What Is Induced Breeding (hypophysation)?
Induced breeding is the process in which fishes are stimulated to reproduce under controlled conditions when natural spawning does not occur in captivity. It is the technique where hormonal preparations are used to activate the maturation of gonads and to release the gametes. This is referred to as hypophysation because the pituitary gland (hypophysis) extract was the first hormone source used for this purpose.
It is the process in which crude pituitary gland extract, human chorionic gonadotropin (HCG), or synthetic releasing hormones (LHRH analogues) are injected into the brood fish to initiate ovulation and spermiation.
In this method, the hormone is given in one or two doses depending on the species. A small priming dose is applied first and after few hours a second resolving dose is given to complete the maturation of dormant eggs.
It is the action of these hormones that overcome the failure of natural spawning inside ponds or tanks where environmental cues such as rainfall, temperature, and current are absent. The injected fish are either stripped after ovulation or allowed to spawn inside a confined enclosure like a hapa or tank. The eggs obtained are fertilized with milt collected from the male brooders.
It is the process which helps in maintaining a regular and reliable supply of fish seed because the natural seed availability is uncertain and seasonal. By using induced breeding the hatcheries can synchronize the spawning, increase fry survival, and also produce seed even outside the normal breeding season. This is the major source of seed production in many carp hatcheries and it ensures better control over quality and quantity of seed produced.
History of induced breeding
The history of induced breeding is the gradual development of hormonal techniques to overcome the difficulties of natural spawning in captive fishes. It is the process that began in the early part of the twentieth century when researchers first attempted to control reproduction under artificial conditions. The earliest work was done in Argentina in 1930 by Houssay who used pituitary extract to stimulate viviparous fishes to give premature birth.
This success showed that reproductive activities can be induced by hormones and the method was soon repeated in Brazil in 1934, and later in America and Russia by Merlin, Hubs and Gerebilisky. It is referred to as hypophysation because crude pituitary glands collected from mature donor fishes were injected into brooders in the initial procedures.
In Asia the application of this technique became important for aquaculture. In India, Khan reported induced breeding in Cirrhinus mrigala in 1937. Later Dr. Hiralal Choudhuri applied this process in minor carps like Pseudeotropius atherinoides and Esomus danricus in 1955. A major step was reached in 1957 when Dr. Choudhuri successfully induced breeding of Indian major carps such as Cirrhinus mrigala, C. reba and Labeo rohita.
After this, Chinese carps like Ctenopharyngodon idella and Hypophthalmichthys molitrix were induced to spawn in India by Parameswaran and Alikuni in 1963. These works established induced breeding as the main method for seed production in freshwater aquaculture.
The traditional method used crude pituitary extract, human chorionic gonadotropin (HCG), or semi-purified fish gonadotropins. It is the process in which brood fishes were given two injections, a small priming dose and later a higher resolving dose. The time gap between these doses varied from 3 to 24 hours depending on the species.
During the 1970s a major shift occurred when synthetic hormones like GnRH and LHRHa started to be used. Chinese researchers around 1977 showed that synthetic LHRH analogues can be used to induce spawning in cultured carps.
It was observed that LHRHa alone was not effective in many cyprinids. Because dopamine has an inhibitory effect on gonadotropin release, the technique was improved by combining LHRHa with dopamine antagonists such as pimozide or domperidone. This combined technique was standardized in China as the LinPe method.
Later developments included the use of sustained-release polymer implants of GnRH agonists that release hormone slowly over several days. It is the method that reduces the need for repeated injections and is used for inducing multiple spawning in species like seabass and milkfish.
Recent studies are directed toward regulators like Kisspeptin, a neuropeptide that controls the onset of puberty and reproductive cycles. Synthetic Kisspeptin analogues have shown improvement in fecundity, fertilization, and hatching in experimental fishes such as goldfish.
The main objective in this long history has been to produce a steady and good quality seed supply, to obtain better fry survival inside hatcheries, and to remove the limitations caused by natural seasons so that aquaculture can be performed in a controlled and predictable manner.
Principle of Induced Breeding (hypophysation)
The principle of induced breeding is based on the artificial activation of the fish reproductive system by supplying the hormonal signal that is normally produced inside the body. It is the process in which the natural environmental cues like temperature, rainfall, and water current are replaced by hormone injections so that spawning can occur inside captivity. In natural condition these external factors act on the brain and stimulate the hypothalamus to secrete releasing hormones. These releasing hormones then activate the pituitary gland.
It is the pituitary gland that produces the gonadotropic hormones, mainly FSH and LH, which are responsible for the growth and maturation of gonads. In hypophysation this hormonal step is artificially supplied by injecting pituitary extract or synthetic hormones into the brood fish. The hormone injected acts in the same way as the natural gonadotropins and it is carried to the gonads through the blood. Here it accelerates the final maturation of ova and sperm.
The matured gonads now respond by releasing the gametes. In females the fully ripened eggs are liberated either by stripping or by natural spawning inside a confined enclosure. In males the hormone helps in the release of milt. This is the method that completes the spawning cycle without depending on environmental conditions. The principle therefore lies in substituting the natural endocrine pathway by supplying an external dose of hormone so that controlled breeding can be achieved under hatchery conditions.
