What is the vaccine?
- It is biochemical preparation that gives you an active, acquired immunity to an infectious disease.
- A typical vaccine has an ingredient that resembles the microorganism that causes illness and is typically made from weak or dead forms from the microbes, their toxicants, as well as one or more of the surface proteins.
- The drug stimulates the immune system of the body to recognize that the substance is a danger and eliminate it, and further identify and eliminate any microorganisms that are associated with the agent they may come across in the future.
- Vaccines are either prophylactic (to reduce or prevent the effects of an infection caused by a natural or “wild” pathogen), or therapeutic (to combat a disease that already has occurred like cancer).
- Certain vaccines provide complete sterilization immunity, meaning that infection is totally prevented.
- The administration of vaccinations is known as vaccination. It is the most efficient method of preventing the spread of disease.
- The terms “vaccine” and “vaccine” are both derived from Variolae vaccinatione (smallpox that affects cows) The term was invented by Edward Jenner (who both developed the concept of vaccines as well as developed the very first vaccination) to describe cowpox.
Ingredients of a vaccine
Vaccines are made up of very small pieces of organism responsible for the disease or the blueprints used to create tiny fragments. Additionally, they contain components to ensure that the vaccine is secure and efficient. These ingredients are present in the majority of vaccines, as well as used over years in millions of doses of vaccine.
Each component of the vaccine serves an exact purpose and every ingredient is tested during the manufacturing process. Each ingredient is tested for security.
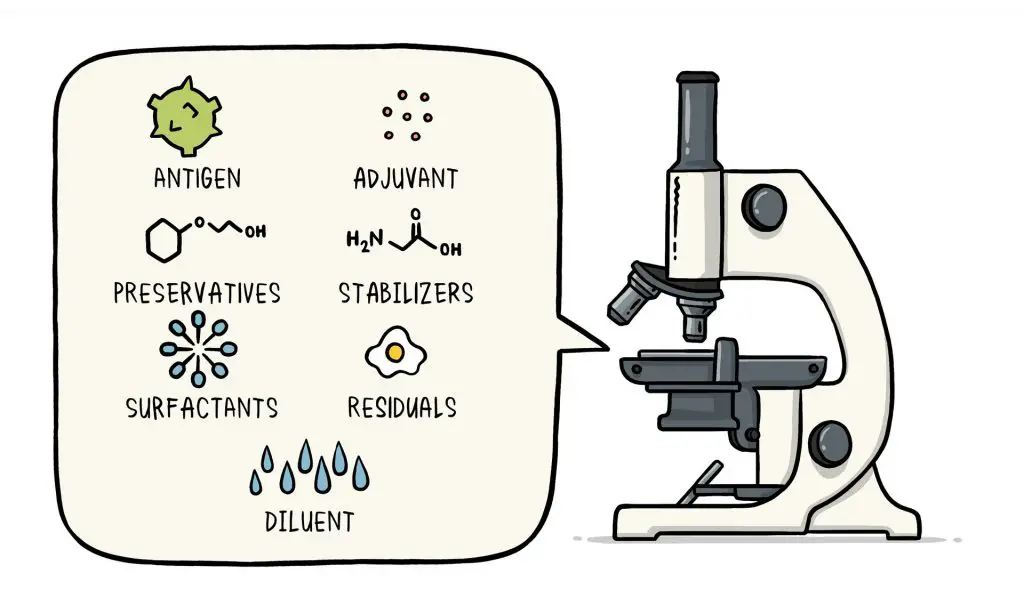
1. Antigen
- Every vaccine contains an active component , or antigen that causes an immune response or the blueprint that is used to create that active part.
- The antigen could be a tiny portion of the disease-causing organism like sugar or a protein or the entire organism that is weak or inactive state.
2. Preservatives
- Preservatives stop the vaccine from getting infected after the vial is opened, especially if it is going to be used for vaccination of several people.
- Certain vaccines do not contain preservatives since they are stored in single-dose vials and then disposed of when the dose has been given.
- The most frequently used preservative is 2-phenoxyethanol.
- It has been used for a number of years in various vaccines. It’s used in a variety of baby products and is safe to use in vaccines as it poses no risk to humans.
3. Stabilizers
- Stabilizers block chemical reactions that can occur in the vaccine, and prevent the vaccine’s components from adhering to the vial of vaccine.
