Hepatitis C is a viral infection that causes liver inflammation, sometimes leading to serious liver damage. The hepatitis C virus (HCV) is spread through contaminated blood, and it can be acute or chronic. Acute hepatitis C is a short-term illness that occurs within the first 6 months after someone is exposed to the virus. Chronic hepatitis C is a long-term illness that can last for years or even decades. Chronic hepatitis C can lead to serious health problems, including liver damage, liver failure, and liver cancer.
There is no vaccine for hepatitis C, but it can be treated with medications that can cure the infection. If you think you might have been exposed to hepatitis C, it is important to get tested and seek medical attention as soon as possible.
Hepatitis C virus is a member of the genus Hepacivirus and the family Flaviviridae. Prior to 2011, it was believed to be the only species in its genus. However, a member of this genus, canine hepacivirus, has been detected in dogs. This genus also contains at least one virus that infects horses. Multiple more viruses belonging to the genus have been identified in bats and rodents.
Geographical distribution of Hepatitis C virus
It is estimated that HCV infects around 3% of the global population. According to recent estimates, 170 million people are living with HCV infection over the world. It’s the leading reason why people need parenteral NANBH treatment. Parental anti-trypanosomal treatment has been linked to a 22% prevalence incidence in Egypt. Similarly high rates of prevalence are seen in healthy blood donors. Central Europe, the Middle East, Spain, Italy, and Japan all have exceptionally high rates of hepatitis C virus infection.
Structure of Hepatitis C virus
- Hepatitis C virus is the only member of the RNA-containing virus family Flaviviridae belonging to the genus Hepacivirus.
- HCV appears to share tight ties with hepatitis D, dengue, and yellow fever viruses. Due to its structural and organisational similarities with flavivirus, it has been classified as a new genus within the Flaviviridae family: Hepacivirus.
- The Hepatitis C virus exhibits the following morphological characteristics:
- It is an enclosed, spherical, 9.4 kb, single-stranded RNA virus with a 55 nm diameter.
- The genome encodes ten structural and regulatory proteins using roughly 9500 base pairs.
- The structural proteins consist of the core and two envelope proteins, E1 and E2. These two envelope proteins vary during infection because their genes contain hypervariable regions.
- The viruses are susceptible to both ether and acid.
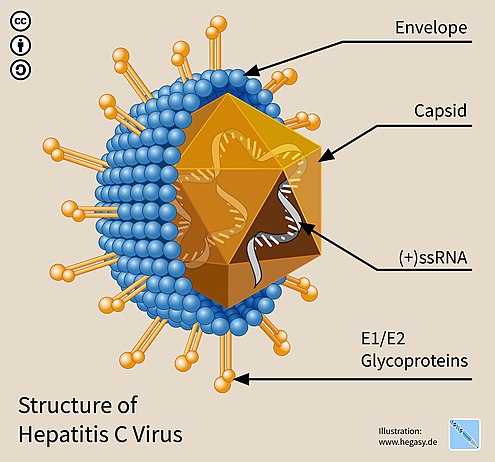
E1 and E2 glycoproteins
- When embedded in the envelope of HCV, E1 and E2 form covalent bonds and are maintained by disulfide bonds. According to electron microscope photos, E2 appears to protrude 6 nm from the envelope membrane and has a globular shape.
- These glycoproteins play a crucial role in hepatitis C’s interactions with the immune system. Hypervariable region 1 (HVR1) is a hypervariable region discovered on the E2 glycoprotein.
- HVR1 is very flexible and accessible to neighbouring molecules. HVR1 assists E2 in protecting the virus from the immune system. It stops CD81 from attaching to its appropriate viral receptor.
- Moreover, E2 can protect E1 from the immune system. Although the amino acid sequence of HVR1 is relatively varied, this region of many E2 glycoproteins shares similar chemical, physical, and structural properties.
Genome Organization of hepatitis C virus
- The HCV genome is a 9.6 kb positive-polarity single-stranded RNA molecule. It is bordered by two highly organised noncoding regions (NTRs).
