What is Hydrogen Sulfide (H2S) Test?
- This test determines whether sulfur-containing chemicals are converted to sulphides during the microbe’s metabolic activity.
- When sulphide is generated, it reacts with iron compounds to form FeS, a black precipitate.
- Multiple iron-containing media permit the detection of hydrogen sulphide generation. Sulfide-Indole-Mobility (SIM) medium is utilised.
- This nutrient medium enables the detection of three distinct characteristics in bacteria: (1) it contains sulphates, which serve as the substrate for detecting sulphide production; (2) it contains an abundance of tryptophan, which serves as the substrate for indole production; and (3) its 0.5% agar content is sufficient to permit bacterial motility, thereby enabling the detection of motility.
- Triple sugar iron agar (TSIA) is a second medium used to determine the microbe’s ability to ferment up to three sugars. In any situation, the test’s chemistry and interpretation are same.
- A pure culture’s inoculum is transferred aseptically to a sterile TSIA (triple sugar iron agar) slant.
- After incubating the infected tube at 35-37 degrees Celsius for 24 hours, the results are determined. The TSIA contains an iron compound.
- Sulfide ions are strongly attracted to iron ions (Fe2+). As a result, H2S reacts with iron to form FeS, a dark chemical. In tubes of TSIA containing bacteria that produce hydrogen sulphide, the FeS causes the agar to turn black.
- Please note that this medium can also be used to observe the fermentation capabilities of the unknown microorganism. TSIA contains three sugars: glucose, sucrose, and lactose. If any of the available sugars can be utilised, the bacteria will produce acidic byproducts.
- In a positive test, the normal red hue of the pH indicator in the medium turns to yellow, suggesting acid production. Refer to the discussion of TSIA agar slants for information on how to interpret these modifications.
Hydrogen sulfide (H S)-producing bacteria
- The hydrogen sulphide generating bacteria (H2S) are diverse types of bacteria and archaea that obtain energy by converting sulfur-containing organic and inorganic molecules (sulphur amino acids, sulphate, sulfite, thiosulfate, tetrathionates, and elemental sulphur) to H2S. Hydrogen sulphide is an odourless, poisonous gas that smells strongly like rotten eggs.
- It is present in the environment as one of the compounds resulting from the degradation of organic matter.
- It is prevalent in untreated air and water mostly as a result of natural emissions, and industrial operations enhance its concentration.
- Depending on the mineralogy of the originating rock and the microorganisms present, H2S is notably abundant in some groundwater. A multitude of foods and beverages, including water, may contain sulphides.
- In food, H2S-producing bacteria stand out as distinct microorganisms of spoilage in some fish products and red meats, especially if processed.
- As stated previously, the bacteria that produce H2S are a diverse and heterogeneous species, with sulfate-reducing bacteria (SO) standing out as the primary agents of H S generation in anaerobiosis.
- Frequently, the reducing sulphate activity of bacteria is connected to the oxidation capacity of other oxidised sulphur compounds.
- In addition to sulfur-reducing bacteria (S) and sulfite-reducing bacteria (SO), thiosulfate (SO O)-reducing non-sulfate-reducing bacteria have been discovered. The properties of the most prevalent forms of bacteria are then discussed.
- Sulfate-reducing bacteria (SRB) are chemolithotropic bacteria that employ sulphate as the last electron acceptor in the breakdown of organic materials, a process known as sulphate reduction that produces H2S. More than 220 species of 60 genera of SRB have been described so far. They belong to five divisions or phyla within the bacteria (the spore formers Desufotomaculum, Desulfosporomusa, and Desulfosporosinus within the division Firmicutes, Deltaproteobacteria; the Thermodesulfovibrio species within the division Nitrospira; and two phyla represented by the species Thermodesulfobi (the genus Archaeoglobus of the phylum Euryarchaeota and the two genera Thermocladium and Caldivirga of the phylum Crenarchaeota, affiliated to the order Thermoproteales).
