condition that requires immediate medical attention.
- Sinusitis and Otitis Media: Influenza A virus can also lead to sinusitis and otitis media, which are infections of the sinuses and ears.
- Coinfections with Other Pathogens: Influenza A virus can also contribute to coinfections with other viral or bacterial pathogens, leading to more severe illness.
- Rare Complications: Although rare, influenza A virus infection has been associated with complications such as encephalopathy, transverse myelitis, myositis, myocarditis, pericarditis, and Reye’s syndrome.
Laboratory diagnosis of Influenza A Virus
Laboratory diagnosis of Influenza A Virus involves different methods, such as:
- Molecular Tests: These tests use a technique called polymerase chain reaction (PCR) to detect the genetic material of the virus in respiratory specimens. This is considered the most accurate method for diagnosing influenza.
- Rapid Influenza Diagnostic Tests (RIDTs): These tests can provide results within 15-30 minutes by detecting viral antigens in respiratory specimens. However, they are less sensitive than molecular tests and may produce false-negative results.
- Viral Culture: This involves growing the virus in a laboratory setting using respiratory specimens. It is considered the gold standard for influenza diagnosis, but it is time-consuming and may take up to several days to obtain results.
- Serologic Tests: These tests detect antibodies produced by the body in response to the virus. They are useful for identifying past influenza infections but are not recommended for routine diagnosis due to their limited accuracy and delayed results.
Treatment of Influenza A Virus
The treatment of Influenza A Virus can involve several strategies, including:
- Antiviral medications: These medications can be used to treat and prevent influenza. They work by stopping the virus from replicating in the body. Examples include oseltamivir (Tamiflu) and zanamivir (Relenza).
- Symptomatic treatment: Medications such as acetaminophen or ibuprofen can be used to relieve fever and muscle aches. Decongestants and cough suppressants may also be used to alleviate respiratory symptoms.
- Rest and hydration: Getting plenty of rest and staying hydrated can help the body fight off the virus and recover faster.
- Hospitalization: In severe cases of influenza, hospitalization may be necessary for treatment with intravenous fluids and antiviral medications.
It’s important to note that antibiotics are not effective against the influenza virus, as they only work against bacterial infections. Additionally, antiviral medications are most effective when taken within the first 48 hours of symptom onset.
Prevention of Influenza A Virus
Prevention of Influenza A Virus can be done through vaccination and by practicing good hygiene.
- Vaccination: The most effective way to prevent Influenza A Virus is through annual vaccination. The vaccine is typically given as a shot, but there is also a nasal spray option available. It is recommended for everyone aged 6 months and older, especially for those who are at a higher risk of complications from the virus, such as the elderly, young children, pregnant women, and people with certain medical conditions.
- Good hygiene practices: In addition to vaccination, practicing good hygiene can also help prevent the spread of Influenza A Virus. This includes:
- Washing hands frequently with soap and water for at least 20 seconds or using alcohol-based hand sanitizer.
- Covering mouth and nose with a tissue or elbow when coughing or sneezing.
- Avoiding close contact with people who are sick.
- Staying home when feeling sick to avoid spreading the virus to others.
- Avoiding touching the face, especially the nose and mouth, to prevent the virus from entering the body.
Types of vaccine of Influenza A Virus
There are four types of vaccines available for Influenza A Virus:
- Killed vaccines: These vaccines contain inactivated virus particles and are administered through an injection. They are safe for individuals with weakened immune systems.
- Split virus vaccines: These vaccines contain split virions (partially disrupted virus particles) and are also administered through an injection. They are effective in inducing an immune response, but may not be suitable for individuals with egg allergies.
- Subunit virus vaccines: These vaccines contain only the antigenic components of the virus and are also administered through an injection. They are safe for individuals with egg allergies and are often used for high-risk populations.
- Live attenuated vaccines: These vaccines contain a weakened form of the virus and are administered through a nasal spray. They are not recommended for individuals with weakened immune systems or pregnant women.
Control of Influenza A Virus
Controlling Influenza A virus involves a combination of preventive measures, treatment, and surveillance. Some of the key control measures are:
- Vaccination: Annual vaccination with the most recent strains of the virus is the best way to prevent influenza. Vaccines are available in various forms, such as inactivated or live attenuated vaccines.
