- Hepatitis E is a kind of viral hepatitis caused by infection with the hepatitis E virus (HEV). Hepatitis E is transmitted mostly through a fecal-oral route, similar to hepatitis A, although the viruses are unrelated.
- In hindsight, the earliest known hepatitis E pandemic occurred in New Delhi in 1955, but the virus was not discovered until 1983 by Soviet scientists studying an outbreak in Afghanistan.
- HEV is a positive-sense, single-stranded, envelopeless, RNA icosahedral virus and one of the five known human hepatitis viruses A, B, C, and E.
- Similar to hepatitis A, hepatitis E typically follows an acute and self-limiting course of illness (the condition is temporary and the individual recovers) with low death rates in resource-rich areas; however, it can be significantly more severe in pregnant women and individuals with a compromised immune system, with much higher death rates.
- In pregnant women, particularly during the third trimester, the condition is typically more severe and is linked with a clinical state known as fulminant liver failure, with mortality rates of approximately 20%.
- Unlike pregnant women, organ transplant recipients who use immunosuppressants to avoid organ rejection might develop a slower and more persistent form of hepatitis E called chronic hepatitis E, which is identified after three months of continuous viremia.
- HEV can be clustered genetically into eight genotypes, with genotypes 3 and 4 typically causing chronic hepatitis in immunocompromised individuals. In 2017, it was predicted that more than 19 million people have hepatitis E.
- Men aged 15 to 35 are at the highest risk of HEV infection. In China, the HEV 239 vaccine has been licensed for use.
Hepatitis E Virus
- Hepatitis E virus (HEV) is the leading cause of enterically transmitted non-A non-B hepatitis virus (NANBH), which is particularly prevalent in poor nations, such as India. The virus is extremely similar to HAV.
- The virus was identified for the first time by electron microscopy of feces contaminated with intestinal NANBH. Hepatitis E virus is now categorized as a member of the Calciviridae family. It is similar to calciviruses like Norwalk virus.
- Yet, the HEV genome is distinct from those of other calciviruses, and examination of the genome sequence reveals that it is more comparable to rubella virus.
- Thus, HEV must still be classified. It is a spherical virus without an envelope approximately 32–34 nm in diameter. On the surface of the virion are pits and spikes. It’s an icosahedron. Around 7.6 kb of positive-sense, single-stranded RNA is included within the virus.
- The genome of the virus has three open reading frames (ORFs). ORF1 is the largest open reading frame, encoding the nonstructural protein responsible for viral replication. ORF2 encodes the capsule protein. ORF3’s function remains unknown.
- The virus is resistant to heat. The cloning of the viral genome revealed that there is only one serotype of HEV. With HEV infection, the IgM antibody titer rises first and rapidly declines following infection, becoming nearly undetectable within six months.
- Nevertheless, anti-HEV IgG persists for longer than six months. Antibody IgG appears to provide protection against reinfection with HEV. Hepatitis E virus typically causes an acute, self-limiting illness comparable to that caused by HAV.
- Initially, it was misidentified as HAV due to clinical and epidemiological similarities. Currently, Hepatitis E virus infection is recognized as a unique clinical entity, distinct from HAV infection. Clinically, HEV infection differs from HAV infection in that it causes acute disease and symptoms appear much later than in HAV disease.
- The HEV infection incubation period ranges from 2 to 9 weeks, with an average of 35 days. In pregnant women, the Hepatitis E virus produces a dangerous infection. It causes severe disease in pregnant women, particularly in the latter trimester, and has a high fatality rate of 15–20%. The most significant causes of death are encephalopathy and disseminated intravascular coagulation.
- Very high rates of fulminant hepatic failure are observed in infected pregnant women. Infection with the Hepatitis E virus does not appear to induce persistent liver disorders. HEV infection is characterized by portal tract infiltration by lymphocytes and polymorphonuclear leukocytes, inflated hepatocytes, development of acidophilic bodies, and intralobular necrosis of hepatocytes.
- The Hepatitis E virus is spread globally. It is mainly prevalent in poorer nations. There have been reports of HEV epidemics in India, Pakistan, Nepal, China, Burma, North Africa, and Mexico. Between 1986–1988, a similar outbreak affecting approximately 10,000 persons was reported in north-east China.
- The greatest HEV pandemic in India occurred in Delhi during the winter of 1955–1956, affecting over 30,000 people. Anti-HEV antibodies are detected in the serum of up to 60% of children younger than 5 years old in India.