- SRBs are strictly anaerobic bacteria that convert sulphate, the most oxidised form of sulphur, into sulphur, the most reduced form.
- This non-assimilable sulphate reduction is a large-scale process restricted to SRBs. In addition to sulphate, SRBs can employ other oxidised sulphur compounds, such as sulphites and thiosulfate, as terminal electron acceptors, therefore lowering sulphate intermediates.
- Among the SRBs are also some facultative sulfur-reducing bacteria, which utilise elemental sulphur as a respiratory substrate in the absence of other potential terminal electron acceptors such as sulphate, sulfite, thiosulfate, nitrite, or nitrate.
- Some bacteria of the genera Desulfomicrobium and Desulfovibrio utilise sulphur as an alternate electron acceptor, despite the fact that most SRBs cannot develop by elemental sulphur reduction.
- Under anaerobiosis, SRB can have a heterotrophic, autotrophic, lithoautotrophic, or respiratory metabolism. In addition, it was recently shown that several SRB species traditionally thought to be strict anaerobes may in fact be microaerophilic. SRBs can utilise over a hundred compounds as electron donors (sugars, amino acids, monocarboxylic acids, dicarboxylic acids, alcohols, and aromatics) and are the microorganisms that reduce the greatest number of distinct end-acceptor terminal electrons, including sulfur-containing inorganic compounds. Due to this, its ecological and metabolic role in the natural world is of considerable significance.
- SRBs are widely distributed in anoxic aquatic and terrestrial habitats.
- They can develop in a variety of physical-chemical circumstances, allowing them to survive in the most severe ecosystems on Earth, such as salty, hot, cold, and/or alkaline ecosystems.
- The most thoroughly investigated genus is Desulfovibrio, which is prevalent in watery habitats or flooded soils with ample organic matter and sufficient sulphate levels.
- The ability of sulfur-reducing microorganisms to convert elemental sulphur to sulphide is referred to as sulforeduction. Several types of archaea and chemoorganotrophic bacteria may oxidise organic substrates (mostly short peptides, glucose, and starch) anaerobically, using S° as the ultimate electron acceptor.
- Anaerobic respiration is mostly observed in the archaeal genera Thermococcus and Thermoproteus, and to a lesser extent in Desulfurococcus, Thermofilum, and Pyrococcus.
- In addition, chemolithotrophic archaea belonging to the genera Acidians, Pyrodictium, and Thermoproteus are capable of autotrophic growth at the expense of CO, H, and S°.
- Desulfuromonas, Desulfurella, and Campylobacter are the eubacterial genera responsible for anaerobic respiration of sulphur dioxide. The oxidation of substrates such as acetate and ethanol is coupled with the reduction of elemental sulphur to hydrogen sulphide by these bacteria.
- In addition to being able to reduce elemental sulphur, chemoreganotrophic facultative aerobic bacteria of the genera Proteus, Pseudomonas, and Salmonella may also reduce sulphur compounds such as thiosulfate, sulfite, and dimethylsulfoxide.
- Sulfite-reducing anaerobes are connected with Clostridium species. As such, they are classified as Gram-positive, anaerobic, spore-forming organisms.
- They are present in a variety of environmental sources, including soil, marine sediments, decomposing plants, human and animal intestines, excrement and infected wounds of humans and animals, surface waters, and food, particularly when hygiene standards during preparation are inadequate.
- They are degrading because they emit foul odours and frequently blacken iron-containing products by precipitating iron sulphide.
- These microorganisms can convert sulfites to sulphides using amino acids and sulphur compounds.
- These microorganisms have been recommended as indications of water pollution with high danger. Their spores are resistant to disinfection and can be discovered in some water samples even after pre-disinfection, flocculation, sedimentation, filtration, and terminal disinfection. This is the most significant advantage of these organisms.
- They are also useful as markers of recent faeces contamination in food.
- The detection of H2S-producing bacteria in the laboratory is predicated mostly on the detection of hydrogen sulphide production in culture.