Events of natural breeding
- It is initiated by environmental factors like day length, rainfall, temperature and the flow of water which act on the brain and stimulate hormonal activity.
- These factors influence the pituitary gland, and gonadotropins are released which help in the maturation of gonads in both males and females.
- Indian major carps usually spawn in submerged river stretches during monsoon when fresh floodwater creates suitable conditions for eggs and developing fry.
- The presence of fresh current, rising water level and improved food availability (microflora and microfauna) support the spawning process.
- Spawning takes place when favourable water currents and rainwater inflow create an appropriate environment for releasing eggs.
- Static water bodies like ponds often fail to provide these conditions and therefore major carps do not breed naturally in confinement.
- Any change in natural factors such as temperature fluctuation or poor water quality can disturb hormonal secretion and block the breeding cycle.
- The pituitary gland secretes hormones like FSH which regulate maturity of reproductive organs and initiate spawning when all cues are properly aligned.
- In wild conditions these cues are naturally present, but in captivity they are absent, so induced breeding is required to supply the hormonal signals artificially.
Mechanism of induced breeding
The mechanism of induced breeding is completed in several steps. These steps are arranged in a sequence because spawning depends on proper preparation of breeders and hormone administration.
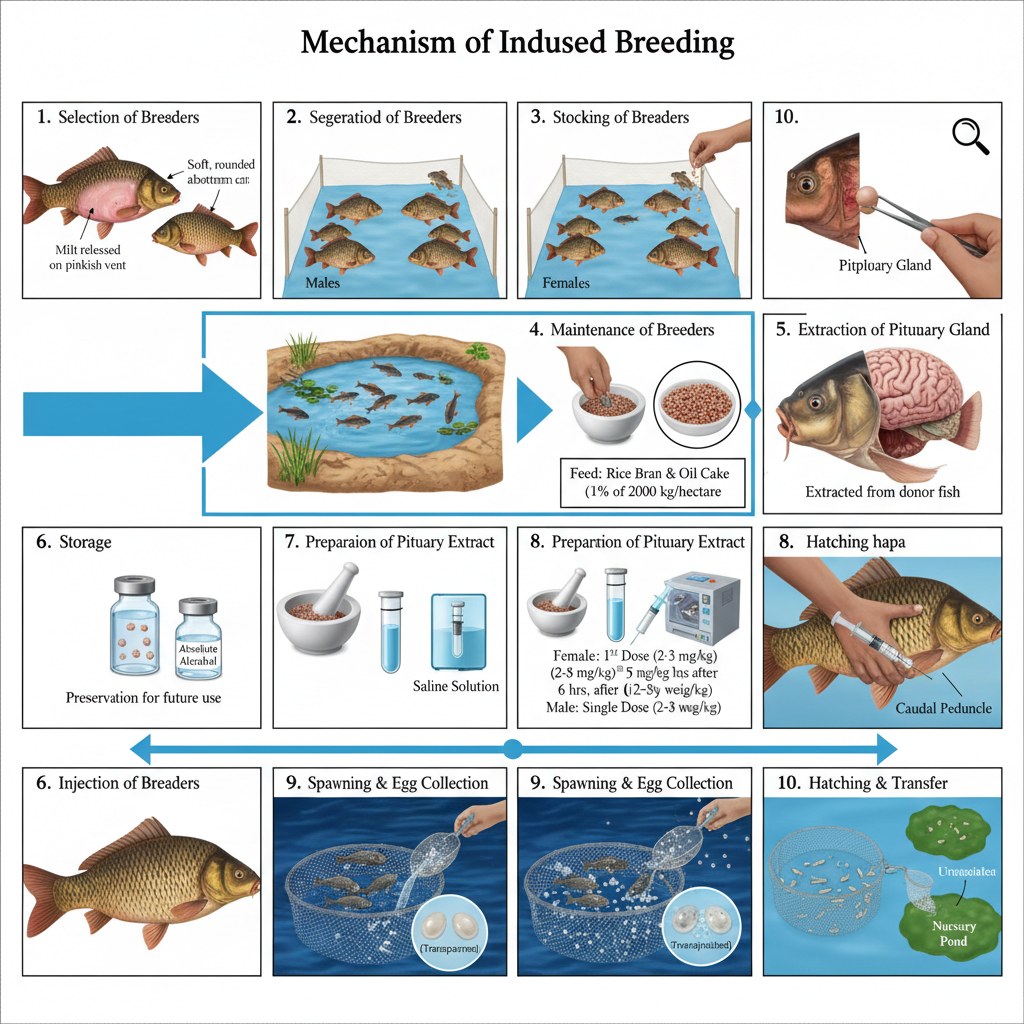
1. Selection of Breeders
It is the first step in which healthy and active breeders are selected. The fishes are collected before the breeding season and kept in fertilization ponds.
Ripe males show rough pectoral fins and milt comes out when slight pressure is given. Ripe females have soft rounded abdomen and pinkish swollen vent. The success of the process is dependent on proper selection.