- In poorer countries, climate, sanitation, and personal hygiene all contribute to the outbreak of the disease. Due to fecal pollution of water in endemic areas, Hepatitis E virus is primarily transmitted via the fecal–oral route.
- Water contaminated by feces is the primary source of illness. The reservoir of HEV is unknown, but transmission may occur through animals.
Taxonomy and Classification of Hepatitis E Virus
- Based on its superficial similarities in shape and genetic organization to caliciviruses, HEV was previously classified as a member of the Caliciviridae family.
- However, additional research indicated that the HEV genome lacks considerable sequence similarities with caliciviruses and that its codon use and genomic structure are distinct from those of caliciviruses.
- In the Eighth Report of the International Committee on the Taxonomy of Viruses (ICTV), HEV was therefore declassified from the family Caliciviridae and placed in the only genus Hepevirus. ICTV has not yet officially recognized the proposed family name Hepeviridae.
- The species in the genus Hepevirus comprises the four recognized major genotypes of HEV: genotype 1 (primarily Burmese-like Asian strains), genotype 2 (a single Mexican strain), genotype 3 (strains from rare endemic cases in the United States, Japan, and Europe, and swine strains from pigs in industrialized nations), and genotype 4. (variant strains from sporadic cases in Asia, and swine strains from pigs in both developing and industrialized countries).
- The discovery of avian HEV from chickens represents a possible new species in the genus Hepevirus. The identity of the nucleotide sequence varies by 20–30% between genotypes and by 40–50% between avian HEVs and mammalian HEVs. Despite the vast nucleotide sequence differences, it appears that only one serotype of HEV exists.
Structure of Hepatitis E Virus
- HEV is a symmetrical, icosahedral, spherical, approximately 32–34 nm in diameter, envelope-less viral particle.
- The sole known structural protein on the virion is the capsid protein expressed by the open reading frame (ORF) 2 gene of HEV.
- When produced in baculovirus, the N-terminal shortened capsid protein, consisting of amino acid residues 112–660, can self-assemble into empty virus-like particles.
- HEV virions had a buoyant density of 1.35–1.40 g cm–3 in CsCl and 1.29 g cm–3 in potassium tartrate and glycerol. The sedimentation coefficient of virion is 183 S.
- According to reports, HEV virion is stable when exposed to trifluorotrichloroethane but vulnerable to low-temperature storage and iodinated disinfectants.
- HEV virion is more heat-labile than HAV, another hepatitis virus transmitted enterically. At 60 C for 1 hour, HAV was only 50% inactivated, whereas at 66 C, it was nearly completely inactivated.
- In contrast, HEV was approximately 50% inactivated at 56 C and nearly completely (96%) inactivated at 60 C. The feces-to-mouth transmission pathway implies that HEV is resistant to inactivation by acidic and somewhat alkaline intestinal conditions.
Genome of Hepatitis E Virus
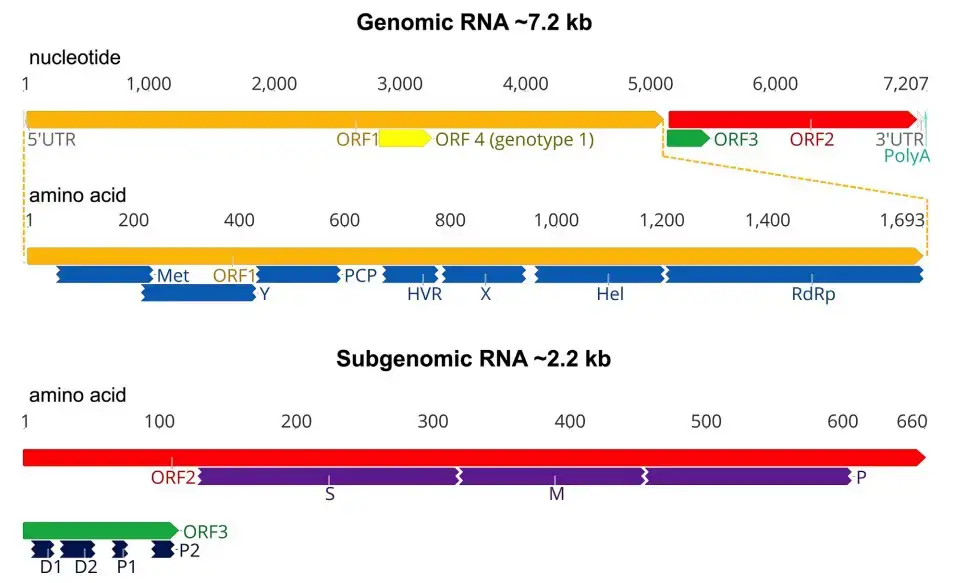
- The genome of HEV is a single-stranded, positive-sense RNA molecule of roughly 7.2 kb in length, with a cap structure at its 50 end. A brief 50 noncoding region (NCR) of around 26 bp, three open reading frames (ORFs 1–3), and a 30 NCR make up the viral genome. ORF2 and ORF3 do not overlap with ORF1.