- Adjustments are made to the source of sulphur, the sulphide indicators, and the incubation conditions of the culture in order to detect the various types of bacteria through isolation in culture.
- As sulphur suppliers, several species of H2S-producing bacteria utilise various inorganic chemicals or sulphur amino acids (such as protein digests – peptone -, sulphur amino acids – cysteine or methionine – and thiosulfate).
- On the market, there are numerous culture media containing various sulphur compounds, where gaseous H2S produced by the reduction of an inorganic sulphur source such as thiosulfate or by the reduction of the organic sulphur as provided by the functional group R-SH of the amino acid cysteine present in peptones has been detected.
- H2S generation is identified when the gas reacts with particular metals, such as lead, iron, or bismuth, to generate sulphides (black).
- Peptonized iron, ferrous sulphate, ferrous or ferric ammonium sulphate, ferric citrate, sodium thiosulfate, bismuth sulfite, and lead acetate are different sulphur indicators for various media.
- Iron salts are commonly employed to detect members of the Enterobacteriaceae family, but lead acetate techniques are the most sensitive for detecting minute quantities of H2S in bacteria that are not members of the Enterobacteriaceae family.
Principle of Hydrogen Sulfide (H2S) Test
- The hydrogen sulphide test is designed to detect the formation of sulphide gas. To do so, a substance containing iron and sulphur is introduced to the test medium.
- When a specific strain of bacteria lowers the sulphur component, hydrogen sulphide is produced.
- A specific type of bacteria produces hydrogen sulphide by reducing sulfur-containing amino acids such as methionine and cysteine, or by reducing inorganic sulphur compounds such as sulphates, thiosulfates, and sulfites, during protein degradation or when anaerobic respiration converts electrons to sulphur instead of oxygen.
- Hydrogen sulphide gas is created and interacts with the iron component to precipitate ferric sulphide as a dark substance. Because of the colour (black), which serves as an indicator, you will be able to detect the presence of hydrogen sulphide.
Objective of Hydrogen Sulfide (H2S) Test
- Determine the organism’s ability to create hydrogen sulphide.
Media for the detection of Hydrogen Sulfide (H₂S)
Commonly used media for detecting hydrogen sulphide generation, sulphur sources, and sulphide indicators include the following:
| Media | Sulfur source | H₂S indicator |
| Bismuth sulfite | Peptones plus sulfite | Ferrous sulfate |
| Citrate sulfide agar | Sodium thiosulfate | Ferric ammonium citrate |
| Deoxycholate citrate agar (DCA) | Peptones | Ferric citrate |
| Lysine iron agar (LIA) | Sodium thiosulfate | Ferric ammonium citrate |
| Kligler iron agar (KIA) | Sodium thiosulfate | Ferrous sulfate |
| Triple sugar iron (TSI) agar | Sodium thiosulfate | Ferrous sulfate |
| Lead acetate agar | Sodium thiosulfate | Lead acetate |
| Salmonella-Shigella (SS) agar | Sodium thiosulfate | Ferric citrate |
| Sulfide-indole-motility (SIM) Medium | Sodium thiosulfate | Peptonized iron |
| Xylose-lysine-deoxycholate (XLD) agar medium | Sodium thiosulfate | Ferric ammonium citrate |
| Hektoen enteric agar | Sodium thiosulfate | Ferric ammonium citrate |
Microbiologists can select a specific detection system based on their demands and the features of the test isolate, as many types of medium with differing degrees of sensitivity are available for detecting H2S generation. For instance, when testing bacteria that produce only minimal levels of H2S, the most sensitive indicator, lead acetate, should be employed.
A lead acetate-impregnated filter paper should be draped beneath the cap of a culture tube during testing, rather than adding lead acetate into the culture medium. This is because lead acetate may hinder the development of many fastidious bacteria when added to culture media.
