A pH meter is an instrument that is used to measure the pH of a solution, and it is the value that shows how acidic or alkaline the solution is. It is the process where a glass electrode and a reference electrode are placed in the sample, and the potential difference formed between them is converted into a pH value.
It is sensitive to hydrogen ion activity, so even small changes in the solution can affect the reading. With time, the electrode becomes weak because of fouling, aging of glass membrane, and exposure to different chemicals, so the reading is not the true value.
For this reason, calibration is required because it is the process of adjusting the meter with buffer solutions of known pH, and this helps in correcting the slope and the offset of the electrode. It is also important because temperature changes can affect the sensitivity of the electrode, so calibration ensures that the pH meter is giving accurate and reliable values before it is used.
Why pH Meters need to Calibrate?
A pH meter needs to be calibrated because the electrode does not remain the same with time, and its response slowly changes due to aging and continuous exposure to different chemical conditions. It is the process where the electrode begins to show drift, so the pH value shown by the meter becomes slightly higher or lower than the actual value.
This is corrected only when the meter is adjusted with standard buffer solutions of known pH, and these buffers help the instrument to find the true slope and the offset of the electrode. It is also important because temperature affects both the electrode sensitivity and the pH of the buffer, so calibration is required to match the reading with the correct value under that condition. In this way, calibration ensures that the pH meter is giving accurate and reliable measurements each time it is used.
Preparation of Standard Buffer for Calibration of a pH Meter
It is based on the fact that each buffer maintain a constant pH when it is dissolved properly and the solution is allowed to mix uniformly. The buffer act as the reference medium during the adjustment of the instrument, and any variations in dissolution or contamination is reflected in the reading.
Preparation of pH 4.00 Buffer
The pH 4.00 buffer at about 20°C is prepared by transferring the capsule powder into a 100 ml volumetric flask. It is observed that purified water help in dissolving the contents, which is necessary for correct calibration. In this step, nearly 80 ml water is added and stirred until the powder is dispersed.
After mixing, the volume is made up to the mark producing an uniform solution. It is the process where small fragments sometimes float before complete dissolution.
Preparation of pH 7.00 Buffer
In this step, the pH 7.00 buffer capsule is poured into the 100 ml flask. It is used to provide the mid-range reference point. About 80 ml purified water is added so that the powder is dissolved forming a homogeneous mixture.
The final volume is adjusted to 100 ml although the alignment of the meniscus is sometimes tricky. Gentle swirling is helpful in reducing the undissolved granules that appear first.
Preparation of pH 9.20 Buffer
The pH 9.20 buffer is prepared next. It is the process where the tablet is placed inside the volumetric flask giving a slightly alkaline solution needed for upper pH calibration. The addition of nearly 80 ml water allow the contents to break down.