- ORF1 at the 50 end encodes nonstructural viral proteins that are involved in viral replication and protein processing. Based on sequence analysis and comparability with other single-strand positive-sense RNA viruses, ORF1 was shown to have functional motifs typical of methyltransferases, papain-like cystein proteases, helicases, and RNA-dependent RNA polymerases.
- ORF2, which is positioned at the 30 end, encodes the main capsid protein, which comprises a signal peptide sequence and three possible glycosylation sites. The capsid protein possesses the highest immunogenic epitope, generates neutralizing antibodies against HEV, and is the focus of HEV vaccine development.
- A tiny phosphoprotein linked with the cytoskeleton is encoded by ORF3. The N-terminal cysteine-rich region of ORF3 binds HEV RNA and forms a complex with the capsid protein. The C-terminal portion of the ORF3 protein is a multifunctional domain that may contribute to HEV virion formation and viral pathogenicity.
- Antibodies to ORF3 protein have been found in rhesus monkeys and hens experimentally infected with HEV; nevertheless, it is unknown whether ORF3 is a structural protein in the virion.
- The lack of an effective cell culture method for the propagation of HEV has hindered the understanding of the transcription and translation mechanisms of HEV genes. Using an in vitro HEV replicon system, a bicistronic subgenomic mRNA encoding both ORF2 and ORF3 proteins was discovered and shown to encode both proteins.
- In infected liver tissues, subgenomic mRNAs were also discovered. The ORF3 gene contains a cis-reactive element and encodes a protein necessary for virus infectivity in vivo. Recent research has demonstrated, however, that the production of ORF3 protein is not necessary for virus replication, virion assembly, or infection of hepatoma cells in vitro.
- The translation and post-translational mechanisms of the ORF1 polyprotein are not well understood. It was discovered that the nonstructural ORF1 polyprotein, when expressed in baculovirus, processes into smaller proteins that correspond to the ORF1 polyprotein’s expected functional domains. In contrast, no processing of the ORF1 polyprotein was observed in bacterial or mammalian expression systems.
Epidemiology of Hepatitis E Virus
- Hepatitis E virus (HEV) causes around 20 million new infections and over 55,000 deaths annually, per the World Health Organization (WHO).
- The incidence of HEV infection is universal, however it is most prevalent in underdeveloped nations. Asia, Africa, the Middle East, and Central America have the highest frequency of HEV infection.
- In North Africa and the Middle East, HEV is the second leading cause of sporadic hepatitis. There have been sporadic reports of HEV infection in western nations, primarily among travelers to HEV-endemic regions.
- Between 2009 and 2010, the total seroprevalence of HEV in the United States was reported to be 6%. The probability of HEV seropositivity increases with age, international birth, Hispanic ethnicity, and meat eating (> 10 times per month).
- Although HEV was originally believed to be endemic exclusively in developing countries in Asia and Africa, it has been shown that it is a prevalent zoonotic illness in Europe’s high-income countries, where it is acquired through pigs.
Transmission of Hepatitis E Virus
- Hepatitis E virus (HEV) is typically transmitted through the fecal-oral route via contaminated water, leading to large waterborne outbreaks, especially in developing countries.
- HEV can also be a rare fecally transmitted zoonotic infection, and person-to-person transmission is uncommon.
- In endemic areas, HEV can be transmitted via blood transfusion, and vertical transmission from infected mothers to their infants can result in significant perinatal mortality and fetal loss.
- Transmission of HEV via breast milk is not well-studied, but the virus has been isolated in breast milk, and serum titers were found to be comparable.
- The incubation period of HEV infection ranges from 28 to 40 days.
- After ingestion, HEV is absorbed through the gastrointestinal mucosa into the portal circulation, eventually reaching the liver where it replicates.
