What is Hymenolepis nana?
- Hymenolepis nana, commonly referred to as the dwarf tapeworm, is a significant member of the cestode family and is particularly prevalent in temperate regions. It is often categorized as one of the most common intestinal helminths affecting humans, with a notable incidence in children. This species has several synonyms, including Rodentolepis nana, Vampirolepis nana, Hymenolepis fraterna, and Taenia nana, which reflect its diverse historical classifications.
- The life cycle of Hymenolepis nana is intricate and can occur both with and without an intermediate host. Typically, the adult tapeworm resides in the intestines of its definitive host, where it attaches to the intestinal wall using its scolex, or head. The body of the tapeworm, composed of segments known as proglottids, can grow to a length of up to 4 centimeters, with each segment containing reproductive structures that produce eggs. These eggs are expelled in the feces and can be ingested directly by a new host, initiating infection.
- Besides its capacity for direct transmission, Hymenolepis nana can also use various species of beetles as intermediate hosts, wherein the larvae develop before infecting the definitive host. This dual life cycle facilitates its widespread occurrence in various environments. Once inside the host, the eggs hatch, and the larvae penetrate the intestinal lining, maturing into adult tapeworms. This ability to thrive in both humans and rodents contributes to its high prevalence and cosmopolitan distribution.
- Clinically, infections caused by Hymenolepis nana are often asymptomatic, but some individuals may experience gastrointestinal symptoms such as abdominal pain, diarrhea, and nausea. The diagnosis typically involves identifying eggs in the stool through microscopic examination. Treatment usually entails the use of anthelmintic medications, which effectively eliminate the tapeworm from the host’s system.
History and Distribution of Hymenolepis nana
Hymenolepis nana, known as the dwarf tapeworm, has a significant historical and geographical context that sheds light on its prevalence and impact on human health. The name “Hymenolepis” originates from the Greek words for membrane and covering, referring to the thin shell surrounding its eggs, while “nana” denotes its diminutive size. The initial discovery of this species occurred in 1857 by the parasitologist Theodor Bilharz, marking the beginning of its recognition in scientific literature.
- The distribution of Hymenolepis nana is cosmopolitan, indicating its presence across diverse geographic regions; however, it is notably more prevalent in warmer climates than in colder ones.
- This tapeworm is particularly widespread in temperate areas, where it has been identified as the most common cestode infecting humans, especially in school-aged children and institutionalized populations.
- As the smallest tapeworm species found in the human intestine, Hymenolepis nana typically measures up to 4 centimeters in length, making it a significant pathogen among helminths.
- An important aspect of Hymenolepis nana is its unique life cycle, which can be completed entirely within a single host—humans—without the necessity of an intermediate host. This contrasts with most other tapeworms that require multiple hosts to complete their life cycles.
- Its life cycle includes the maturation of eggs into larvae within the human intestine, allowing for rapid transmission and increased infection rates within susceptible populations.
- Epidemiologically, Hymenolepis nana is the leading cause of cestode infections worldwide, with the highest incidence reported among children and individuals in institutional settings.
- While Hymenolepis diminuta is another related tapeworm species that can infect humans, it occurs less frequently compared to Hymenolepis nana and has been recorded in various global locations.
Habitat of Hymenolepis nana
- Natural Habitat in Humans:
- The adult H. nana resides predominantly in the proximal ileum, which is the final section of the small intestine. This location is favourable due to the nutrient-rich environment conducive to the survival and reproduction of the tapeworm.
- The proximal ileum provides ample access to digested food, facilitating the tapeworm’s absorption of nutrients through its cuticle.
- Habitat in Rodents:
- H. nana var. fraterna, a variant of the dwarf tapeworm, is typically found in rodent populations, specifically in the posterior part of the ileum.
- Rodents such as mice and rats serve as critical hosts, enabling the transmission and proliferation of this parasite.
- The posterior ileum in rodents offers a suitable habitat for larval development and attachment to the intestinal walls, promoting the worm’s growth and lifecycle completion.
- Environmental Conditions:
- The habitat of H. nana is characterized by a warm, moist environment, which is typical of the intestinal tracts of both humans and rodents.
