Despite all the technical advances in scientific laboratories, the Neubauer Chamber is still the most widely used method for cell counting worldwide. This technical data sheet was created to assist experienced and novice researchers in performing a cell count using a Neubauer or Hemocytometer. These principles can be applied to any cell-counting chamber. However, the volumes and dimensions of each chamber might differ. This technical data sheet outlines the best practices, recommendations, and applications for cell counting.
What is Hemocytometer?
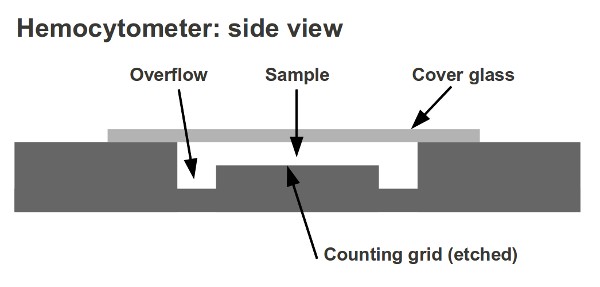
The hemocytometer, also known as haemocytometer, is a counting chamber device that was originally created and used to count blood cells.
- Louis-Charles Malassez invented the hemocytometer. It consists of a thick microscope slide made from glass with a rectangular indentation. This creates a precise volume chamber.
- This chamber has a laser-etched grid with perpendicular lines.
- The lines are carefully placed so that the device’s area is well-defined, as is the chamber’s depth.
- It is possible to count the cells or particles within a certain volume of fluid by observing the grid. This allows you to calculate the total concentration of cells in the fluid.
- The Neubauer counting chamber is a well-known hemocytometer.
- There are many types of hemocytometers available with different rulings. Fuchs-Rosenthal rulings are used commonly for spinal fluid count, Howard Mold rulings are used for mold on food packaging products, McMaster Egg Slide ruling is used to count microbial eggs within fecal material. Nageotte Chamber ruling is used to count low levels of white cells and white-reduced platelet component components. Palmer Nanoplankton ruling is used for counting smaller plankters.
- Petroff-Hausser counter with Improved Neubauer rulings can be used to count bacteria and sperm. It is available in varying chamber depths.
- The hemocytometer’s Sedgwick-Rafter Cell ruling is intended for microscopy of water.
What is Haemocytometry?
This is a method used to determine the total number of cells in blood or other biological fluids. You can do this using a haemocytometer, or an electronic cell counter.
What is Purpose of Haemocytometry?
There may be a variation in the values of certain types of cells due to pathological conditions. It is possible to determine if someone is healthy or sick by counting the cells found in blood and body fluids. The cell count is essential:
- To determine the normal and abnormal cell counts
- To confirm and support the clinical diagnosis of the patient
- To determine the patient’s response to treatment
Principles
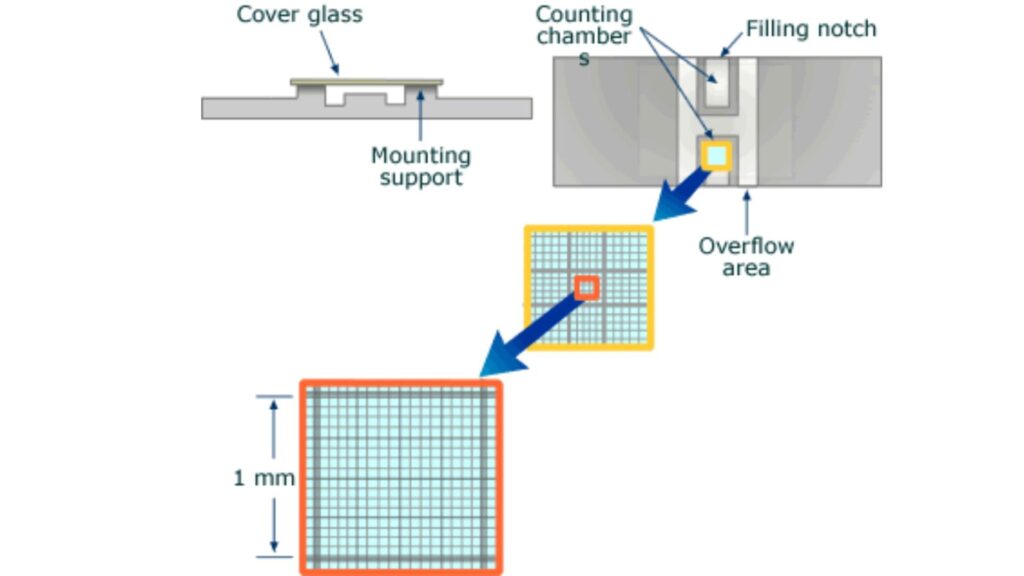
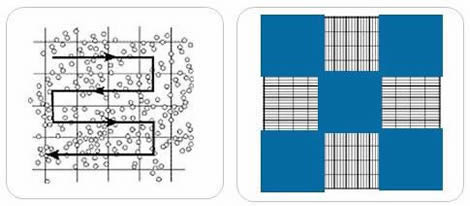
Nine 1×1 mm (1.mm2) squares make up the gridded area for the Improved Neubauer Ruled Hemocytometer. These can be subdivided into three directions: 0.25x 0.25mm (0.0625mm2), 0.25x 0.20mm (0.05mm2) and 0.20x 0.20mm (0.04mm2). Further subdivide the central square into 0.05×0.05mm (0.0025mm2) squares. The hemocytometer’s raised edges hold the coverslip at 0.1 mm from the grid. This gives each square a defined volume (see figure to the right).