As H2S detected in one medium may not be detected in another, when interpreting identification charts, it is vital to know the test system employed. SIM, KIA, or TSI tubes are often used in diagnostic microbiology to detect H2S generation. The least sensitive of these three biochemical test media is TSI. It is hypothesised that the sucrose included in this test media inhibits hydrogen sulphide formation. SIM is more delicate than KIA and TSI. The absence of carbohydrates to inhibit H2S creation and the utilisation of peptonized iron as the indicator make SIM a superior test medium for detecting H2S production.
The endpoint of all H2S detection methods is an insoluble heavy metal sulphide, which generates a black precipitate in the media or on the filter paper strip. Because hydrogen ions must be present for H2S creation, blackening appears first in test medium in where acid formation is greatest, i.e. along the inoculating line, within the depths of slanted agar media, or in the cores of colonies developing on agar surfaces.
Procedure of Hydrogen Sulfide Test
Tube Media Method
- Warm to room temperature, then inspect for cracks. Do not use if there are cracks.
- Using a sterile inoculating needle, contact the core of a colony that is well-isolated.
- Stabbing to within 3 to 5 mm of the tube’s base.
- Remove the needle.
- For KIA or TSI, stripe the entire agar slant surface.
- Optional for fussy organisms: put a 1 in.-long piece of lead acetate paper to the top of the tube and secure it in place with the tube’s cap. within the tube
- Place the top on the tube loosely. Do not tighten the cap to allow gas in the tube to escape.
- 18 to 24 hours of aerobic incubation at 35 to 37°C.
- Observe for black precipitate, which indicates the generation of hydrogen sulphide.
- Extend incubation solely to detect H2S generation, if required. Campylobacters may require three days to produce H2S.
Plate Media Method
- In order to obtain isolated colonies, streak a plate.
- Incubate aerobically between 35 and 37 degrees Celsius
- Observe for colonies with a black hue
Procedure of Hydrogen Sulfide Test based on types of media is used
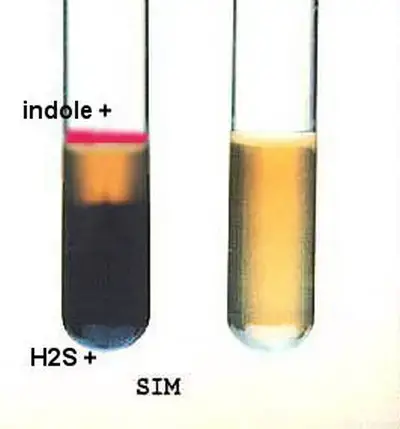
Using SIM (sulphite indole motility) as a medium
SIM media for hydrogen sulphide gas detection. Ferrous ammonium sulphate and sodium thiosulfate are components of SIM medium. They serve as indicators of the production of hydrogen sulphide. The presence of black precipitate indicates the formation of hydrogen sulphide. It is a chemical reaction between ferrous ammonium sulphate and hydrogen sulphide gas.
- Using a stab inoculation procedure, the organism to be examined is introduced into a designated test tube.
- The infected tube must be incubated at 37 degrees Celsius for a minimum of one to two days.
- Beware the production of a black precipitate.
Using iron agars such as triple sugar iron agar and Kligler iron agar as a medium
This medium is ideal for detecting the presence of hydrogen sulphide gas produced by enterobacteria. When ferric citrate is present in a medium, hydrogen sulphide gas becomes positive.
- The organism to be examined must be inoculated onto Kligler iron agar and incubated overnight at the appropriate temperature.
- Check for any changes in the medium, particularly if it turns black.
Using Hydrogen Sulfide Test Strips (Lead acetate test strips, H2S test strips)
Principle
These strips are used to detect microorganisms that produce hydrogen sulphide. Large numbers of bacteria in carbohydrate-rich environment can create tiny amounts of hydrogen sulphide from sulfur-containing amino acids such as cysteine. Hydrogen sulphide creates a black precipitate upon contact with lead acetate, as demonstrated by a visible black reaction on the hydrogen sulphide paper strip. The lead acetate process is more sensitive than any other method for detecting hydrogen sulphide formation; even amounts of hydrogen sulphide can be detected.