- Optimal temperatures and pH levels in the ileum support the metabolic processes necessary for the parasite’s survival.
- Transmission Dynamics:
- Transmission typically occurs through the ingestion of eggs, which can contaminate food and water supplies. This process highlights the importance of hygiene in preventing the spread of H. nana.
- In rodent populations, fecal contamination plays a significant role in the life cycle, as eggs shed in the feces can infect other hosts.
- Public Health Implications:
- Given its prevalence in both human and rodent hosts, monitoring the habitats of H. nana is essential for disease control and prevention strategies.
- Infestations in humans can lead to a range of gastrointestinal symptoms, necessitating awareness of its habitat and life cycle.
- Research and Monitoring:
- Ongoing research into the habitats of H. nana and H. nana var. fraterna is vital for understanding their ecological roles and impacts on public health.
- Surveillance of rodent populations and sanitation measures in areas prone to human infestation can mitigate the risks associated with these parasites.
Morphology of Hymenolepis nana
The morphology of Hymenolepis nana, the smallest intestinal cestode infecting humans, is a key aspect of its biology and life cycle. A thorough understanding of its structure aids in identifying the parasite and recognizing its potential impact on human health.
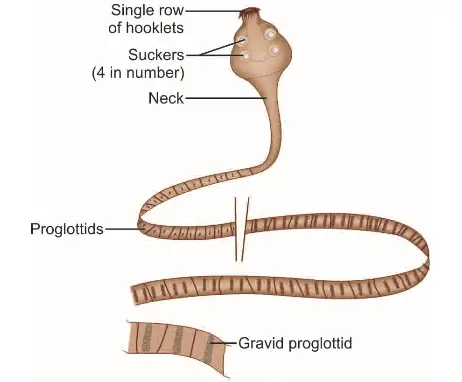
- Adult Worm Characteristics:
- Size: H. nana measures between 5 mm and 45 mm in length, with a thickness of less than 1 mm. This diminutive size allows for its survival and proliferation within the confined environment of the human intestine.
- Scolex Structure: The scolex, or the head of the worm, is equipped with four prominent suckers, facilitating attachment to the intestinal wall. Additionally, it features a retractile rostellum, which is adorned with a single row of hooklets. This adaptation enhances the worm’s ability to anchor itself securely within the host’s gut.
- Neck and Strobila: Following the scolex, a long, slender neck connects to the strobila, which consists of approximately 200 or more proglottids. These segments are significantly broader than they are long, a characteristic that distinguishes H. nana from other cestodes.
- Genital Pores: The proglottids exhibit a unique arrangement, with genital pores located along the same side of the segments’ margins. This anatomical feature is essential for reproductive functions, facilitating the release of eggs.
- Reproductive Structures: The uterus within the adult worm has lobulated walls, which maximizes the surface area for egg production. In addition, there are three round testes located throughout the proglottids, contributing to the worm’s reproductive capacity.
- Egg Release Mechanism: Eggs are expelled into the intestine through the disintegration of the distal gravid segments, ensuring that the lifecycle continues as the eggs exit the host.
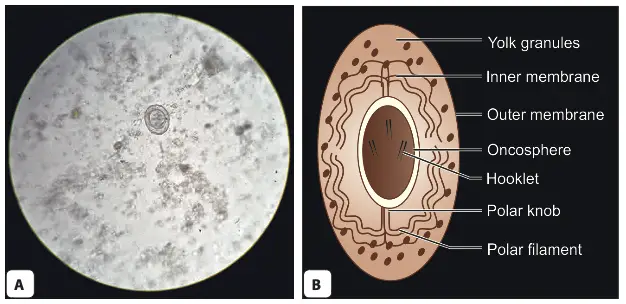
- Egg Morphology:
- Shape and Size: The eggs of H. nana are roughly spherical to ovoid, measuring approximately 30 to 40 micrometres in diameter. Their size and shape are critical for identifying the species in diagnostic examinations.
- Outer Membrane: Each egg is surrounded by a thin, colorless outer membrane, which serves as a protective barrier.