- Antiviral medication: Antiviral drugs can be used for treatment and prevention of influenza. These drugs work by inhibiting the virus from replicating and spreading, reducing the severity and duration of symptoms.
- Hygiene practices: Frequent hand washing and covering your mouth and nose while sneezing or coughing can help prevent the spread of the virus.
- Social distancing: Maintaining distance from people who are sick or have flu-like symptoms can help prevent the spread of the virus.
- Isolation and quarantine: Individuals who are infected with the virus may need to be isolated or quarantined to prevent further spread of the virus.
- Surveillance: Monitoring the spread and evolution of the virus is important for developing effective control strategies. Health officials use surveillance data to track outbreaks, identify high-risk groups, and make recommendations for prevention and treatment.
FAQ
What is Influenza Virus?
Influenza Virus is a highly contagious respiratory virus that causes flu. It is a common illness that affects millions of people worldwide each year.
What are the symptoms of Influenza Virus?
The symptoms of Influenza Virus include fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills, and fatigue.
How is Influenza Virus transmitted?
Influenza Virus is transmitted through respiratory droplets when an infected person coughs or sneezes. It can also be contracted by touching surfaces contaminated with the virus and then touching your mouth or nose.
Who is at risk for Influenza Virus?
Anyone can get Influenza Virus, but certain groups are at higher risk of complications, including young children, older adults, pregnant women, and people with underlying health conditions.
How is Influenza Virus diagnosed?
Influenza Virus is diagnosed through laboratory testing, such as a rapid influenza diagnostic test (RIDT) or a viral culture.
What are the complications of Influenza Virus?
Complications of Influenza Virus can include pneumonia, bronchitis, sinus and ear infections, and worsening of chronic medical conditions, such as asthma or diabetes.
How is Influenza Virus treated?
Influenza Virus is treated with antiviral medications, which can help shorten the duration of the illness and prevent complications. Supportive care, such as rest, hydration, and fever reduction, is also important.
How can Influenza Virus be prevented?
The best way to prevent Influenza Virus is by getting an annual flu vaccine, practicing good hand hygiene, and avoiding close contact with sick individuals.
Can Influenza Virus be deadly?
Yes, Influenza Virus can be deadly, particularly for those at high risk of complications. It is estimated to cause between 290,000 to 650,000 deaths worldwide each year.
Can you get Influenza Virus even if you have been vaccinated?
Yes, it is possible to get Influenza Virus even if you have been vaccinated, as the vaccine does not provide 100% protection. However, getting vaccinated can still reduce the severity of the illness and prevent complications.
References
- Arbeitskreis Blut, Untergruppe «Bewertung Blutassoziierter Krankheitserreger». Influenza Virus. Transfus Med Hemother. 2009;36(1):32-39. doi: 10.1159/000197314. PMID: 21048819; PMCID: PMC2928832.
- Lamb, R. A. (2008). Influenza. Encyclopedia of Virology, 95–104. doi:10.1016/b978-012374410-4.00654-3
- Grant EJ, Chen L, Quiñones-Parra S, Pang K, Kedzierska K, Chen W. T-cell immunity to influenza A viruses. Crit Rev Immunol. 2014;34(1):15-39. doi: 10.1615/critrevimmunol.2013010019. PMID: 24579700.
- Belshan, M. A., Knoop, F. C., & Huggett, K. N. (2014). Influenza A☆. Reference Module in Biomedical Sciences. doi:10.1016/b978-0-12-801238-3.05048-0
- https://www.efsa.europa.eu/en/topics/topic/new-influenza-h1n1
- https://www.metropolisindia.com/blog/preventive-healthcare/influenza-a-vs-b-virus/
- https://media.hhmi.org/biointeractive/click/virus-explorer/influenza.html
- https://www.cusabio.com/infectious-diseases/influenza-virus.html
- https://www.cdc.gov/flu/about/viruses/types.htm
- https://www.mayoclinic.org/diseases-conditions/flu/symptoms-causes/syc-20351719
- https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- https://www.healthline.com/health/influenza-a-symptoms