Then the volume is taken to 100 ml. It is sometimes seen that slow mixing is preferred because rapid shaking produce micro-bubbles which affect the reading during calibration.
pH Meter Calibration Procedure
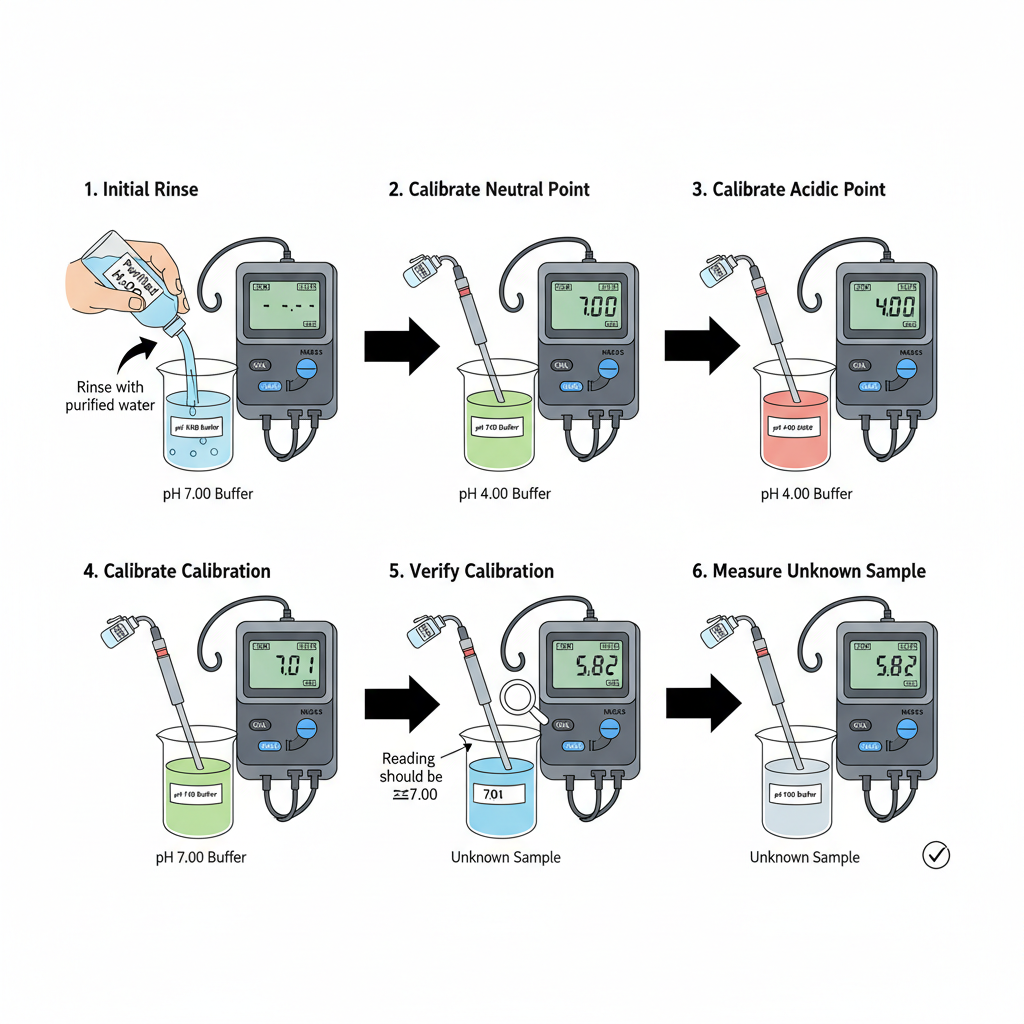
- Switching on the instrument– The pH meter is switched on first, and it is kept for some time so that the display comes to a stable condition. It is seen that proper warming gives steady readings.
- Rinsing of the electrode– The electrode is rinsed with purified water. It is the process that remove any previous solution, and the extra water is gently shaken off without touching the bulb.
- Selection of the neutral buffer -The pH 7.00 buffer is used first. It is considered the midpoint reference, and the solution is taken freshly to avoid contamination.
- Immersing the electrode in pH 7.00 buffer– The electrode is dipped inside the buffer so that the sensing bulb is fully covered. A slight stirring is done to produce uniform contact between electrode and solution.
- Allowing the reading to stabilize– The meter is kept undisturbed until the reading stop drifting. After stabilization, the displayed value is adjusted to 7.00. This is referred to as the initial point setting.
- Rinsing before next point– The electrode is taken out and washed again using purified water. It is important because leftover droplets may change the next buffer’s value.
- Calibration with pH 4.00 buffer– The electrode is placed in the pH 4.00 buffer. The solution is slightly acidic, and the reading usually changes slowly at first. When the value become steady, the meter is set to 4.00.
- Cleaning after acidic buffer– The electrode is washed with purified water to remove the acidic residues that sometimes cling on the glass surface.
- Calibration with pH 9.20 buffer– The electrode is then dipped in pH 9.20 buffer. It is the process for upper pH range. The reading may take some extra time because alkaline buffers is often slow to settle. After stabilization, the value is adjusted to 9.20.
- Final rinsing– The electrode is rinsed once again with purified water. It is shaken lightly to remove the attached droplets, avoiding dilution of the sample.
- Verification step– Sometimes the electrode is placed again into pH 7.00 buffer to check whether the instrument gives nearly the same reading. This indicate that calibration is correct.
- Instrument ready for use– After these steps, the pH meter is considered calibrated, and the electrode is now ready for measuring the unknown solutions.
How Often To Recalibrate pH Meter?
The typical recalibration interval is often carried out daily, especially in laboratories where readings’ drift quickly because of probe aging or residue’s that collect on the glass surface, which leads to variability.
In practice many instruments are recalibrated before every measurement session, and this process involves verifying the slope / offset to confirm the meter is considered stable.
A freshly cleaned electrode may hold its accuracy for a few hours, however some units’ drift sooner, resulting in the need for mid-day check’s.
When high-precision tasks are done, e.g. buffer preparation or culture work, it is observed that recalibration is required every 2–4 hours, which gives a look into how sensitive the electrode’s behavior is under even minor temperature shifts.
