What is Immunoglobulin G (IgG Antibody)?
- Immunoglobulin G (IgG) is the most common type of antibody found in the human body. It represents approximately 75% of the total antibodies present in the serum, which is the clear part of the blood. IgG antibodies are produced and released by plasma B cells, a type of white blood cell.
- Antibodies, also known as immunoglobulins, are glycoproteins that play a crucial role in the immune system’s defense against foreign substances and microorganisms. They have a Y-shaped structure, with two arms called paratopes that can bind to specific antigens, which are molecules present on the surface of pathogens.
- In humans, there are five different classes of antibodies: IgG, IgA, IgM, IgD, and IgE. Among these, IgG is the most abundant and versatile antibody class. It is found not only in the blood circulation but also in all other body fluids, including saliva, tears, and breast milk.
- One of the primary functions of IgG antibodies is to protect against bacterial and viral infections. They can neutralize pathogens by binding to their antigens and preventing them from entering or damaging the body’s cells. IgG antibodies can also activate other immune cells, such as natural killer cells and macrophages, to destroy the pathogens directly through processes like Antibody-Dependent Cellular Cytotoxicity (ADCC) and Antibody-Dependent Cellular Phagocytosis (ADCP).
- Additionally, IgG antibodies play a crucial role in the immune system’s complement system, which is a group of proteins that work together to eliminate pathogens. IgG antibodies can trigger a process called Complement-Mediated Cytotoxicity (CMC), where the complement proteins are activated to form a membrane attack complex that punctures and kills the targeted cells.
- The concentration of IgG in the serum is typically around 10 to 16 mg/mL, making it the major immunoglobulin in the extracellular spaces. This high concentration reflects the important role of IgG in providing long-term immunity against infections. IgG antibodies can persist in the bloodstream for an extended period, providing a memory response to previously encountered pathogens. This memory response allows the immune system to mount a faster and more effective defense if the same pathogen is encountered again in the future.
- In summary, Immunoglobulin G (IgG) is the most common antibody found in the human body. It plays a critical role in protecting against bacterial and viral infections by neutralizing pathogens, activating immune cells, and participating in the complement system. With its high concentration and ability to provide long-term immunity, IgG is a vital component of the immune system’s defense mechanisms.
Definition of Immunoglobulin G (IgG Antibody)
Immunoglobulin G (IgG) is the most common type of antibody in the human body, representing approximately 75% of serum antibodies. It plays a crucial role in protecting against bacterial and viral infections by binding to specific antigens and activating immune responses.
Structure of Immunoglobulin G (IgG Antibody)
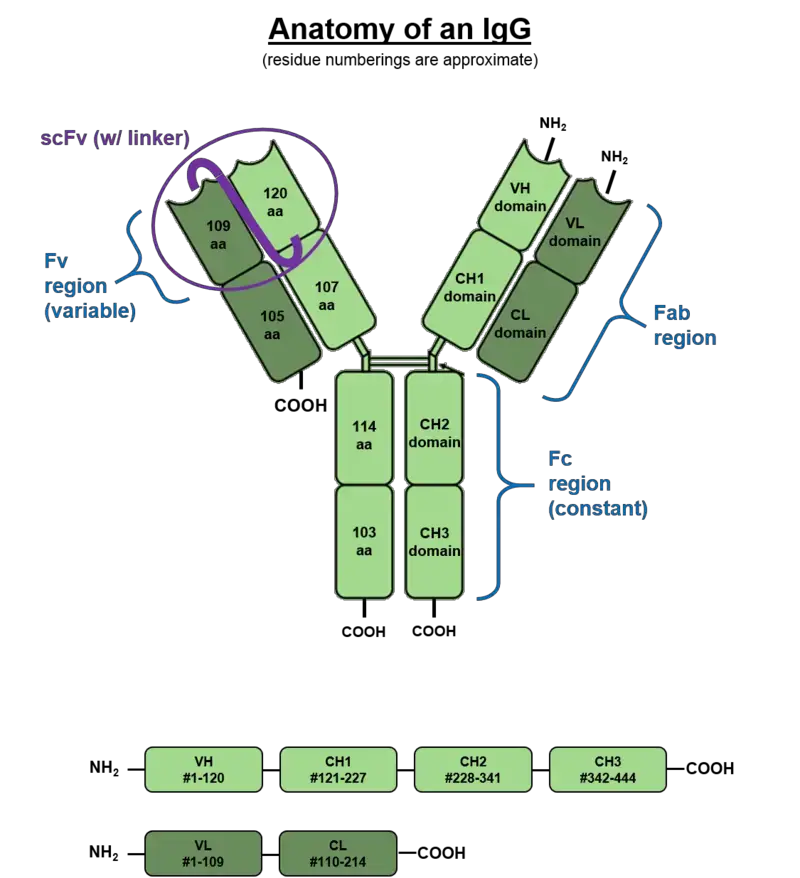
- Immunoglobulin G (IgG) antibodies have a specific structure that contributes to their function in the immune system. IgG antibodies are large monomeric molecules weighing approximately 150 kDa and have a tetrameric quaternary structure.