- The 50 NTR features an internal ribosome entry site (IRES), which facilitates cap-independent translation of a polyprotein of approximately 3000 amino acids. The 30 NTR consists of a 40-nt-long variable region, a polypyrimidine tract (length diverse), and a 98-nt-long highly conserved 30 terminal X-tail.
- Both the 50 and 30 NTRs contain essential cis-acting RNA elements (CREs) for viral replication. Three more CREs are positioned inside the 30 terminal region of the NS5B gene (5BSL3.1–5BSL3.3), of which 5BSL3.2 is essential for viral RNA replication by forging a long-distance RNA–RNA interaction with the middle loop in the X-tail.
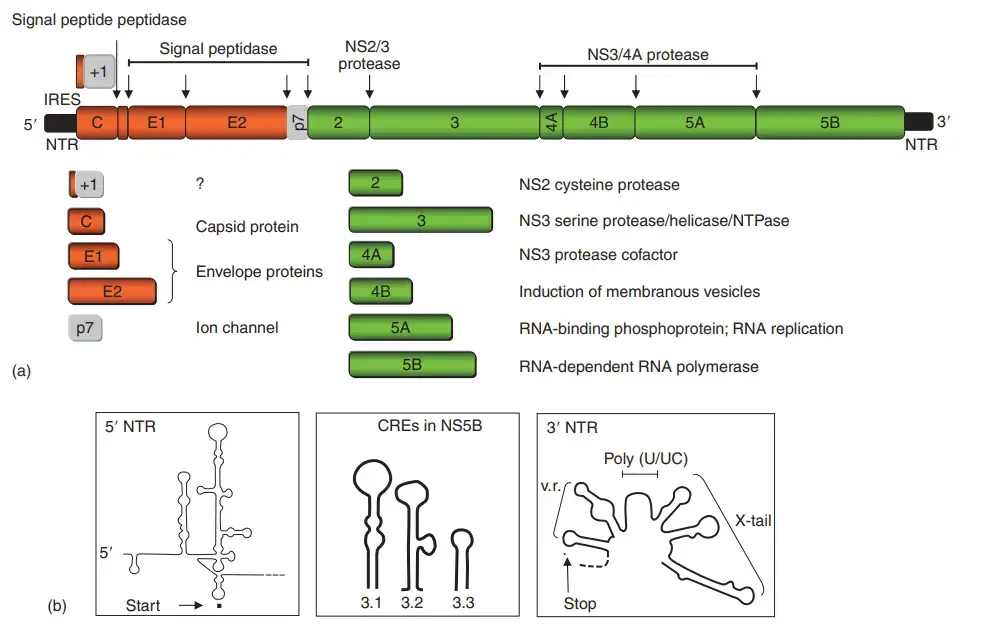
- Figure depicts the structure of the HCV polyprotein and the functions of the different gene products (a). The structural proteins, core and envelope proteins 1 (E1) and 2 (E2), are situated in the N-terminal portion of the polyprotein immediately before the p7 protein, which appears to be an ion channel and was therefore classified as a member of the viroporin protein family.
- The remainder encodes the nonstructural (NS) proteins NS2, NS3, NS4A, NS4B, NS5A, and NS5B. Figure 2(a) depicts the autocatalytic cleavage of the NS2–NS3 junction by the NS2 protein and the N-terminal protease domain of NS3.
- It is a dimeric cysteine protease whose active site is composite. The same NS3 protein domain contains a serine-type protease that, upon interaction with its cofactor NS4A, cleaves the residual NS protein junctions.
- In addition, the same protease cleaves two signalling molecules involved in the activation of the innate immune response (see below).
- In the C-terminal two-thirds of NS3, two additional enzymatic activity (RNA helicase and nucleoside triphosphatase) reside. NS4B, an integral membrane protein of 27 kDa, is primarily responsible for modifying intracellular membranes, namely membranous vesicles.
- These vesicles amass in the perinuclear region, are referred to as the membranous web, and serve as the replication site for viral RNA. Uncertainty surrounds the function of the phosphorylated zinc metalloprotein NS5A in the viral replication cycle.