- HEV replication has not been observed in tissues other than the liver.
- HEV can cause liver damage with morphologic changes resembling both cholestatic and classic acute hepatitis, but these features are not specific to hepatitis E.
- HEV is excreted in feces.
Genomic Replication Strategy
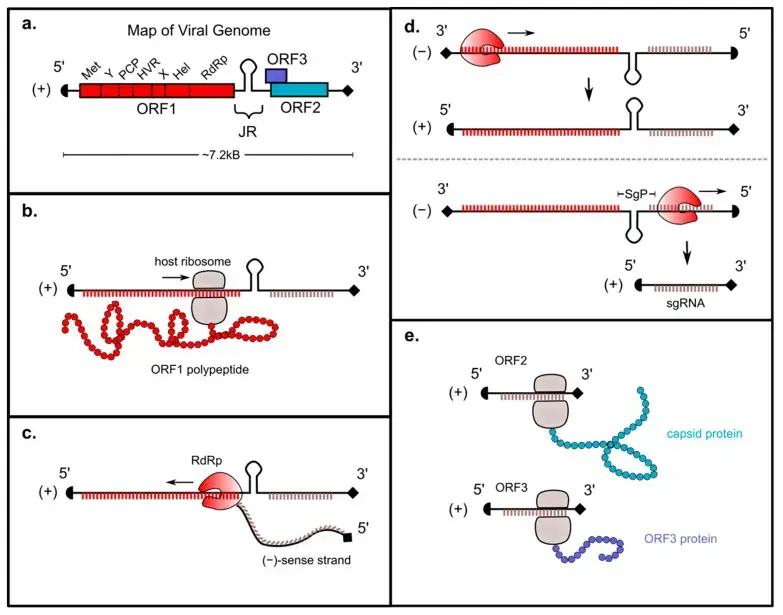
- The (+)-sense HEV genome is capped at the 5′ end and polyadenylated at the 3′ end; consequently, it can be immediately translated by host ribosomes.
- After viral entrance and uncoating, the HEV ORF1 region is translated into a polyprotein containing RdRp. The topic of whether this polyprotein is then digested into smaller subunits will be covered in greater detail later in this review.
- The RdRp then uses the (+)-sense strand as a template to transcribe a full-length ()-sense genome. This ()-sense genome acts as a template for transcription of two distinct (+)-sense RNAs: a full-length (+)-sense transcript that is packed into progeny virions, and a smaller “subgenomic” (sg) (+)-sense RNA encoding ORFs 2 and 3.
- The subgenomic RNA is translated into the capsid protein and HEV viroporin, which are respectively necessary for viral packaging and release.
Replication of Hepatitis E Virus/Life cycle of hepatitis E virus (HEV)
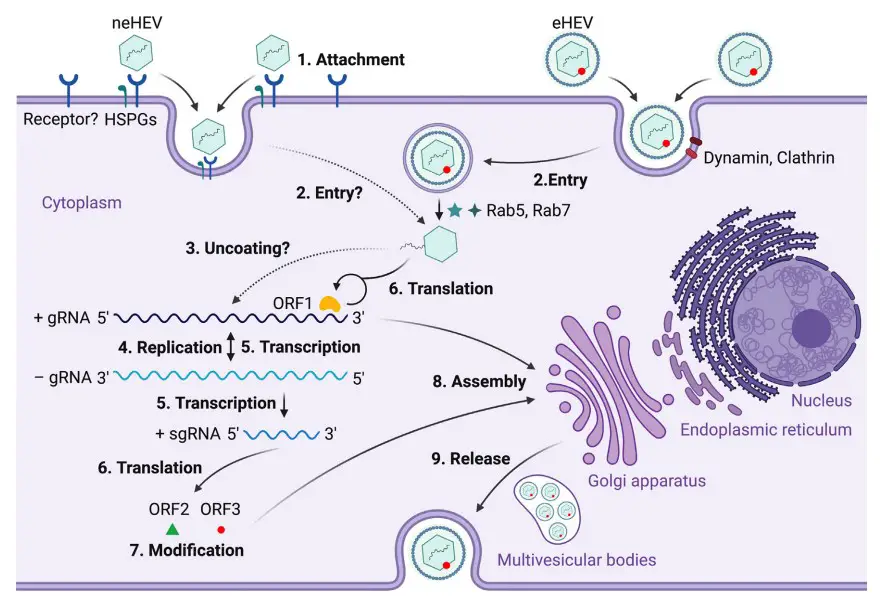
- Non-enveloped HEV (neHEV) particles bind to heparan sulfate proteoglycans (HSPGs) on the surface of liver cells and enter through an unknown specific cellular receptor.