- The basic monomeric structure of an IgG antibody consists of two identical Heavy (H) chains and two identical Light (L) chains. The Heavy chains, which are about 50 kDa in weight, are of the gamma (𝞬) type, while the Light chains are approximately 25 kDa. Disulfide bonds link the two Heavy chains to each other and to a Light chain, forming a Y-like shape.
- Each fork of the Y-shaped IgG antibody contains an identical antigen binding site, allowing for the binding of specific antigens. This means that each IgG antibody has two antigen binding sites.
- The Heavy chains of the IgG antibody consist of several components, including CH1, CH2, CH3, hinge, and the VH (variable region of the Heavy chain). The Light chains consist of the CL (Constant region of the Light chain) and either the κ or λ chains.
- One notable feature of IgG antibodies is the presence of a highly conserved N-glycosylation site in the Fc (crystallizable fragment) region. This site allows for the attachment of carbohydrate molecules to the Fc region.
- In summary, IgG antibodies have a tetrameric quaternary structure and are composed of two identical Heavy chains and two identical Light chains. They form a Y-like shape with two antigen binding sites. The Heavy chains have different components, and the Fc region of IgG antibodies contains a conserved N-glycosylation site. This unique structure enables IgG antibodies to bind to specific antigens and carry out their immune functions.
Subclasses of Immunoglobulin G (IgG Antibody)
Immunoglobulin G (IgG) antibodies have four subclasses in humans: IgG1, IgG2, IgG3, and IgG4. The size of the hinge area, the position of interchain disulfide links, and the molecular weight distinguish these subclasses. Each subclass has distinct properties and functions in the immune system.
- IgG1 is the most abundant subclass, comprising approximately 60 to 65% of the total IgG antibodies. It is primarily involved in the immune response against protein and polypeptide antigens. IgG1 antibodies play a crucial role in thymus-mediated immune responses, opsonization (marking pathogens for destruction), and activation of the complement cascade. Deficiency in IgG1 isotype is often associated with hypogammaglobulinemia, a condition characterized by low levels of immunoglobulins.
- IgG2 is the second most common subclass, representing around 20 to 25% of the main IgG subclass. It is particularly important in the immune response against carbohydrate and polysaccharide antigens. Deficiency in IgG2 is the most frequently observed among all IgG isotype deficiencies and is often linked to recurrent airway and respiratory infections, especially in infants.
- IgG3 makes up approximately 5 to 10% of total IgG. It plays a significant role in immune responses against protein and polypeptide antigens. IgG3 antibodies are known for their ability to efficiently activate complement, which is a group of proteins involved in pathogen destruction. IgG3 is associated with strong inflammatory responses and is generally short-lived compared to other IgG subclasses.
- IgG4 is the least abundant subclass, usually constituting less than 4% of total IgG antibodies. Unlike the other subclasses, IgG4 does not bind to polysaccharides. Elevated levels of IgG4 in the bloodstream have been observed in patients with certain conditions such as sclerosing pancreatitis, cholangitis, and interstitial pneumonia caused by infiltrating IgG4 positive plasma cells. However, the precise role of IgG4 in the immune system is still not fully understood.
| Name | Percentage | Affinity to Fc receptor on phagocytic cells | Crosses placenta easily | Half life (days) |
| IgG1 | 66% | High affinity | yes | 21 |
| IgG2 | 23% | Extremely low affinity | no | 21 |
| IgG3 | 7% | High affinity | yes | 7 |
| IgG4 | 4% | Intermediate affinity | yes | 21 |
Clinical Significance of Immunoglobulin G (IgG Antibody)
The clinical significance of Immunoglobulin G (IgG) antibody lies in its measurement, which can provide valuable information about a person’s immune status and help diagnose certain conditions. Here are some key points:
- Immunity status: IgG antibody levels can be measured to assess an individual’s immune status against specific pathogens. For example, the measurement of IgG titers can indicate whether a person has developed immunity to diseases such as rubella, measles, mumps, hepatitis B, and varicella (chickenpox). This information is particularly useful in determining vaccination effectiveness or assessing susceptibility to these infectious diseases.
- Autoimmune hepatitis diagnosis: In cases where autoimmune hepatitis is suspected based on certain symptoms, checking IgG antibody levels can be a diagnostic tool. Elevated levels of IgG antibodies in conjunction with other clinical findings can support the diagnosis of autoimmune hepatitis.