- NS5A consists of an N-terminal amphipathic a-helix that serves as a membrane anchor and three domains that are predominantly cytosolic. The X-ray crystal structure of RNAbinding domain I has been determined, revealing that it is composed of homodimers that form a fundamental groove.
- The phosphorylation of NS5A is mediated by cellular kinases, specifically the an isoform of protein kinase CKI. The phosphorylation status of NS5A appears to influence replication efficiency, indicating that NS5A is a crucial replication factor.
- NS5A may potentially contribute to interferon-alpha (IFN-a) resistance, which is frequently reported in viruses of genotypes 1 and 4. NS5B, a 68-kilodalton protein, is the RNA-dependent RNA polymerase (RdRp).
- Similar to other polymerases with palm, finger, and thumb subdomains, it is a membrane-associated enzyme. However, it differs from the majority of other RdRps in that its active site is completely surrounded by subdomains of the finger and thumb.
- In addition to the polyprotein, a heterogeneous set of HCV proteins are expressed through either ribosomal frameshifting into the þ1 ORFor or internal translation initiation.
- The resultant proteins, generally referred to as core þ1, are not required for viral replication and synthesis in cell culture, and their function, if any, in vivo remains unknown.
Replication of Hepatitis C Virus
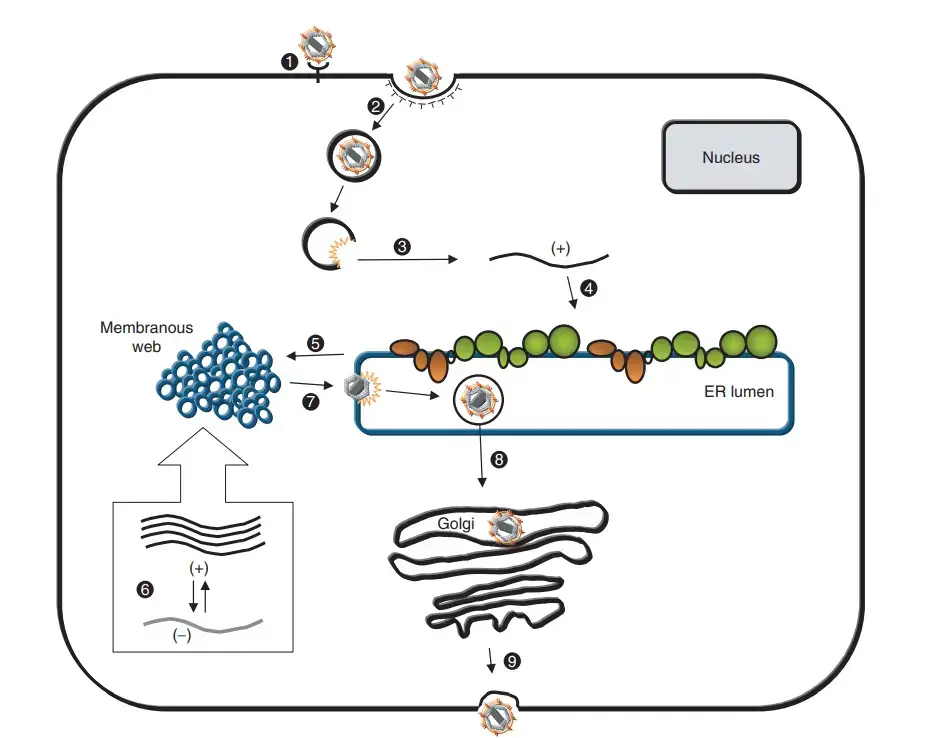
- HCV infection commences by attaching the envelope glycoprotein E1/E2 complex on the surface of the virus particle to its corresponding receptor(s) probably leading to clathrin-mediated endocytosis and a subsequent fusion step from inside an acidic endosomal compartment.
- Cellular components implicated in virus binding and entrance are glycosaminoglycans, scavenger receptor class B type 1 (SR-B1), CD81, and low-density lipoprotein (LDL) receptor.