Field probes also are recalibrated more frequently, because outdoor pH conditions can influence junction flow, they might need a quick 1-point check at each site visited.
If the electrode is older than ~6–8 months, the recalibration intervals become shorter, sometimes after each sample, Oddly the slope flatten’s and the instrument respond in to slow drifts.
After storage in wrong solution (i.e. water instead of proper KCl), the pH meter calibration is supposed to be repeated immediately before any reading, At this stage the glass membrane is rehydrating, which leads to unstable signals.
Some rugged industrial meters’ allow weekly calibration, but this is recognized as being acceptable only when environmental changes are minimal, Still many operators also perform quick verification in-between.
Failure to calibrate a pH meter can have several consequences:
- It gives inaccurate reading’s, and During this process the drift can be observed quietly, which leads to mistaken interpretations in sensitive pH-based analyses.
- It results in compromised experiment’s because the sample conditions’ are not actually known, however the data still look “correct”, producing false conclusions or repeating entire trials.
- They create product quality issue’s in food / beverage or pharma sectors, the inconsistency is considered to be dangerous for stability and safety.
- It may generate health and safety risk’s, like incorrect pool pH where bacterial growth can occur, and the system is affected by even small deviations.
- They cause economic implication’s because wasted batches, recalls, or re-testing increase operational cost’s rapidly, leading to unnecessary troubleshooting.
- It influences environmental assessments, and inaccurate soil/water pH is recorded, resulting in wrong ecological decisions that can affect wildlife, plants’, etc.
- It can cause equipment Damage, corrosion or scaling appear in reactors / pipes, and At this stage maintenance escalate’s.
- They produce legal and regulatory non-compliance since required pH ranges are not met, which is associated with fines or penalties.
- It leads to loss of trust, clients’ question the reliability, however the core problem was simply uncalibrated readings.
- They waste time, troubleshooting goes on for hours, forming long cycles of repeated evaluations that prevail correct workflow.
- It misguides educational environments, students learn incorrect values, then the misconception’s become embedded.
Why We Need to Calibrate a pH Meter?
- To obtain accurate readings– A pH meter without calibration does not show the true value. It is necessary because the electrode response change with time, and only calibration bring the reading back to correct scale.
- To maintain stability of measurement– The electrode sometimes drift slowly. Calibration help in bringing the electrode to a stable condition, so the reading does not fluctuate during use.
- To compensate electrode aging– The electrode performance reduce naturally. The glass bulb become slow, and the reference junction get deposited with salts. Calibration is needed to adjust these changes.
- To match the instrument with standard reference points– The buffers (pH 7, pH 4, pH 9.20) act as fixed points. When the meter is calibrated, the instrument is aligned with these known values, and this alignment is essential for further measurements.
- To avoid errors in solution preparation– Many laboratory procedures depend on exact pH. If the meter is not calibrated, the prepared buffers or media will not reach the intended pH, causing problems in experiments.
- To ensure reproducibility– Calibration help in maintaining similar readings each time. Without this, two measurements of the same sample may give different values, which makes the procedure unreliable.
- To support biochemical reactions– Some enzymes and biological systems is highly sensitive to pH. Proper calibration ensure that the experimental conditions remain suitable.
- To fulfil laboratory standards– Most laboratories require calibrated instruments for record keeping and quality control. It is the step that ensures the data is acceptable.
- To prevent false interpretation – When the pH meter is calibrated, the sample is not misjudged as too acidic or too alkaline. This avoid wrong conclusions in practical work.
Consequences of Not Calibrating a pH Meter
- Incorrect pH readings– When the meter is not calibrated, the displayed value shift from the true value. It is the process that cause wrong interpretation of acidity or alkalinity, and the error increase with time.
- Unstable measurement– The electrode response becomes unstable. The reading may drift continuously, and the instrument is unable to settle at one point.
- Poor reproducibility– Results obtained from repeated measurements show large variations because the reference points (pH 7, pH 4, pH 9.20) are not set beforehand. These inconsistencies is commonly noted in practical records.
- Inaccurate preparation of solutions – Many laboratory solutions require specific pH for proper functioning. If the meter is not calibrated, the prepared buffers, media, or reagents will not reach the required pH, affecting their performance.
- Enzyme and reaction errors– Some biochemical reactions is highly sensitive to small pH changes. Incorrect pH measurement lead to reduced activity of enzymes or unexpected behaviour of the reaction mixture.