- Quasi-enveloped HEV (eHEV) particles enter liver cells through dynamin-dependent, clathrin-mediated endocytosis, which requires small GTPases Ras-related proteins Rab5 and Rab7.
- The capsid protein of HEV is uncoated, and the viral genomic RNA is released to the cytosol through an unknown process.
- The viral genomic RNA serves as mRNA for ORF1 polyprotein translation and also synthesizes a complementary negative-sense RNA to serve as a template for HEV replication.
- The intermediate negative-sense RNA acts as a template for the transcription of full-length genomic as well as subgenomic mRNAs (sgRNAs).
- ORF1 polyproteins are translated from the full-length genomic RNA, and ORF2 capsid protein and ORF3 multifunctional protein are translated from sgRNAs.
- ORF2 and ORF3 undergo post-translational modifications such as glycosylation, phosphorylation, and palmitoylation.
- ORF2 capsid protein self-assembles into virus-like particles (VLPs) and binds to newly synthesized positive-sense genomic RNA to form progeny HEV virions.
- ORF3 regulates the host environment by interacting with several cellular proteins to promote viral replication and virion secretion. ORF3 binds to TSG101 involved in the ESCRT pathway, facilitating the budding of nascent virions into multivesicular bodies. These bodies fuse with the plasma membrane, and the virions are released from the liver cells either into the bloodstream as eHEV or in the bile duct as neHEV.
Pathogenesis of Hepatitis E Virus
- The mechanism of how hepatitis E virus (HEV) reaches the liver and its pathogenesis is not well understood. It is assumed to be transmitted through the fecal-oral route, but it is unclear how the virus travels to the liver. There is evidence of extra-hepatic HEV replication in the small intestine, lymph nodes, colon, and liver of pigs, suggesting the possibility of viral replication in the intestinal tract before reaching the liver. Once in the liver, HEV replicates in the cytoplasm of hepatocytes and is released into both the blood and bile.
- Liver damage caused by HEV infection may be immune-mediated by cytotoxic T cells and natural killer cells since HEV is not directly cytopathic. HEV is shed in the stool, and a serological anti-HEV response is typically detected in patients at the onset of illness. Anti-HEV IgMs can persist for several months and are detected in the early phase of clinical illness, while anti-HEV IgG appears shortly after the IgM response and can last several years. The existence of only one serotype of HEV makes cross-protection possible.
- The exact reasons behind why a hepatitis E infection becomes fulminant are still unclear. However, research suggests that both Th1 and Th2 type immune responses could play a role in liver failure. It is believed that host factors, rather than virus genotype, variants, or specific amino acid substitutions, are responsible for the development of fulminant hepatitis.
- In patients with fulminant hepatitis E, there are higher levels of anti-HEV IgM and IgG titers, along with increased concentrations of IFN-γ, TNF-α, IL-2, and IL-10. CD4+ T cells are also more frequent in the liver, and CD8+ T cells have been shown to infiltrate the liver of these patients. Therefore, cytotoxic CD8+ T cells could be particularly important in the pathogenesis of fulminant hepatitis.
- It has been observed that women with acute liver failure (ALF) exhibit a reduced expression of toll-like receptor (TLR) 3/TLR7/TLR9. This impaired monocyte-macrophage function in pregnant women with ALF could contribute to an inadequate innate immune response, leading to the development and severity of ALF. Additionally, high concentrations of cytokines such as TNF-α, IL-6, IFN-γ, and TGF-β1 may also be associated with an adverse pregnancy outcome.
- In HEV-positive pregnant women with fulminant hepatic failure, the concentrations of estrogen, progesterone, and β-human chorionic gonadotrophin are higher than in HEV-negative pregnant women with the same condition. An in vitro study has shown that serum from pregnant women, especially those in the third trimester, enhances the replication of HEV by inhibiting estrogen receptor and type I IFN expression.
Clinical Manifestations of Hepatitis E Virus
- HEV typically causes a self-limiting, acute, icteric hepatitis with symptoms like jaundice, fever, anorexia, abdominal pain, and malaise.
- Clinical disease is more common in adults than children.
- Mortality rates associated with HEV are low, estimated to be around 1% in the general population.