- However, for most of these criteria, the specific role is not well understood and one or more additional factors may be necessary for productive entry.
- Upon release of the RNA genome, the polyprotein is expressed through IRES-dependent translation happening at the rough endoplasmic reticulum (rER) where host cell signal peptidases, signal peptide peptidases, and viral proteases catalyse polyprotein cleavage.
- During or after cleavage, the membrane-associated replication complex, which catalyses the RNA amplification via negative-strand RNA intermediates, is generated.
- These membrane-associated complexes are composed of viral RNA, viral proteins, and most likely host cell components.
- Newly generated positive-strand RNAs either are used for translation or serve as templates for additional RNA synthesis or interact with the core protein to construct the viral nucleocapsid.
- The E-proteins are maintained at rER membranes showing that viral envelopes are formed by budding into the lumen of this organelle.
- Progeny particles are assumed to be exported by the secretory route and after fusion of the transport vesicle with the plasma membrane, virions are liberated.
- However, most of these phases are poorly understood and various assumptions were made that are based on studies with heterologous expression systems and similarities to closely related viruses.
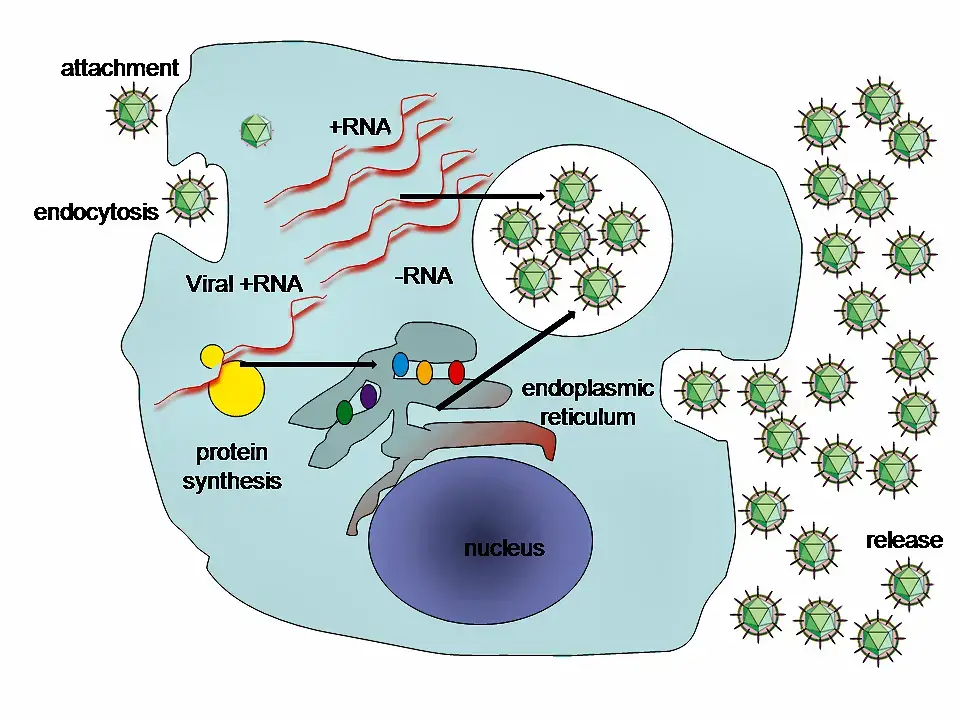
Molecular biology of Hepatitis C Virus
This virus’s proteins are located in the following order along the genome: Nonstructural protein 2 (NS2), core, envelope (E1), envelope (E2), p7, NS3, NS4A, NS4B, NS5A, NS5B, and finally the C terminal. Virus-encoded proteinases are essential for the production of fully formed nonstructural proteins (NS2 through NS5B). Encoded in both NS2 and the N-terminus of NS3, this proteinase cleaves the NS2/NS3 junction in the presence of metal. A serine protease located in NS3’s N-terminal region catalyses the subsequent cleavages downstream from this position.