2. Segregation of Breeders
In this step, males and females are separated. It is done to make sure that ovulation and milt release occur at the same time. Fishes are observed for any injury and treated with dilute KMnO₄ solution. Suitable water conditions are maintained in this phase for keeping the breeders active.
3. Stocking of Breeders
The breeders are then stocked in bundhs or ponds. These ponds are made to resemble natural spawning grounds. It is stocked at the density of 1000–2000 fishes per hectare. The fishes may also be collected from natural water bodies during the season.
4. Maintenance of Breeders
The fishes are fed with rice bran and oil cake mixture (about 1% of body weight). They are kept under regular observation for maturity. Males and females are kept separately so that controlled breeding can be performed when hormones are applied.
5. Extraction of Pituitary Gland
The pituitary gland is the source of the spawning hormone. It is taken from donor fish either through the foramen magnum or by cutting the skull. The first method is generally used. Care is taken so that the gland is not damaged during removal.
6. Storage of Pituitary Gland
After extraction, the glands are preserved. These are kept in absolute alcohol, acetone, or frozen condition. These chemicals help in dehydration of the gland and maintain hormone activity for future use.
7. Preparation of Pituitary Extract
The preserved glands are ground with small volume of distilled water or saline. The mixture is centrifuged and the supernatant is taken as the hormone extract. The dosage depends on the size and maturity of the receiving fish.
8. Injection of Pituitary Extract
The extract is injected into the caudal peduncle region.
Females are injected twice. The first dose is about 2–3 mg/kg and second dose is about 5–8 mg/kg given after six hours. Males receive single dose of 2–3 mg/kg. After injection the fishes are kept in breeding hapa or cistern.
9. Spawning Observation and Egg Collection
Spawning usually occurs within six hours of the second injection. It takes place during night. Fertilized eggs are transparent and float while unfertilized ones are opaque. Eggs are collected carefully and placed in hatching hapa for incubation.
10. Hatching and Transfer of Hatchlings
After incubation period the hatchlings come out. These hatchlings are shifted to outer chamber of the hapa. The inner chamber holds the egg shells. The hatchlings are maintained carefully in order to prepare them for nursery ponds.
Procedure of induced breeding technique
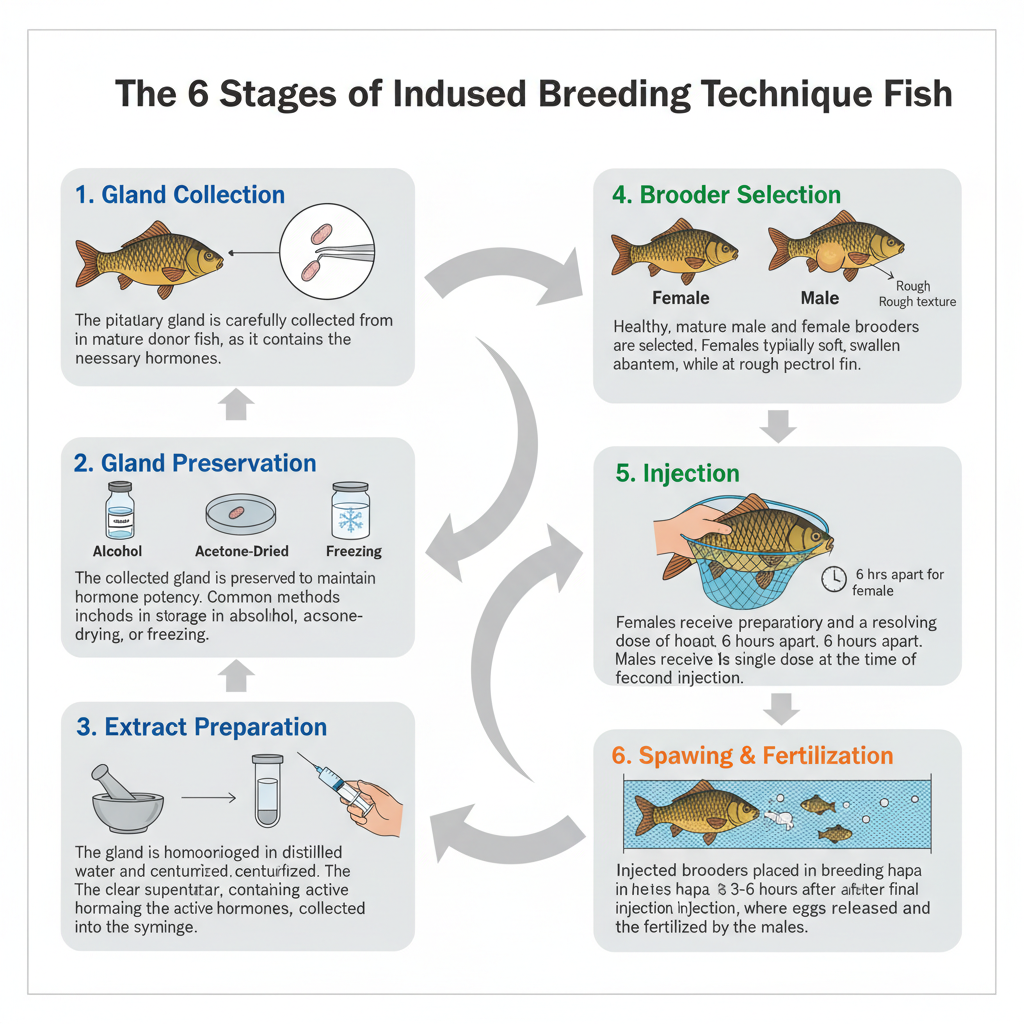
1. Collection of Pituitary Gland from Fish
The pituitary gland is the main organ used in induced breeding and it is collected from a fully mature donor fish. It is usually taken from common carp (Cyprinus carpio) because this species remains available throughout the year and gives a regular supply of mature individuals. It is collected very carefully so that the gland is not damaged. The collection is done mainly by two methods.