- Inner Embryophore: Inside the outer membrane lies the embryophore, which encases the hexacanth oncosphere—a crucial stage for infection.
- Yolk Granules and Filaments: The space between the outer membrane and the embryophore contains yolk granules, providing nutrients for the developing oncosphere. Additionally, there are 4 to 8 thread-like polar filaments extending from two knobs on the embryophore, which may play a role in the attachment of the egg to the intestinal wall of a host.
- Buoyancy and Infectivity: H. nana eggs are known to float in a saturated salt solution, making them easier to identify in laboratory settings. They are non-bile stained, indicating their specific developmental status. Importantly, these eggs are immediately infective upon release but do not survive for more than ten days outside the host’s environment, which underscores the need for effective transmission routes.
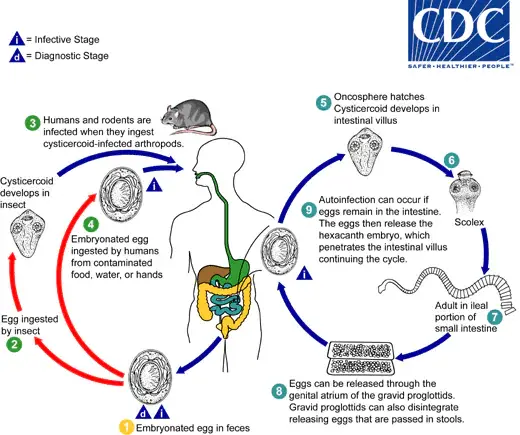
Life Cycle of Hymenolepis nana
The life cycle of Hymenolepis nana, a common intestinal cestode, demonstrates its unique ability to infect both humans and rodents. This life cycle is characterized by direct transmission and internal autoinfection, making it a significant concern for public health.
- Eggs in the Environment:
- The eggs of Hymenolepis nana are immediately infective upon being passed with the stool of an infected host.
- These eggs have a limited viability of up to 10 days in the external environment, making conditions for transmission time-sensitive.
- Ingestion by Intermediate Hosts:
- Various species of arthropods, including beetles and fleas, serve as intermediate hosts. When these arthropods ingest the eggs, the contained oncospheres (hexacanth larvae) are released.
- Inside the arthropod, the oncospheres develop into cysticercoids.
- Transmission to Definitive Hosts:
- The definitive hosts, primarily humans and rodents, acquire the infection by ingesting these infected arthropods or contaminated food and water.
- Release of Oncospheres:
- Upon ingestion of contaminated food, water, or hands (especially in children), the oncospheres are released in the small intestine.
- Development in the Intestinal Mucosa:
- The oncospheres penetrate the intestinal villi, where they develop into cysticercoid larvae.
- As the cysticercoids mature, they cause rupture of the villus, allowing them to return to the intestinal lumen.
- Evagination and Attachment:
- Once in the intestinal lumen, the cysticercoids evaginate their scoleces and attach firmly to the intestinal mucosa using their four suckers.
- This attachment allows the cysticercoids to develop into adult tapeworms.
- Maturation and Egg Production:
- The adult worms reside primarily in the ileal portion of the small intestine, where they produce gravid proglottids. These proglottids mature and begin to produce eggs.
- Egg Release:
- Eggs are expelled with the host’s feces either through the genital atrium of the proglottids or when the proglottids disintegrate in the small intestine.
- Internal Autoinfection:
- An alternative mode of infection is internal autoinfection. Here, the eggs released in the intestine hatch, and the hexacanth embryos penetrate the intestinal villi again, continuing the cycle without needing to exit into the external environment.
- Longevity of Adult Worms:
- Adult Hymenolepis nana worms have a lifespan of approximately 4 to 6 weeks, but internal autoinfection can allow the infection to persist for years.
Life Cycle of Hymenolepis diminuta
The life cycle of Hymenolepis diminuta, commonly known as the rat tapeworm, illustrates a complex interaction between the definitive host and an intermediate host, typically arthropods. Understanding this life cycle is essential for comprehending the transmission dynamics and potential public health implications associated with this parasite.