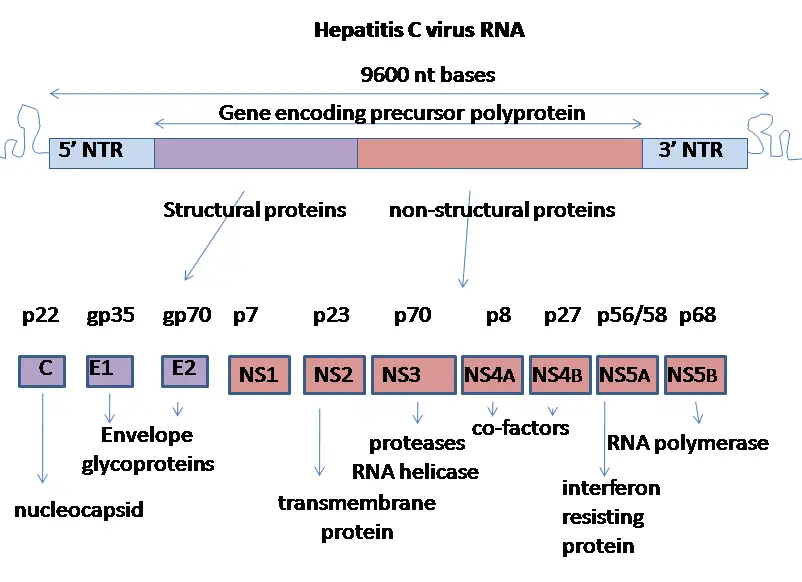
- The core protein consists of 191 amino acids and can be broken down into three domains based on their hydrophobicity. Domain 1 (residues 1-117) is mostly basic with two short hydrophobic regions; domain 2 (residues 118-174) is less basic and more hydrophobic; its C-terminus is at the end of p21; and domain 3 (residues 175-191) is highly hydrophobic and acts as a signal sequence
- E1 and E2 are two envelope proteins that play crucial roles in cell entrance and are heavily glycosylated. Fusogenic subunit E1 and receptor binding protein E2 are both components of the complex. Compared to E2, which has 11 N-glycosylation sites, E1 only has 4-5 N-linked glycans.
- However, the NS1 (p7) protein is essential for virus morphogenesis but not for genome replication. This protein, which consists of 63 amino acids, is found in the endoplasmic reticulum and is a membrane-spanning protein. Signal peptidases in the endoplasmic reticulum are responsible for p7 cleavage. P7 has two transmembrane domains that are joined by a cytoplasmic loop and face the lumen of the endoplasmic reticulum.
- The NS2 protein is a transmembrane enzyme that ranges in size from 21 to 23 kiloDalton (kDa).
- NS3 is a 67 kDa protein with NTPase/helicase activity at its carboxy terminus and serine protease activity at its amino terminus. It is a 54-amino-acid membrane protein that works as a cofactor of the proteinase and is found in the endoplasmic reticulum, where it forms a heterodimeric complex with NS4A.
- As a cofactor of the proteinase, NS4A is a membrane protein of 54 amino acids.
- A tiny (27 kDa), hydrophobic integral membrane protein with four transmembrane domains, NS4B is a key component of the NS4A protein complex. It helps bring in additional viral proteins and is found in the endoplasmic reticulum. As a result, the endoplasmic reticulum undergoes morphological modifications, culminating in the formation of a structure known as the membranous web.
- Hydrophilic phosphoprotein NS5A is involved in viral replication, regulation of cell signalling pathways, and the interferon response. It has been shown to interact with human VAP proteins that are located in the endoplasmic reticulum.