Collection of Glands from the Foramen Magnum
It is the process where the foramen magnum is exposed by removing the vertebral portion attached with the skull. The surrounding fat is taken out with the help of forceps and cotton pieces. Two forceps is inserted dorsally inside the foramen magnum so that the anterior brain part becomes separated. The remaining portion of brain is gently lifted upward through the foramen magnum and the pituitary gland is now exposed at the base. The gland is identified and removed carefully so that no injury occurs to the tissue.
Collection of Glands by Dissecting Heads
This method is commonly preferred because the fish head can still be used for consumption, making it more economical. The head is cut with a butcher knife and a portion of the scalp is removed. Fat which surrounds the brain is removed using cotton. The olfactory and optic nerves are now cut and the brain comes out easily from the skull cavity. In some cases the gland remains attached with the brain, and in some cases it stays on the floor of the brain cavity under a thin membrane. In both situations, the pituitary gland is taken out gently, keeping it intact and undamaged.
2. Preservation of Gland
Preservation of the pituitary gland is an important step after its collection because the gland must remain active for future use in induced breeding. It is necessary that the gland is preserved in such a way that the gonadotropins do not lose their potency. Different methods are used in different regions for preserving the gland and each method helps in maintaining the structure and activity of the tissue.
Immediate Storage in Absolute Alcohol
It is the process where the collected gland is kept immediately in sterile vials containing absolute alcohol (ethanol). This method is commonly used in India. The alcohol helps in preventing microbial contamination and stops the gland from getting degraded. The gland can be kept for several days or even a week in this condition. It also keeps the gland in stable condition for later extraction.
Acetone-Dried Preservation
In temperate regions like USSR and USA, the acetone-dried method is used widely. The gland is dehydrated in acetone which removes water completely. It is the process that prevents tissue breakdown and keeps the gland for a long period. This method is useful when the gland needs to be stored for extended duration.
Freezing Method
Freezing is another method where the glands are kept under low temperature. Freezing stops enzymatic activities and thus maintains the potency of the gland. It is especially used when the gland is not required for immediate extraction. It can preserve the gland for much longer time compared to simple alcohol storage.
Glycerine Preservation
In some cases glycerine is used for preserving the gland. Glycerine provides protection to the gland by preventing drying and maintaining the structural form. It creates a suitable medium where the gland does not lose moisture.
3. Preparation of Hormonal Extract from Pituitary Gland
Preparation of hormonal extract is an important step in induced breeding because the extract contains the active gonadotropic hormones needed for spawning. The extract must be prepared carefully so that the hormones remain effective. The procedure is simple but each step needs proper handling.
Measurement of Pituitary Gland
It is the process where the required amount of pituitary gland is calculated according to the body weight of the brooder fish. This measurement is important because the correct dosage depends on total body weight. The amount is adjusted so that the hormone concentration remains suitable for inducing spawning.
Drying of Pituitary Gland
After collection, the gland is dried on blotting paper. It removes extra moisture from the tissue. Drying ensures that the gland does not lose its potency and helps in making a proper homogenized extract. It is done gently so that the gland remains intact.
Homogenization of the Gland
The dried gland is placed inside a tissue homogenizer. A small amount of distilled water is added and the gland is homogenized into fine suspension. The extract is prepared in such a way that its dilution remains controlled. Usually it is adjusted so that 0.2 ml extract is given per kg body weight of fish. It is the step where the active hormone becomes released into the solution.
Centrifugation
The homogenized mixture is centrifuged to separate the solid tissues from the liquid portion. After centrifugation, the clear supernatant contains the active hormones. This supernatant is used for injection in brooders. The solid residue is discarded because it does not contain useful material. Only the purified extract is used for inducing spawning.
4. Brooders Selection
Brooder selection is an important step in induced breeding because the success of spawning depends on using fully mature and healthy brooders. It is necessary that the brooders are in proper condition so that the hormonal extract can induce ovulation or spermiation effectively. Selection is done by observing maturity, health, weight, and external features.
Maturity and Health
It is essential that the brooders are fully mature and free from diseases. Immature or weak fishes do not respond properly to hormonal treatment. Usually fishes of 2 to 4 years are taken because this age group shows better reproductive activity. The brooders should have active movement and no sign of injury on the body.