- Quality control failure– In research and industrial laboratories, uncalibrated meters produce data that cannot be accepted. The final results is rejected because the pH value is outside the allowed range.
- Damage to the electrode– When calibration is skipped, the electrode may be used in unsuitable conditions. This sometimes cause salt-build up, slow response, and loss of electrode efficiency.
- Misinterpretation of sample condition– The sample may appear acidic or alkaline when it is actually not. This false conclusion affect the entire experiment or production batch.
- Trouble in standardizing procedures– Without correct pH reference, procedures such as titration, culture preparation, and analytical testing is not comparable between days or between operators.
- Increased cost and time loss– Wrong pH readings lead to repeated experiments, wastage of chemicals, and unnecessary troubleshooting. It is a step that cause both time delay and material loss.
FAQ
What is a pH meter?
A pH meter is an instrument used to measure the acidity or alkalinity of a solution, providing a value on a scale from 0 (very acidic) to 14 (very alkaline), with 7 being neutral.
How often should I calibrate my pH meter?
The frequency of calibration depends on the level of precision required and the conditions of use. For high-precision tasks, it’s advisable to calibrate before each use. For regular use, calibrating once a day or week is often sufficient.
Why is calibration important for a pH meter?
Calibration ensures that the pH meter provides accurate and consistent readings, adjusting for any drift or changes in the electrode’s efficiency.
Can I use tap water to clean the pH electrode?
It’s recommended to use deionized, distilled, or reverse osmosis water to clean the electrode to prevent contamination.
What are pH buffer solutions?
pH buffer solutions are solutions with a known and stable pH value, used for calibrating pH meters.
Why is my pH meter giving erratic readings?
Erratic readings can be due to a dirty electrode, a damaged electrode, or the need for recalibration. It’s essential to clean the electrode regularly and ensure it’s in good condition.
How should I store my pH meter when not in use?
The pH electrode should be stored in a storage solution or a pH buffer solution to keep it hydrated and maintain its efficiency. Avoid storing it in distilled water.
What is the lifespan of a pH electrode?
The lifespan varies based on usage, care, and the specific model. With proper care, a pH electrode can last 1-3 years or even longer.
Can I measure the pH of solid substances with a pH meter?
Directly measuring the pH of solids is challenging. Typically, a slurry or solution is made from the solid, and then its pH is measured.
Are there different types of pH meters?
Yes, there are various types, including benchtop pH meters for laboratory use, portable pH meters for field use, and pen-style pH meters for quick and convenient measurements.
- Atlas Scientific. (2025). How To Calibrate a pH Meter Correctly. Retrieved from [Atlas Scientific website].
- Atlas Scientific. (2025). ph Probe Calibration Explained. Retrieved from [Atlas Scientific website].
- BOQU. (2024, April 7). The Influence of Ionic Strength on pH Meter Readings in Industrial Settings. Retrieved from [BOQU Instrument website].
- Broadley-James. How do I test my pH electrode? Retrieved from [Broadley-James website].
- Cheng, K. L., & Zhu, D. M. (2005). On Calibration of pH Meters. PMC. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC3933894/
- GMP Insiders Expert Team. (2024, June 20). Calibration, Handling and Maintenance of pH Meters. Retrieved from [GMP Insiders website].
- M4 Knick. (2020, May 21). A Better pH Calibration when Switching to Automatic Mode. Retrieved from [M4 Knick website].
- Metrohm. How to calibrate a pH meter. Retrieved from [Metrohm website].
- Reagecon Technical Dept. How does a pH meter measure the response of a pH electrode. Retrieved from [Reagecon Knowledge website].
- Reagecon Technical Dept. What are the acceptable ranges for the slope and asymmetry potential of a pH electrode. Retrieved from [Reagecon Knowledge website].
- Source Authors. (n.d.). Calibration of pH Measurement Systems: Principles, Metrology, and Quality Assurance. [Synthesized Technical Document]. [Used for comprehensive background principles, non-ideal behavior, and thermal effects].
- Thermo Fisher Scientific. (2009). pH Calibration Procedure for Optimal Measurement Precision (Technical Note T-PHCAL-E 0808 RevA). Retrieved from [Thermo Fisher Scientific website].
- U.S. Environmental Protection Agency, Region 4 Science and Ecosystem Support Division. (2013, January 29). FIELD pH MEASUREMENT (SESD Operating Procedure SESDPROC-100-R3).
- Yokogawa America. Successful pH Troubleshooting. Retrieved from [Yokogawa America website].