- The RNA-dependent RNA polymerase of viruses is the NS5B protein (65 kDa). For HCV, NS5B is essential for RNA replication because it catalyses the polymerization of ribonucleoside triphosphates (rNTP) using the viral positive sense RNA strand as a template. In several crystal forms, the structure of NS5B polymerase has been established using the same consensus sequence (BK) (HCV-BK, genotype 1). A right hand, complete with fingers, palm, and thumb, is a convenient metaphor for the structure. The palm structure of NS5B houses the protein’s ringed active site, which is exclusive to this enzyme. Recent structural analyses of the NS5B protein from the 1b genotype of the J4 (HC-J4) strain suggest the presence of an active site, which may regulate nucleotide binding and initiate de novo RNA synthesis. For RNA replication to begin, de novo synthesis is required to supply the essential primers. Researchers are currently trying to bind structures to this active site in an effort to modify its activity, hence blocking the replication of viral RNA.
- Moreover, a 11 protein has been identified. Frameshifting by one base pair in the capsid gene encodes this protein. It seems to have antigenic properties, although its role is unclear.
Pathogenesis and Immunity
The ability of HCV to remain cell-associated and prevent host cell death is the primary determinant of viral pathogenicity, which is responsible for persistent infection in the liver. The presence of a population of closely related but diverse viral genomes is one of the major reasons responsible for the persistence of HCV infection in the liver.
Pathogenesis of hepatitis C virus infections
Hepatocytes and potentially B lymphocytes are HCV’s natural targets. Recent research indicates that at least fifty percent of hepatocytes in patients with chronic hepatitis may be infected with HCV. Viremia persists and is associated with varying degrees of hepatic inflammation and fibrosis in the majority of infected individuals. Chronic hepatitis is distinguished by lymphocyte infiltration within the portal tract or liver lobule, as well as portal and periportal fibrosis. Chronic hepatitis induced by HCV is characterised by portal inflammation, interphase hepatitis, and lobular necrosis, among other histological characteristics.
Host immunity
Immunity against HCV may not be permanent, however HCV-specific blood antibodies are typically protective. Primarily cytotoxic T cells contribute to liver inflammation and, ultimately, tissue necrosis via cell-mediated immunity.
Epidemiology of Hepatitis C Virus
- Hepatitis C is a common viral infection, and it is estimated that about 71 million people worldwide are living with chronic hepatitis C. In the United States, it is estimated that about 2.4 million people are living with chronic hepatitis C.
- Risk factors for hepatitis C include injection drug use, receiving a blood transfusion or organ transplant before 1992, being on long-term hemodialysis, and having HIV. People who have tattoos or piercings, especially if they were done with non-sterile equipment, are also at increased risk of hepatitis C.
- Hepatitis C is more common in certain groups of people, such as baby boomers (born between 1945 and 1965), people who have ever injected drugs, and people with HIV. It is also more common in certain parts of the world, such as Eastern Europe, North Africa, and Central and East Asia.
Transmission of Hepatitis C Virus
The hepatitis C virus has never been detected in any other species. Infected patients provide a crucial reservoir of the virus. The most common routes of transmission are through contact with infected blood or blood products, as well as diseased organs. Among other ways, the hepatitis C virus might spread are:
- Blood transfusion: The most common way HCV is spread is through transfusions of blood. It is now believed that there is a risk of one case of transfusion-derived HCV for every 100,000 units transfused.
- Parenteral transmission: Transfusion of infected blood or blood products, organ transplantation from infected donors, and needle sharing among IV drug users all fall under the category of parenteral transmission of HCV. About half of all acute and chronic infections are linked to the use of intravenous medications.
- Sexual transmission: About 20% of hepatitis C cases are thought to be the result of sexual transmission. It suggests that the chance of transmitting a sexually transmitted disease like HIV is increased when there is already an infection in the body.
- Perinatal transmission: Transmission during pregnancy is conceivable, although it only happens in about 5% of babies delivered to women with HCV. Infants born to moms who are co-infected with HCV and HIV are at a greater risk of contracting the virus during pregnancy.
- Other methods of transmission: Other, less common, routes of transmission of HCV include hemodialysis, tattooing, body piercing, and acupuncture performed using unsterile equipment. Nearly 4% of all new infections are contracted by healthcare workers due to needle stick injuries and subsequent exposure to infectious blood. After a needle stick injury with an infected patient, the risk of contracting HCV appears to be between 0% and 7%.