- Pregnant women are at higher risk and may have mortality rates as high as 20%.
- The incubation period ranges from 2 to 9 weeks.
- In developed countries, HEV infections have a similar presentation to those in endemic regions but with a higher mortality rate of 8% to 11%.
- Most autochthonous HEV infections are self-limiting, but 8% to 11% of infected individuals can develop fulminant hepatitis and liver failure.
- Individuals with underlying chronic liver disease may have a poor outcome, with mortality approaching 70%.
- Patients with chronic liver disease and HEV superinfection have a 1-year mortality rate of 70%.
- Chronic HEV infection has been observed in patients receiving immunosuppressive therapy after organ transplantation.
Laboratory Diagnosis of Hepatitis E Virus
Hepatitis E virus (HEV) can be diagnosed through laboratory testing. Here are some common methods used for laboratory diagnosis of HEV:
- Serological tests: These tests detect the presence of anti-HEV antibodies (IgM and IgG) in the blood. IgM antibodies appear in the blood during the acute phase of infection, while IgG antibodies appear later and persist for a longer time. Serological tests are useful for diagnosing both acute and past HEV infections.
- Nucleic acid tests: These tests detect the viral RNA in blood or stool samples. Nucleic acid tests are highly sensitive and specific for detecting HEV RNA during the acute phase of infection. They are also used to monitor chronic HEV infection in immunocompromised patients.
- ELISA assays: These tests are used to detect HEV antigens in blood or stool samples. ELISA assays are highly specific and sensitive for detecting HEV during the acute phase of infection.
- PCR (polymerase chain reaction): This test amplifies and detects the viral RNA in blood or stool samples. PCR is highly sensitive and specific for detecting HEV RNA during the acute phase of infection.
- Liver function tests: These tests measure levels of liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin. Elevated levels of these enzymes may indicate liver damage caused by HEV infection.
- Imaging studies: Imaging studies, such as ultrasound, can be used to assess the liver and detect any abnormalities, such as liver enlargement or fluid accumulation in the abdomen.
It is important to consult with a healthcare provider for proper interpretation of test results and diagnosis of HEV infection.
Treatment of Hepatitis E Virus
- Acute infection with the Hepatitis E virus (HEV) is often self-limiting and requires only supportive care. Within one to six weeks following the commencement of the illness, abnormal biochemical tests typically normalize; however, individuals who develop fulminant liver failure require a liver transplant.
- Uncertain is the function of antiviral therapy, such as Ribavirin, in the treatment of acute HEV infection in immunocompromised patients.
- Due to the danger of teratogenicity, pregnant individuals with acute HEV infection should not receive ribavirin. In immunocompromised individuals with chronic HEV infection, it is advisable to modify immunosuppressive treatment and administer antiviral medicines such as ribavirin, peginterferon, or both.
Prevention and Control of Hepatitis E Virus
Prevention and control of hepatitis E virus (HEV) infection can be achieved through several measures, including:
- Improving sanitation: HEV is primarily transmitted via the fecal-oral route. Therefore, improving sanitation and hygiene practices, such as ensuring access to clean water and proper disposal of sewage, can help prevent HEV infection.
- Safe food handling: HEV can be transmitted through contaminated food, particularly undercooked or raw meat from infected animals. Therefore, it is important to practice safe food handling, including cooking meat thoroughly and washing fruits and vegetables before consumption.
- Vaccination: In areas where HEV is endemic, vaccination can be an effective preventive measure. Two vaccines are currently available for use in China and several other countries. However, the vaccines are not widely available and are not approved for use in many other countries.
- Blood safety: Screening of blood and blood products for HEV can help prevent transmission of the virus through blood transfusion.
- Education and awareness: Education and awareness campaigns can help promote safe practices and prevent HEV infection. This can include information on safe food handling, proper sanitation practices, and vaccination.
- Treatment of sewage: Treatment of sewage can help in the prevention and control of HEV. This is particularly important in areas where HEV is endemic, as the virus can persist in sewage and infect individuals who come into contact with contaminated water or food.
Overall, a multifaceted approach is needed for the prevention and control of HEV infection. This includes improvements in sanitation, safe food handling, vaccination, blood safety, education and awareness, and treatment of sewage.
FAQ
What is Hepatitis E virus?
Hepatitis E virus (HEV) is a RNA virus that causes liver inflammation and can lead to acute and chronic hepatitis.
How is HEV transmitted?