Composition
- Sterile filter paper strips impregnated with lead acetate
Procedure
- Inoculate Peptone Water with the organism being tested.
- Insert a lead acetate paper strip between the stopper and the inner tube wall, above the infected mixture, and incubate at 35 degrees Celsius for 18 to 24 hours.
Quality control
The cultural reaction after 18 to 24 hours at 35 degrees Celsius. A good reaction appears as a darkening of the strip’s lowest portion. A negative reaction reveals no discoloration.
Result and Interpretation of Hydrogen Sulfide (H2S) Test
Positive reactions
- H2S production in tube media: black coloration throughout the medium, a black ring at the intersection of the butt and slant, or the presence of any black precipitate in the butt. Typically, discoloration develops near the inoculation line.
- H2S production on plate media: black colonies bordered by a brownish-black zone or a metallic sheen
- Lead acetate paper: the paper strip’s brownish-black hue

Negative reactions
- H2S production in tube media: no blackening in tube
- H2S production in plate media: no blackening and no metallic-sheen colonies
- Lead acetate paper: no change in color of the strip




| Organisms | Result |
| Escherichia coli (25922) | Negative |
| Salmonella serotype Enteritidis (13076) | Positive |
| Citrobacter freundii | Positive |
| Proteus mirabilis | Positive |
| Proteus vulgaris | Positive |
| Edwardsiella tarda | Positive |
| Salmonella serotype Typhimurium (14028) | Positive |
Limitations
- Ensure that the media does not touch the lead acetate paper strip when using it. Lead acetate is poisonous to bacteria and may limit the growth of some bacterial species.
- TSI may suppress the generation of hydrogen sulphide gas by bacteria that utilise sucrose. It can inhibit the enzyme process that leads to hydrogen sulphide gas generation.
- Other testing procedures, such as molecular, mass spectrometry, immunological, and biochemical testing, should be utilised for the comprehensive identification of organisms.
Uses
- The test is conducted to identify and distinguish Enterobacteriaceae members from other Gram-negative bacilli.
- It helps detect Francisella, Salmonella, and Proteus species, among others.
- It is utilised to distinguish microorganisms such as Bacteroidessps and Brucella species and to identify members of the Enterobacteriaceae family.
- On TSI, organisms that utilise sucrose and repress the enzymatic process that results in H2S formation may have their H2S production inhibited.
Keynote
- Due to its semisolid form, absence of interfering carbohydrates, and utilisation of peptonized iron as an indicator, SIM is more sensitive than either TSI or KIA for the detection of H2S.
- Paper made from lead acetate is ten times more sensitive than other media.
- Lead acetate is poisonous to bacteria and may impede bacterial growth. Do not let media contact with the strip.
Quality Control
- Positive control: Proteus vulgaris
- Negative control: Escherichia coli
References
- https://www.vumicro.com/vumie/help/VUMICRO/Hydrogen_Sulfide_Production_Test.htm
- https://www.intertek.com/petroleum/h2s-test/
- https://www.sigmaaldrich.com/IN/en/product/sial/06728
- https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/263/310/06728dat.pdf
- https://laboratoryinfo.com/hydrogen-sulfide-test/
- https://medicallabtechnology.com/h2s-test-hydrogen-sulfide-principle/
- https://www.grainger.com/category/lab-supplies/lab-consumables/chemical-test-strips?attrs=Testing+Parameter%7CHydrogen+Sulfide&filters=attrs
- https://www.ivami.com/en/food-microbiology/5445-hydrogen-sulfide-h2s-producing-bacteria-sulfate-reducers-sulfite-reducers-sulfur-reducers-and-other-molecules-with-sulfur-qualitative-and-quantitative-culture-molecular-identification-pcr-and-sequencing
- https://mytapscore.com/products/hydrogen-sulfide-h2s-in-water-test
- https://microbeonline.com/hydrogen-sulfide-production-test/
- https://microbenotes.com/hydrogen-sulfide-h2s-production-test/
- https://microbiologyinfo.com/hydrogen-sulfide-test/