Laboratory Diagnosis
There are several laboratory tests that can be used to diagnose hepatitis C virus (HCV) infection. These tests can detect the presence of HCV in the blood, as well as measure the amount of virus present (viral load) and determine the genotype (strain) of the virus. Here are some common laboratory tests used to diagnose HCV infection:
- HCV Antibody Test: This test looks for antibodies to HCV in the blood. Antibodies are proteins produced by the immune system in response to an infection. If the test is positive, it means that the person has been infected with HCV at some point in their life, although it does not necessarily mean that they are currently infected.
- HCV RNA Test: This test looks for the genetic material (RNA) of the HCV virus in the blood. If the test is positive, it means that the person is currently infected with HCV.
- HCV Genotype Test: This test determines the strain of the HCV virus that is present in the blood. There are several different genotypes of HCV, and the specific genotype can affect how the virus is treated.
- HCV Viral Load Test: This test measures the amount of HCV virus in the blood. A high viral load may indicate a more severe infection and a greater risk of liver damage.
It’s important to note that these tests are not always 100% accurate and may give false-positive or false-negative results. If you think you may have been exposed to HCV or are experiencing symptoms of hepatitis, it’s important to talk to a healthcare provider to determine the best course of action.
Clinical manifestations of Hepatitis C Virus
There are three types of hepatitis C infection: (a) acute, (b) chronic, and (c) cirrhosis and various consequences. Hepatitis C has an incubation period of 15–60 days, with an average of 8 weeks.
Acute HCV infection
Acute HCV infections typically cause symptoms but not jaundice in the vast majority of patients. Acute HCV infection typically causes minor symptoms that may be mistaken for HBV symptoms. Less than 25% of acutely infected patients exhibit jaundice, while 1/3 of patients exhibit hepatomegaly. However, the vast majority of cases (80%) show no symptoms and do not progress to jaundice.
Chronic HCV infection
Infectious hepatitis caused by the hepatitis C virus is a major public health problem all over the world. In the absence of hepatic synthesis malfunction, most individuals with chronic hepatitis are asymptomatic, although they may experience nonspecific symptoms such as fatigue or malaise.
Cirrhosis and other complications induced by hepatitis C
The spread of the hepatitis C virus has made it a major factor in the development of chronic liver disease. Cirrhosis develops in around 20% of people with chronic hepatitis. On a global scale, this could take up to 20 years after the initial outbreak. Liver failure, portal hypertension, and other problems might occur as a side effect in patients with this syndrome. In 1-5% of patients with underlying cirrhosis, hepatocellular carcinoma is one of the most serious consequences. Patients with persistent infections and cirrhosis typically develop this syndrome after 30 years. Multiple nations have observed an uptick in fatalities attributable to cirrhosis and other HCV-related diseases.
Treatment of Hepatitis C
Patients with chronic HCV infections currently have one treatment option, which is a combined therapy of pegylated interferon and the antiviral drug ribavirin. Protease inhibitors, ribozymes, and viral vaccinations are three other potential therapeutic approaches.
Prevention and Control of hepatitis C
There is currently no HCV vaccination on the market. The injection of immunoglobulin has been linked to HCV and has no protective effect against the spread of the virus. Donating blood, organs, or sperm from HCV-positive donors should be screened for and ultimately avoided to prevent the spread of the virus.
References
- Aziz, A. A. (2018). Hepatitis C Virus. Hepatitis C in Developing Countries, 3–11. doi:10.1016/b978-0-12-803233-6.00001-1
- Bartenschlager, R., & Bühler, S. (2008). Hepatitis C Virus. Encyclopedia of Virology, 367–374. doi:10.1016/b978-012374410-4.00416-7
- https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- https://www.mayoclinic.org/diseases-conditions/hepatitis-c/symptoms-causes/syc-20354278
- https://www.cdc.gov/hepatitis/hcv/index.htm#:~:text=Hepatitis%20C%20is%20a%20liver,to%20prepare%20and%20inject%20drugs.