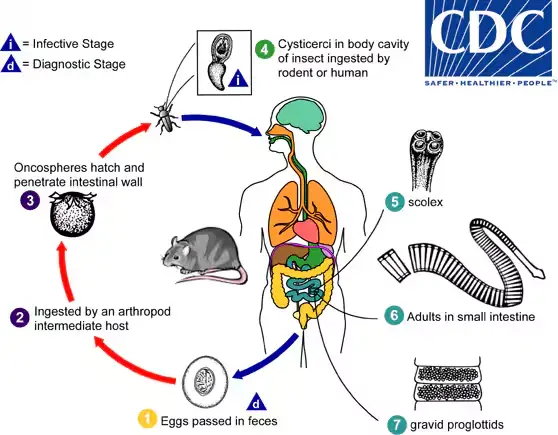
- Hosts Involved:
- Definitive Hosts: Hymenolepis diminuta primarily infects rodents, although humans can also serve as definitive hosts.
- Intermediate Hosts: Various arthropods, including species from the genus Tribolium (such as flour beetles), act as intermediate hosts, facilitating the development of the parasite.
- Lifecycle Stages:
- Egg Passage: Mature eggs are expelled into the environment via the feces of the infected definitive host, which may include both rodents and humans. This stage marks the beginning of the life cycle.
- Ingestion by Intermediate Hosts: The eggs are then ingested by various arthropod adults or larvae, serving as the primary method of transmission to the intermediate host.
- Oncosphere Release: Upon ingestion, the oncospheres (embryonic larvae) are released from the eggs within the arthropod’s intestine. They subsequently penetrate the intestinal wall, initiating the next phase of development.
- Development of Cysticercoid Larvae: The oncospheres develop into cysticercoid larvae within the intermediate host. These larvae persist throughout the arthropod’s growth stages until adulthood, remaining encapsulated in the tissues.
- Infection of Definitive Hosts: Mammals, including humans, acquire infection by ingesting the infected intermediate host containing cysticercoid larvae. This transmission may occur inadvertently through the consumption of contaminated food products, such as precooked cereals, or by direct environmental exposure, particularly in children who explore their surroundings orally.
- Release and Attachment: After ingestion, the digestive processes in the stomach and small intestine break down the tissues of the infected arthropod, releasing the cysticercoid larvae. Shortly after this release, the larvae evaginate their scoleces (the head of the tapeworm) and use the four suckers on the scolex to attach firmly to the intestinal wall of the definitive host.
- Maturation and Egg Production: The maturation of the parasites occurs within approximately 20 days. Adult H. diminuta worms can reach lengths of around 30 cm. Gravid proglottids (segments of the worm containing eggs) disintegrate and release eggs into the small intestine, which are subsequently expelled into the environment through the feces of the mammalian host, thereby completing the life cycle.
Clinical Features
Clinical features of hymenolepiasis, caused primarily by the tapeworm Hymenolepis nana, can vary significantly depending on the severity of the infection and the host’s immune response. This condition is notably more prevalent in children, highlighting the need for awareness and preventive measures in this demographic.
- Prevalence:
- Hymenolepiasis occurs most frequently in children, suggesting a higher susceptibility in this age group, likely due to factors such as hygiene practices and increased exposure to contaminated environments.
- Symptomatology:
- Asymptomatic Cases:
- In many instances, infected individuals exhibit no symptoms, making detection challenging.
- Symptoms in Heavy Infections:
- In cases of heavy infestation, the following clinical features may manifest:
- Nausea: Patients may experience feelings of discomfort and an urge to vomit.
- Anorexia: A loss of appetite is common, potentially leading to weight loss and nutritional deficiencies.
- Abdominal Pain: Patients may report cramping or general discomfort in the abdominal region.
- Diarrhea: Frequent, watery stools may occur, which can contribute to dehydration and electrolyte imbalances.
- Irritability: Especially in children, irritability may be observed, often as a response to discomfort or pain.
- In cases of heavy infestation, the following clinical features may manifest:
- Pruritus:
- In some cases, patients may experience itching (pruritus), which is likely due to an allergic reaction to the tapeworm or its metabolic products.