Body Weight
The brooders selected generally weigh between 1 to 5 kg. This weight range indicates good health and also ensures that the fish can provide sufficient quantity of eggs or milt. Heavier brooders are usually preferred when available.
Identification of Male and Female Brooders
Proper identification of sex is required for successful pairing. The characteristics of male and female fishes can be observed externally.
Male Characteristics
- The inner surface of pectoral fin becomes rough.
- Abdomen is narrow and firm.
- The vent region looks whitish and recessed.
- When slight pressure is given on abdomen, milt comes out indicating maturity.
Female Characteristics
- The inner side of pectoral fin is smooth.
- Abdomen is soft, bulging and larger than male.
- The vent is pinkish and protruding outward.
- On pressing the abdomen gently, eggs are released which shows that the female is ripe for spawning.
5. Injection to the Brooders
Injection of hormonal extract is an important step in induced breeding because the hormones help in inducing ovulation in females and spermiation in males. The success of spawning depends on correct dosage, proper timing, and careful handling during injection. The extract used is mainly prepared from pituitary gland of donor fish.
Homoplastic Injection
It is the process where pituitary extract of one species is used for another species within the same group. For example, extract collected from one carp species can be injected to another carp species. It shows good response because both species are closely related.
Heteroplastic Injection
In this method the extract is taken from a distantly related species. Sometimes catfish pituitary is used for carps or the reverse. It is used when same-group donors are not available, but the response may vary according to species.
Process of Injection in Fishes
- Correct Dosage Determination– The dosage is calculated according to size, weight, and maturity of brooders. The maturity of donor fish also affects the hormone strength. It is important because wrong dosage may not induce proper spawning.
- Injection Methods– The extract is given by hypodermic syringe. It may be intramuscular or intraperitoneal. Intramuscular injection is mostly preferred because it is easier and safer. Intraperitoneal injection requires more care because there is chance of injury to internal organs.
- Dosage and Timing– Female brooders receive two injections. The first dose is 2–3 mg/kg body weight which prepares the fish for final response. After around 6 hours a second dose is given with 5–8 mg/kg which induces ovulation. Male brooders do not get the first dose. They receive only one dose of 2–3 mg/kg at the time when females receive the second dose. The actual dosage may differ according to age, sex, maturity and environmental factors.
- Injection Sites– For intramuscular injection the fish is placed sideways in a hand net and the needle is inserted at the caudal peduncle or shoulder region. For intraperitoneal injection the needle is inserted near the base of pectoral fins but this method needs more experience because the heart and other organs can be injured. So intramuscular injection is generally used.
6. Spawning
Spawning is an important phase in induced breeding and it takes place after the brooders receive the hormonal injections. It is the process where the male and female fishes release eggs and milt inside a prepared breeding hapa. The success of induced breeding depends greatly on proper spawning activity and fertilization of eggs.
Breeding Setup
Brooders are released into the breeding hapa immediately after the hormone injection. Usually one female with two males is kept together to increase the chances of fertilization. The hapa is made of fine mesh cloth like marking cloth or mosquito netting. The fine mesh allows water circulation but prevents escape of eggs and milt. It ensures that fertilization occurs inside the hapa.
Dimensions and Configuration
A breeding hapa commonly measures around 3 m × 1.5 m × 1 m which is suitable for fishes weighing around 3–5 kg. The upper part of the hapa should remain about 20 cm above water level so that fish seedlings do not suffocate. The sides remain enclosed while the top may be kept open or closed depending on the condition.
Behavioral Changes
Within 2–3 hours after the second injection the fishes become more active. Both male and female brooders show excitement. The males start chasing the female and press their snout against the female body. This pressing behaviour is an indication that spawning is about to occur.
Timing of Spawning
Spawning generally occurs 3–6 hours after giving the second dose. When injections are given in the evening, the spawning usually takes place around midnight. Cooler and cloudy weather helps in getting better results because the fishes show more natural activity under such conditions.
Fertilization Process
During spawning the female releases eggs and the males release milt which fertilizes the eggs inside the hapa. Fertilized eggs are transparent, crystalline and look like pearls. Slight water movement helps them float upward. Unfertilized eggs appear whitish and opaque. After spawning, the fertilized eggs are collected and transferred to hatching hapas for incubation.
Factors Affecting Spawning Success
The success of spawning depends on the quality of hormonal extract, correct dosage, maturity of brooders and proper environmental condition. The type of inducing agent used also affects the spawning response. Careful handling and proper technique are important for achieving good fertilization rate.
Role of other natural and synthetic hormones in induced breeding
The use of natural and synthetic hormones is important in induced breeding because these substances help in regulating the reproductive activity of fishes. It is the process where the hormones assist in stimulating ovulation and spermiation when natural conditions are not sufficient. These hormones also allow controlled breeding in different aquaculture systems.
1. Gonadotropin Releasing Hormone (GnRH) Analogues
GnRH analogues are used widely in induced breeding. It is used along with dopamine antagonists so that the reproductive inhibition is reduced. These hormones help in releasing gonadotropins which support the spawning response. Many carp species show good reaction under these hormones.