- Stabilizers could be sugars (lactose and sucrose) amino acids (glycine) gelatin as well as protein (recombinant human albumin, which is derived by yeast).
4. Surfactants
- Surfactants help keep the various ingredients in the vaccine mixed together.
- They stop the settlement and clumping of the components that are present within the form of liquids in vaccine.
- They are also used in food items such as Ice cream.
5. Residuals
- Residuals are small quantities of the various substances that are used in the production or manufacturing of vaccines. They do not constitute active components in the finished vaccine.
- The substances used will differ based on the manufacturing method used and could comprise egg proteins, yeast or antibiotics.
- The residual traces of these substances which could be found in a vaccine are very small amounts that they must be measured in portions per million, or even parts/billion.
6. Diluent
- A diluent is a fluid that is used to dilute the contents of a vaccine to the proper concentration prior to its use.
- The most frequently used diluting agent is sterile drinking water.
7. Adjuvant
- Certain vaccines may also contain adjuvants. A adjuvant can boost the immune response of the vaccine, usually because it keeps the vaccine near the injection site for few minutes or by activating local immune cells.
- The adjuvant could consist of a small amount of aluminum salts (like aluminium phosphate, aluminum hydroxide, or potassium sulphate of aluminium).
- It has been proven that aluminium does not to cause permanent health issues Humans consume aluminum regularly from food and drinking.
How are vaccines made?
The majority of vaccines have been used for a long time with millions of people getting the vaccines in a safe manner each year. Like all medicines every vaccine must undergo exhaustive and rigorous tests to make sure it’s safe before it is able to be included into a nation’s vaccination program.
Every vaccine being developed has to undergo screenings and assessments to determine which antigens should be used to trigger an immunological response. The preclinical stage is conducted without the need to test on human beings. A vaccine that is experimental is initially test on animals to determine its safety as well as its potential to stop diseases.
If the vaccine stimulates an immunological response it’s later studied on humans in clinical trials. three phases.
Phase 1
- The vaccine is administered to a select group of patients to evaluate its safety, verify that the immune response it creates and to determine the appropriate dosage.
- Typically, in this stage, the vaccines are evaluated in healthy adults who are young and healthy.
Phase 2
- It is administered to a number of volunteers to determine its efficacy and safety. create an immune response.
- Participants in this stage have similar characteristics (such as gender, age) similar to the individuals who are the ones for whom the vaccine was designed.
- There are often several trials during this phase to assess different age groups as well as different types of vaccines.
- A group of people who did not get the vaccine is normally part of the phase as an illustrative group to determine if the changes observed in the group of people who have been vaccinated can be attributed to the vaccine or are the result of random chance.
Phase 3
- The vaccine is then given to thousands of people and it is then compared to a comparable group of people who did not receive the vaccine, however, they received a comparator to see if the vaccine is efficient against the diseases it was designed to guard against, as well as to determine the safety of the vaccine in a more extensive group of individuals.
- Most often, studies in phase 3 are conducted across several countries and at multiple locations within a nation to ensure the vaccine’s effectiveness are applicable to a variety of groups.
- In the Phase 2 and 3 trials, both the participants and the scientists who conduct the research are not aware of who taken the vaccine that is being evaluated or the comparator. This is known as “blinding” and is necessary to ensure that neither participants nor the researchers are influenced in their evaluation of effectiveness or safety because of knowing who received the product.
- Once the trial has ended and the final results have been finished, the trial participants as well as the trial scientists are informed of who got the vaccine and which were given the comparator.
- If the findings of all clinical trials are released the next step is needed that include reviews of the efficacy and safety to obtain public health and regulatory policy approvals. Officials from every country examine the results of the study and decide if they want to approve the use of the vaccine.
- The vaccine must be shown that it is safe and efficient in an entire population before it is accepted for inclusion in an immunization program for the entire nation.
- The standards for the safety and effectiveness of vaccines is extremely high, and it is important to recognize the fact that vaccines are offered to those who are healthy and free of the disease.
The monitoring continues on a regular basis following the introduction of the vaccine. There are methods to check the effectiveness and safety of every vaccine. Scientists can monitor the impact of vaccines and safety while they are utilized by an extensive number of people over a lengthy period of time. The data is used to alter the rules regarding the use of vaccines to maximize the impact of these vaccines, and allow the use of vaccines to be tracked throughout the duration of its usage. When a vaccine is put in use, it has to be monitored continuously to make sure that it remains secure.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.