- Asymptomatic Cases:
- Impact on Quality of Life:
- The symptoms associated with hymenolepiasis can significantly affect the daily lives of affected individuals, particularly children, by disrupting normal activities, schooling, and social interactions.
Laboratory Diagnosis of Hymenolepis diminuta
The diagnostic process involves various techniques to identify the characteristic eggs in fecal samples.
- Direct Microscopy:
- The primary method for diagnosis is through direct microscopic examination of fecal samples.
- This technique involves the following steps:
- Sample Collection: Fecal samples are collected from the patient, ensuring that they are fresh to increase the likelihood of detecting viable eggs.
- Preparation: The samples are prepared for examination, often requiring a wet mount or smear technique.
- Identification: The technician observes the samples under a microscope, searching for the distinctive eggs of H. diminuta.
- Concentration Methods:
- To enhance the detection of eggs, concentration techniques can be employed, which help separate eggs from fecal matter. Common methods include:
- Salt Flotation: This technique utilizes a saturated salt solution to allow the eggs to float, making them easier to identify.
- Formalin-Ether Concentration: In this method, formalin is used to preserve the sample, and ether is added to facilitate the separation of eggs from debris, improving the yield of egg recovery.
- To enhance the detection of eggs, concentration techniques can be employed, which help separate eggs from fecal matter. Common methods include:
- Enzyme-Linked Immunosorbent Assay (ELISA):
- An ELISA test has been developed as a supplementary diagnostic tool.
- This method demonstrates an 80% sensitivity in detecting H. diminuta infections, providing a reliable alternative to microscopy, especially in cases where egg recovery is challenging.
Treatment of Hymenolepis diminuta
- Praziquantel:
- Praziquantel is the first-line treatment for Hymenolepis diminuta infections, typically administered as a single dose of 25 mg/kg.
- Mechanism of Action: This anthelmintic agent effectively targets both the adult worms and the cysticercoids present within the intestinal villi, leading to their expulsion from the host’s body.
- Nitazoxanide:
- An alternative treatment option is Nitazoxanide, which can be prescribed at a dosage of 500 mg twice daily for three days.
- Efficacy: This drug works by interfering with the energy metabolism of the parasite, thereby facilitating its elimination.
Prophylaxis of Hymenolepis diminuta
- Personal Hygiene and Sanitation:
- Maintaining high standards of personal hygiene is crucial. This includes regular hand washing, especially before meals and after using the restroom, to prevent fecal-oral transmission of the parasite.
- Avoiding Contaminated Food and Water:
- Ensuring that food and water sources are free from contamination is vital. This can be achieved by:
- Drinking clean, treated water.
- Thoroughly cooking food, particularly grains and cereals that may be infested with cysticercoids.
- Ensuring that food and water sources are free from contamination is vital. This can be achieved by:
- Rodent Control:
- Given that rodents are significant reservoirs for H. diminuta, effective rodent control measures should be implemented. This can include:
- Using traps or bait to reduce rodent populations in and around human habitats.
- Ensuring that food storage areas are rodent-proof to minimize the risk of contamination.
- Given that rodents are significant reservoirs for H. diminuta, effective rodent control measures should be implemented. This can include:
- Hench J, Cathomas G, Dettmer MS. Hymenolepis nana: A case report of a perfect IBD camouflage warrior. Medicine (Baltimore). 2017 Dec;96(50):e9146. doi: 10.1097/MD.0000000000009146. PMID: 29390318; PMCID: PMC5815730.
- https://www.sciencedirect.com/topics/medicine-and-dentistry/hymenolepis-nana
- https://www.msdmanuals.com/en-in/professional/infectious-diseases/cestodes-tapeworms/hymenolepis-nana-dwarf-tapeworm-infection
- https://www.msdmanuals.com/en-in/professional/infectious-diseases/cestodes-tapeworms/hymenolepis-nana-dwarf-tapeworm-infection
- https://emedicine.medscape.com/article/998498-overview
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5544084/
- https://en.wikipedia.org/wiki/Hymenolepis_nana
- https://www.cdc.gov/dpdx/hymenolepiasis/index.html