Materials
These are the materials required to count cells in a Neubauer chamber.
- Measure cellular dilution
- Hemocytometer or Neubauer chamber
- Optical microscope
- Cover glass
- Pipette/Micropipette using disposable tips
- If necessary, dilution buffer/PBS

Hemocytometer or Neubauer chamber
- The Neubauer chamber is thick, crystal-slide size (30 x 70mm) and 4 mm thick (4 mm).
- The central area of a simple counting chamber is where cell counts are done. There are three parts to the chamber: The central area, which is where the counting grid was placed on the glass; and the double chambers/two areas that can be loaded separately.
- Neubauer chamber’s count grid measures 3 mm x3 mm.
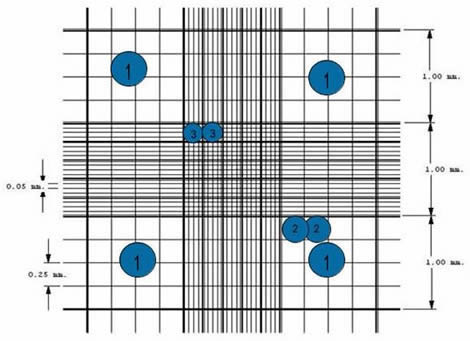
- There are 9 subdivisions with a width of 1mm. The squares at the corners can be used to count white cells in blood cell counts.
- Because their concentrations are lower than red blood cell’s, a greater area is needed to do the cell count.
- The central square is used to store platelets and red blood cells.
- This square is divided into 25 squares with a width of 0.2mm (200 um). Each of the 25 central squares can be subdivided into 16 smaller squares. The central square is therefore made up of 400 smaller squares.
Glass cover
The glass cover is squared glass with a width of 22 mm. The glass cover covers the Neubauer chamber’s central area and is placed on top. The glass cover allows for cell concentration to be maintained between the chamber’s bottom and the cover. The chamber is designed to have a minimum of 0.1mm between the cover and the bottom. The glass cover can sometimes lift slightly when liquid is introduced into the chamber. Some counting chambers have two clamps that prevent the glass cover from lifting to avoid this. The distance between the chambers and the cover glass will exceed 0.1mm and cell counts won’t be accurate if it is lifted.
Pipette
The Neubauer chamber can be filled with liquid precisely using the pipette. They were traditionally made from glass. Today, micropipettes are available that can calibrate with a maximum capacity between 20,200 and 2000 ul.
Step 1 – Sample preparation
- Cell counting requires a preparation of a diluted solution with the right concentration depending on the sample.
- The concentration range for Neubauer chamber cell counts is typically between 250.1 and 2.5 million cells/ml.
- The recommended dilution concentration should be approximately 106 cells per ml (1,000,000 cells/ml), after applying the necessary dilutions. Concentrations below 250,000 cells/ml (2.5×105 cells/ml) will make it difficult to calculate the original concentration.
- Above 2.5 million cells/ml (2.5×106), the likelihood of counting errors increases, as does the time and effort needed to get a reliable count.
- If the concentration is greater than 2.5 million, diluting the sample will be more effective to get a final concentration of 1 million per milliliter. It is important that you record the amount of dilution done to the original sample.
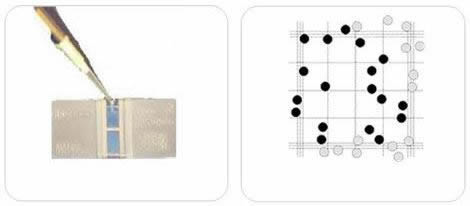
Step 2 – Introducing the sample into the Neubauer chamber
Prepare 10 ul of the dilution as per step 1 using the micropipette.