It’s important to note that while IgG antibody measurement is valuable in certain contexts, it is not generally indicated for diagnosing allergies or determining food intolerances. There is no evidence to suggest a relationship between IgG levels and these conditions.
Functions of Immunoglobulin G (IgG Antibody)
- Infection control: IgG is the main type of antibody found in blood and extracellular fluid. It binds to various pathogens, such as viruses, bacteria, and fungi, helping to immobilize them. This antibody-coated pathogen is then recognized and ingested by phagocytic immune cells, leading to the elimination of the pathogen. IgG also activates the classical complement pathway, releasing immune proteins that aid in the destruction of pathogens. Additionally, IgG can bind and neutralize toxins produced by pathogens.
- Transfer of immunity: IgG is the only class of immunoglobulin that can cross the placenta in humans. This property allows IgG to provide protection to the developing fetus during the first months of life, as it passes from the mother to the baby. IgG in breast milk also provides humoral immunity to the infant before their own immune system develops.
- Role in immune responses: IgG is a major immunoglobulin found in blood, lymph fluid, cerebrospinal fluid, and peritoneal fluid. It plays a key role in the humoral immune response, which involves the production of antibodies to combat pathogens. IgG is involved in opsonization, where the Fc portion of the antibody binds to a receptor on phagocytic immune cells, facilitating the recognition and ingestion of antibody-coated pathogens.
- Longevity and passive immunization: IgG antibodies have a long lifespan in the body, providing prolonged protection against infections. This longevity makes IgG useful for passive immunization, where antibodies are transferred from one individual to another to confer immediate immunity. Detection of IgG in the bloodstream often indicates a prior infection or vaccination.
- Diagnostic and research applications: Due to its abundance and high specificity toward antigens, IgG is the primary antibody used in immunological research and clinical diagnostics. It is used in techniques such as ELISA (enzyme-linked immunosorbent assay) and Western blotting to detect the presence of specific antigens or antibodies in biological samples.
FAQ
What is Immunoglobulin G (IgG) antibody?
IgG is a type of antibody, representing the most common class of antibodies found in the bloodstream. It plays a crucial role in immune responses against infections.
What is the structure of IgG antibody?
IgG is a large, monomeric molecule composed of two identical heavy chains and two identical light chains. It has a Y-shaped structure with two antigen binding sites.
What are the subclasses of IgG antibody?
There are four subclasses of IgG: IgG1, IgG2, IgG3, and IgG4. They differ in their abundance, size of the hinge region, position of interchain disulfide bonds, and molecular weight.
What are the functions of IgG antibody?
IgG plays a vital role in controlling infections by binding to pathogens, promoting their elimination through phagocytosis and activation of the complement system. It also neutralizes toxins and participates in antibody-dependent cellular cytotoxicity.
Can IgG cross the placenta?
Yes, IgG is the only class of immunoglobulin that can cross the placenta from the mother to the fetus, providing passive immunity and protection to the newborn.
How is IgG antibody measured?
IgG antibody levels can be measured through blood tests, typically using techniques like enzyme-linked immunosorbent assay (ELISA) or immunofluorescence.
What can IgG antibody measurement indicate?
IgG measurement can indicate a person’s immune status to certain pathogens, such as measles, mumps, rubella, hepatitis B, and varicella. It can also help diagnose conditions like autoimmune hepatitis in conjunction with other clinical findings.
What is the significance of IgG subclasses?
IgG subclasses have distinct properties and functions. IgG1 is responsible for thymus-mediated immune response, IgG2 is important against carbohydrate antigens, IgG3 plays a major role in immune responses against protein antigens, and IgG4 is associated with certain diseases and its precise role is still not fully understood.
Can IgG antibody testing diagnose allergies or food intolerances?
No, IgG antibody testing is not indicated for diagnosing allergies or determining food intolerances. There is no scientific evidence supporting a correlation between IgG levels and these conditions.
Can IgG antibody testing be used for immunological research and clinical diagnostics?
Yes, IgG antibodies are widely used in immunological research and clinical diagnostics due to their abundance, specificity, and versatility. They are often employed in various laboratory techniques, including immunoassays and antibody-based detection methods.
References
- https://www.biolegend.com/en-us/products/purified-anti-rabbit-igg-13827?GroupID=BLG3472
- https://www.thermofisher.com/us/en/home/life-science/antibodies/antibodies-learning-center/antibodies-resource-library/antibody-methods/immunoglobulin-igg-class.html
- https://www.genscript.com/IgG-antibody.html
- https://www.bio-rad-antibodies.com/igg-immunoglobulin-g-antibodies.html
- https://www.sciencedirect.com/topics/neuroscience/immunoglobulin-g
- https://en.wikipedia.org/wiki/Immunoglobulin_G
- http://mainebiotechnology.com/igg-function/