HEV is transmitted primarily through the fecal-oral route, usually via contaminated water or food. Person-to-person transmission is rare.
Who is at risk of HEV infection?
HEV infection is more common in developing countries with poor sanitation and hygiene practices. Travelers to these areas, people with chronic liver disease, and pregnant women are at increased risk.
What are the symptoms of HEV infection?
Symptoms of HEV infection can include jaundice, malaise, anorexia, fever, abdominal pain, and arthralgia. However, many people with HEV infection may not have any symptoms.
How is HEV diagnosed?
HEV can be diagnosed through blood tests that detect antibodies or viral RNA. Liver function tests can also provide information on the severity of liver damage.
Is there a specific treatment for HEV?
There is no specific treatment for HEV, but supportive care such as rest, hydration, and management of symptoms can help improve outcomes. In severe cases, hospitalization may be required.
Can HEV be prevented?
HEV can be prevented by practicing good hygiene and sanitation, avoiding consumption of contaminated water and food, and getting vaccinated (if available).
Is there a vaccine for HEV?
Yes, there are currently two vaccines available for preventing HEV infection. However, these vaccines are not widely available in all countries.
Can HEV lead to chronic liver disease?
HEV can lead to chronic liver disease in immunocompromised individuals, such as organ transplant recipients or people with HIV. Chronic HEV infection can also occur in pregnant women.
Is HEV contagious?
HEV is not highly contagious and is not transmitted through casual contact. However, precautions should still be taken to prevent transmission through contaminated water or food.
References
- Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014 Jan;27(1):116-38. doi: 10.1128/CMR.00057-13. PMID: 24396139; PMCID: PMC3910910.
- Waqar S, Sharma B, Koirala J. Hepatitis E. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532278/
- Khuroo, M. S., & Khuroo, M. S. (2015). Hepatitis E: an emerging global disease – from discovery towards control and cure. Journal of Viral Hepatitis, 23(2), 68–79. doi:10.1111/jvh.12445
- Kenney SP, Meng XJ. Hepatitis E Virus Genome Structure and Replication Strategy. Cold Spring Harb Perspect Med. 2019 Jan 2;9(1):a031724. doi: 10.1101/cshperspect.a031724. PMID: 29530948; PMCID: PMC6314074.
- LeDesma R, Nimgaonkar I, Ploss A. Hepatitis E Virus Replication. Viruses. 2019 Aug 6;11(8):719. doi: 10.3390/v11080719. PMID: 31390784; PMCID: PMC6723718.
- Nimgaonkar, I., Ding, Q., Schwartz, R. et al. Hepatitis E virus: advances and challenges. Nat Rev Gastroenterol Hepatol 15, 96–110 (2018). https://doi.org/10.1038/nrgastro.2017.150
- Meng, X. J. (2008). Hepatitis E Virus. Encyclopedia of Virology, 377–383. doi:10.1016/b978-012374410-4.00418-0
- Tam, A. W., Yarbough, P. O., & Bradley, D. W. (1999). HEPATITIS E VIRUS. Encyclopedia of Virology, 669–676. doi:10.1006/rwvi.1999.0121
- Karnsakul, W., & Schwarz, K. B. (2011). Hepatitis. Infectious Diseases of the Fetus and Newborn, 800–813. doi:10.1016/b978-1-4160-6400-8.00025-0
- Panda, Subrat & Varma, Pavan. (2013). Hepatitis E: Molecular Virology and Pathogenesis. Journal of Clinical and Experimental Hepatology. 3. 114–124. 10.1016/j.jceh.2013.05.001.
- Ahmad, Imran & Holla, Prasida & Jameel, Shahid. (2011). Molecular Virology of Hepatitis E Virus. Virus research. 161. 47-58. 10.1016/j.virusres.2011.02.011.
- Wang, Bo & Meng, Xiang-Jin. (2021). Structural and Molecular Biology of Hepatitis E Virus. Computational and Structural Biotechnology Journal. 19. 10.1016/j.csbj.2021.03.038.
- https://emedicine.medscape.com/article/178140-overview
- https://www.frontiersin.org/articles/10.3389/fmicb.2018.00266/full
- https://www.hopkinsmedicine.org/health/conditions-and-diseases/hepatitis/hepatitis-a
- https://www.cdc.gov/hepatitis/hev/index.htm#:~:text=Hepatitis%20E%20is%20a%20liver,virus%20%E2%80%93%20even%20in%20microscopic%20amounts.
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-e