- Place the Neubauer chamber’s central area with the glass cover. Place the Neubauer chamber on a flat surface such as a table, workbench, or other similar surface.
- Place a disposable tip at one end of the micropipette.
- To draw 10 ul, adjust the micropipette. To adjust the volume, turn the upper plunger roulette.
- Place the tip of the micropipette on the dilution that was previously prepared in step 1.
- Slowly push the plunger of the pipette slowly until it stops moving.
- The pipette tip should be removed from the dilution solution and brought to the Neubauer chamber. The pipette must be kept in a vertical position when loaded
- Place the tip of the pipette near the edge of the glass, in the middle and center of the Neubauer chamber.
- Slowly release the plunger, and observe how the liquid enters uniformly into the chamber, being absorbed through capillarity.
- If bubbles appear or the glass cover is moving, you can repeat the operation.
- The Neubauer chamber is loaded and ready for the cell count!
Step 3 – Microscope set up and focus
- Place the Neubauer chamber onto the microscope stage. Fix the Neubauer chamber to the microscope stage if it has a fixing clamp
- Turn on the microscope.
- You can focus the microscope until you see the cells clearly through the eyepiece. Adjust the stage.
- Find the square in the counting grid where the cell count will begin. In this example, five large squares will be counted from a Neubauer-improved room.
- Start counting the cells within the first square. Although different counting protocols may be used in different laboratories, the general rule is that cells touching the upper or left limits should be counted. Cells touching the lower or right limits should not be counted.
- It is easy to lose count in high-cell concentration situations. This is where zig-zag counting is useful.
- Note the number of cells in the square.
- Continue the process with the remaining squares and write down the results. The accuracy of measurement is dependent on how many cells are counted.
Step 4 – Concentration calculation
We apply the formula for the calculation of the concentration:
Concentration (cell / ml) = Number of cells / Volume (in ml)
The number of cells will be the sum of all the counted cells in all squares counted.
Since the volume of 1 big square is:
0.1 cm x 0.1 cm = 0.01 cm2 of area counted.
Since the depth of the chamber is 0.1mm:
0.1 mm = 0.01 cm
0.01 cm2 x 0,01 cm = 0.0001 cm2 = 0.0001ml = 0.1 µl
So, for the Neubauer chamber, the formula used when counting in the big squares is:
Concentration = Number of cells x 10,000 / Number of squares
In the case that a dilution was applied, the concentration obtained should be converted to the original concentration before the dilution.
In this case, the concentration should be divided by the dilution applied. The formula will be:
Concentration = Number of Cells x 10,000 / Number of square x dilution
Example:
For a 1:10 dilution → Dilution = 0.1
For a 1:100 dilution → Dilution = 0.01
Errors
However, haemocytometer counts can be affected by the following sources:
Non-uniform suspensions
The assumption is that the volume of cell suspension in the chamber represents a random sample. If the suspension is not well distributed and free from cell clumps, this assumption will not hold. Distribution in the Haemocytometer chamber is dependent on the number of particles and not the particle mass. Cell clumps can cause cell distribution problems by distributing in the same manner as single cells. If 90% or more cells are not in direct contact with each other, the count should be repeated using a fresh sample of the original culture. Mix thoroughly before you taste!
Improper filling of chambers
A chamber must be clean in order to allow capillary action to work properly. This is also true for the Pasteur pipette that was used to fill the chamber. Dry-heat sterilization may be possible for new pipettes. First, clean the chamber and cover slip with distilled water. Next, use ethanol to clean them. Finally, wipe dry with a Kimwipe. The chamber should be cleaned if the cell suspension doesn’t flow in smoothly and immediately.
Failure to adopt a convention for counting cells in contact with boundary lines or with each other
Some cells may settle along the border gridlines, making it difficult to determine whether to count them. Is it in? To determine what cells should be counted and which ones not, you need to create a convention that you don’t count half the cells touching a border. You might decide to not count cells that touch the right and bottom borders (or both). While you can choose your own convention, you must be consistent.
Statistical error
The overall error in counting can easily be reduced by paying attention to details.