2. Synthetic Hormones
Due to difficulties in collecting and preserving natural pituitary glands, several synthetic hormones has been developed. These hormones remain stable for long time and are available in purified forms. It is easy to use them in hatcheries.
3. LHRH-a (Luteinizing Hormone Releasing Hormone Agonist)
LHRH-a is used with domperidone. It is effective in inducing oocyte maturation as well as ovulation in many fishes such as bighead carp. It is the process where the hormone directly promotes reproductive functions and gives better response.
4. Human Chorionic Gonadotropin (HCG)
HCG is used for inducing ovulation in the brooders. It is cheap and available in purified form. The hormone is more stable and can be stored for longer duration. In Labeo rohita, HCG works at around 600 IU/kg and in silver carp it works at 630–660 IU/kg. Many species show successful spawning with HCG.
5. WOVA-FH
WOVA-FH is a synthetic GnRH analogue (SGnRH). It is used for Indian major carps, exotic carps and catfishes. It is used in hatcheries because the response is stable and breeding results remain satisfactory.
6. Ovatide
Ovatide is cheap and easy to use. It is used mainly for major carps. The dose for Rohu and Mrigal is around 0.20–0.40 ml/kg and for Catla it is around 0.20–0.30 ml/kg. Fertilization and hatching rate remain around 85–95%. It is effective under hatchery conditions and widely used.
7. Synahorin
Synahorin contains chorionic gonadotropin mixed with mammalian pituitary extract. It works better when combined with pituitary extract. It has not shown good results when used alone, especially in Rohu.
8. Ovaprim
Ovaprim is a modern GnRH analogue and is widely used as substitute of pituitary extract. It increases egg production and in Rohu eggs increase from 1.15 lakh to around 1.41 lakh. Fertilization and hatching rate remain higher. Eggs become larger after water hardening which shows better development. Spawning time remains similar to pituitary injection. Hatchlings appear healthier though more confirmation is needed.
The recommended doses are:
– Catla: 0.40–0.50 ml/kg
– Labeo rohita: 0.30–0.40 ml/kg
– Mrigal: 0.25–0.30 ml/kg
– Silver carp and grass carp: 0.50–0.70 ml/kg
Male fishes generally receive 0.10–0.20 ml/kg.
Brooder mortality after spawning is very low because handling is less.
Factors influencing induced breeding
1. Favourable Climatic and Hydrological Conditions– It is the most important requirement for induced breeding because the spawning activity depends on suitable environmental conditions. It is seen that appropriate temperature, light and water quality help in successful breeding. When the breeders are not selected properly or there is wrong dosage of pituitary extracts then breeding failure occurs. Extreme heat, very high salinity and too much sunlight usually disturb the breeding activity.
2. Light– Light has a direct influence on the reproductive cycle of fishes. It is the photoperiod that affects the maturation and spawning. Some species show early maturity during shorter light periods and some species show delayed maturation when the exposure of light is longer. In India, Cirrhinus reba shows early maturity in daylight. This is referred to as the effect of light on the reproductive behaviour of the fishes.
3. Temperature– Temperature is one of the major factors because it is the process that controls sexual maturation. Every species has its own optimum temperature range. When the temperature goes above or below this range, successful breeding does not occur. Indian major carps generally breed between 24°C to 37°C and the optimum temperature is around 27°C. It is seen that breeding becomes less successful above 30°C. Higher temperature helps in gonadal ripening and stimulates spawning, while lower temperature after injection gives favourable condition for fertilization and embryonic development.
4. Dissolved Oxygen (DO₂)– High dissolved oxygen is required during induced breeding. It is the process which helps in hatching and most fishes need more oxygen for these activities. Water with low oxygen generally suppress breeding behaviour. So water renewal and aeration is needed for stimulating spawning.
5. Water Current and Rain– The rheotactic response is the tendency of fishes to swim along the direction of water current. This response is important in spawning. Rain is one of the major sources that stimulates spawning in major carps. During monsoon, the rainfall increases which increases the water current and this condition helps in maturation and gonadal activity. Even after hormonal injection, rainwater is seen to enhance the spawning process.
6. Cloudy Weather– Cloudy and rainy days are favourable for spawning. After heavy rainfall the fishes show more activity and the cooler temperature with less light intensity creates a suitable environment for breeding.
7. pH Levels– The pH of water is also an important factor in induced breeding. It is observed that carps breed successfully in alkaline pH. Therefore maintaining proper pH is important for better reproductive performance.
Examples of natural and synthetic hormones use in induced breeding of fishes
Natural Hormones
1. Pituitary Extracts– It is the most commonly used natural hormone in induced breeding. The pituitary gland extract contains gonadotropins which help in the final maturation of gametes. It is prepared from mature carp pituitary and used in different doses depending on the size and maturity of the breeders. It is observed that the response is better when the breeders are in fully ripe condition.
2. Human Chorionic Gonadotropin (HCG)– HCG is a natural glycoprotein hormone that is obtained from urine during pregnancy. It is used to induce gamete maturation. When HCG is injected to mature fishes, it promotes ovulation and spermiation. It is the process that becomes more effective when the dose of HCG is given together with pituitary extracts rather than alone.
Synthetic Hormones
1. Sumaach and Synahorin– These are prepared by INFAR (India) Ltd. and are considered cost-effective substitutes for pituitary extracts. The preparation is made by grinding the material with distilled water and centrifuging to obtain the supernatant. It is used in two injections where the first dose is given to the female and the second is given to both male and female. The dose depends on the body weight and the best results occur when breeders are fully ripe and favourable conditions are present. Synahorin consists of chorionic gonadotropin and mammalian hypophysial extract.
2. Ovaprim (Salmon Gonadotropin RH)– Ovaprim is a mixture of salmon gonadotropin releasing hormone (RH) and a dopamine antagonist. It is stabilised in glycerin and alcohol. It is known that dopamine inhibits gonadotropin release from the pituitary. This preparation removes the inhibitory effect and helps in gamete release. The recommended dose is 0.3–0.5 mg/kg for females and 0.01–0.3 mg/kg for males. It is used widely in India and gives high egg production and is stable in tropical conditions.
3. Pimozide and LHRH-A– Pimozide acts as a dopamine antagonist and increases the effect of LHRH-A. It is found to be effective for Indian major carps. LHRH and its analogues are useful for brackish water fishes but they are short-lived and new long-lasting preparations are needed.
4. DOCA (II-Desoxycorticosterone-acetate)-This synthetic hormone is used mainly in catfishes like Clarias and Heteropneustes. It is the process that works for both maturation and ovulation which makes it different from other synthetic hormones.
5. Antiestrogen Tamoxifen– Tamoxifen is used in coho salmon. It is more effective when given with a primer such as pituitary extract. This shows that a variety of compounds can be used to improve the induced breeding performance in fishes.
These natural and synthetic hormones are important because they help in controlling the breeding activity and give successful results when the environmental conditions and breeder quality are suitable.
Substitutes of fish pituitary gland
Human Chorionic Gonadotropin (HCG)– HCG is one of the important substitutes used in place of fish pituitary. It is obtained from human urine during pregnancy. It is seen that HCG can induce spawning in fishes like silver carp when it is used alone. The response becomes better when HCG is given with carp pituitary extract because both hormones act together and help in improving the spawning activity. It is the process that gives more reliable results when the breeders are in proper maturity stage.
Synahorin– Synahorin is another substitute used in induced breeding. It contains chorionic gonadotropin with mammalian hypophysial extract. It is observed that when Synahorin is combined with carp pituitary, it induces breeding in rohu and silver carp. But when Synahorin is used alone, the response in rohu is not satisfactory. This shows that its effectiveness depends on the species and also on the hormonal combination used during the process.
Ovaprim– Ovaprim is a modern hormone preparation used widely as a substitute for fish pituitary extract. It contains salmon gonadotropin releasing hormone with a dopamine antagonist. It is more costly but gives very good results in induced breeding of different carp species. It is the preparation that works more effectively than carp pituitary extract because it stimulates the reproductive activity strongly and shows high spawning success. This is why Ovaprim is used commonly in hatcheries where fast and reliable breeding is needed.
Problems of hypophysation technique
- Measurement of Potency– It is difficult for farmers to measure the exact potency of the pituitary glands that are available to them. It is seen that there is no proper standardization, so the correct dosage cannot be decided easily. This results in irregular spawning response because the dose may become either too low or too high for the breeders.
- Collection and Storage Challenges– The collection of pituitary glands on a large scale is a major problem. It is the process that needs careful handling and proper storage to maintain gland viability. Many farms cannot collect or store enough pituitary material, which limits the breeding operations, especially during the peak season.
- Supply and Demand Gap– There is a wide gap between the supply and demand of pituitary glands. The requirement is high but the availability is low. Because of this, farmers sometimes cannot obtain the required amount and they are forced to use substitutes which may not give equal results.
- Lack of Basic Equipment– Many hatcheries do not have the necessary laboratory equipment like chemical balances, centrifuges and refrigerators. These are important for processing the pituitary extract properly. When these instruments are not available, the hypophysation technique becomes less effective and the breeding success becomes low.
- High Market Costs– Pituitary glands are costly in the market. The high price makes it difficult for small-scale farmers to use this method. It is observed that the high cost reduces the use of hypophysation in many places even though the technique is effective.
Advantages of induced breeding
- It helps in selecting the targeted species for spawning, so unwanted species are not allowed to multiply in the culture ponds.
- It gives a continuous and regular supply of spawn on demand, which is not possible under natural conditions.
- It is the process that allows seasonal and flexible production of seedlings according to market requirements.
- It reduces the holding period because male and female spawners do not need to be kept for long days waiting for natural breeding.
- The technique is simple and can be performed easily by farmers with basic training.
- It is generally cost-effective because the expenses of collecting natural spawn are avoided.
- It gives better control over the reproductive cycle, so the spawning activity can be done at the right time for maximum production.
Precautions for Induce Breeding
- The breeders must be kept free from diseases and parasites, so the environment should be checked regularly for water quality and fish health.
- Before induction, the breeders are cleaned in potassium permanganate (KMnO₄) solution for few minutes. It is the step that removes surface pathogens.
- The fishes should be handled carefully to avoid mechanical injury because any injury creates stress and reduces the spawning response.
- Water temperature should be maintained between 24°C to 31°C, as this range helps in proper maturation and spawning.
- Water turbidity is kept within 100 to 1000 ppm because this condition supports normal breeding activity.
- Flowing water with high dissolved oxygen is needed to provide a suitable environment for gamete release and embryo development.
- Light intensity and duration must be regulated because light has an important role in the spawning behaviour of fishes.
- Pituitary glands used for injection should be collected from the same species or from a closely related species since these glands show better effectiveness in induced breeding.
Why does fish not breed in captivity?
Environmental Influence– It is seen that many fishes depend on natural environmental cues for starting the breeding process. Rainfall, temperature change and water current stimulate the reproductive activity in natural habitat. These conditions are not present properly in captivity, so the hormonal activity needed for spawning does not start.
Hormonal Regulation– The release of hormones from the pituitary gland and gonads is important for reproduction. In natural water, the environmental changes stimulate these hormones. In captivity this stimulation becomes weak, so the fish do not get the required level of hormones for breeding.
Impact of Poor Nutrition– The maturation of ovaries depends on good nutrition. In captive conditions the fishes do not always get proper natural food. This reduces the growth of reproductive organs and the breeding potential becomes low.
Exposure to Contaminants– In culture ponds some chemicals and biocides may enter the water. These substances affect the gonads and create hormonal imbalance. It is the process that stops proper maturation, so the fish fail to breed.
Stress Factors– Captive environment gives different types of stress like limited space, handling and disturbance. Stress affects the physiological activities related to reproduction. Because of this stress the fishes do not show normal spawning behaviour.
Lack of Natural Stimuli– In nature the fishes get seasonal signals and suitable spawning grounds. These natural cues help them to initiate breeding. In captivity these factors are absent, so the fishes cannot respond and do not start spawning naturally.
Why induced breeding is necessary for fish culture?
- It helps in obtaining pure spawn of a selected species, so the unwanted wild species are not mixed with the cultured stock.
- It gives a timely and regular supply of pure seeds, whereas natural sources do not provide seeds at a fixed time.
- It can produce any required quantity of seed depending on the market demand, so the production can be increased or decreased easily.
- The technique is simple and can be learned by farmers without much technical training.
- It is cost-effective because the expenses of collecting spawn from natural waters are avoided.
- It allows control of temperature, water quality and other factors which help in improving the spawning success.
- It is the process that supports the development of desirable traits like fast growth, better disease resistance and good adaptation to culture conditions.
Why induced breeding? – Significance of induced breeding
- It helps to overcome the natural environmental limitations because factors like rainfall, photoperiod, temperature and water current are not present properly in captivity.
- It provides hormonal stimulation when the natural release of hormones becomes weak in captive condition. The injections help in proper maturation and gamete release.
- It supports better nutrition because in captivity the fishes do not always get natural food, and this affects gonadal maturation. Induced breeding improves the reproductive performance under these conditions.
- It allows controlled breeding cycles, so the spawning of the whole stock can be synchronized. This helps in planned hatchery management and steady seed production.
- It increases the production efficiency by giving a regular supply of good quality juveniles, which helps in meeting the market demand.
- It helps in broodstock management because selective breeding can be done for desirable traits like fast growth and disease resistance.
- It reduces pressure on wild fish populations since the seed is produced in hatcheries. This supports sustainable aquaculture and protects natural resources.
- Paul, Monjit. (2014). Induced Breeding.
- Gupta, Sweety & Kumar, Ajay & Bhojyawal, Vishal & Agrahari, Karishma & Chand, Prem. (2024). INDUCED BREEDING IN FISHES: AN OVERVIEW. 10.58532/V3BCAG18P1CH2.
- https://stjohnscollege.edu.in/web/wp-content/uploads/E-Resources/Zoology/INDUCED%20BREEDING%20.pdf
- https://adpcollege.ac.in/online/attendence/classnotes/files/1626673880.pdf
- https://www.shcollege.ac.in/wp-content/uploads/NAAC_Documents_IV_Cycle/Criterion-II/2.3.2/ppt/Ms_LeenaRaphael_Inducedbreeding.pdf
- https://www.ijbio.com/articles/a-review-on-induced-breeding-in-fishes.pdf
- https://www.btkisanwbuafs.org/assets/web/upload/presentation/Anindya-NAyak.pdf
- https://www.slideshare.net/slideshow/induced-breeding-in-fishes/81112027
- http://www.cifri.res.in/aqua/5.pdf
- https://lkouniv.ac.in/site/writereaddata/siteContent/202003251324430605shelly_Role_of_endocrine_glands.pdf
- https://www.scribd.com/document/242622885/Induced-Breeding
- https://www.slideshare.net/slideshow/induced-breeding-in-fishes-240862668/240862668
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.