Types of Counting Chamber
There are present four types of counting chamber such as;
- Old neubauer counting chamber
- Improved neubauer counting chamber
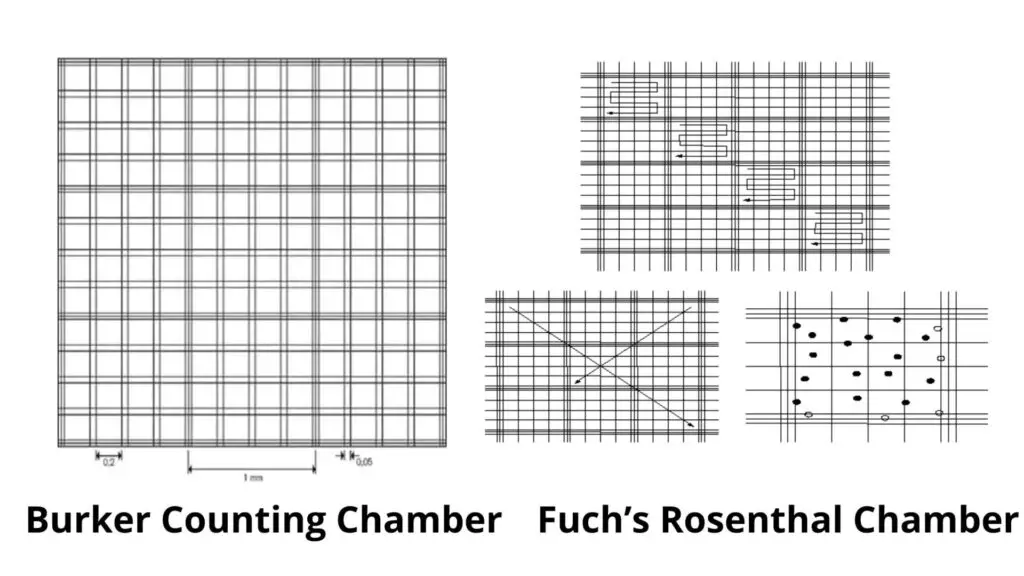
- Burker counting chamber
- Fuch’s rosenthal counting chamber.
Old neubauer counting chamber
- This is where the central platform is located at 0.1 mm.
- Below the level of both sides, the chamber has a depth of 0.01 mm.
- The ruling has a 9 x 9 mm area. Divided into 9 squares of 1 Sq. M. each.
- Each of the four corners squares is subdivided into 16 pieces, each measuring 1/16 of an sq.mm.
- The 1sq.mm. central ruled area is By using a set of triple lines, the larger squares are divided into 16 smaller ones.
- These large squares can be subdivided by single lines into 16 smaller squares.
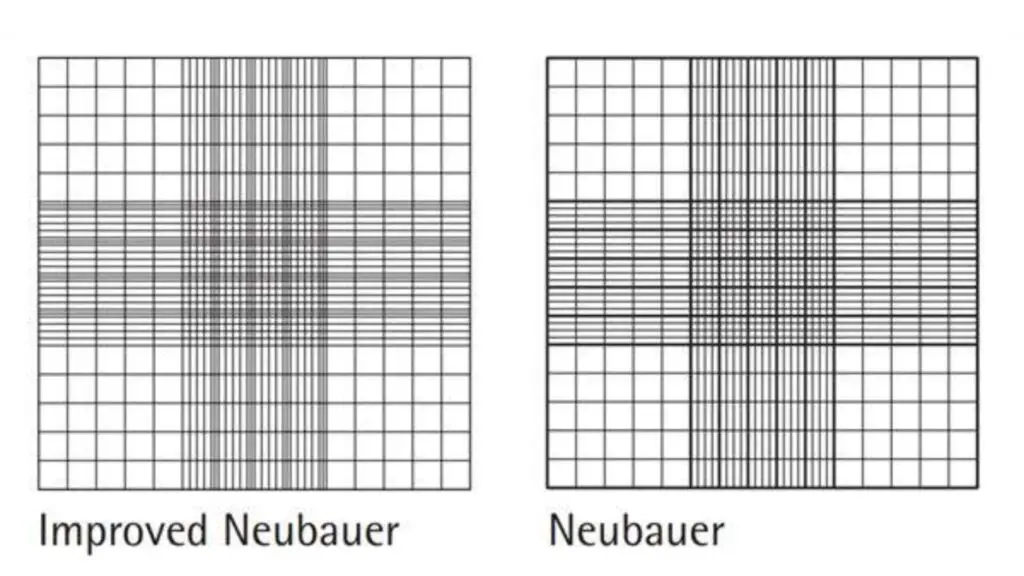
Improved neubauer counting chamber
- The three lines that divide the central large square by the triangle are much closer together.
- The area ruled by the central square is divided into 25 large areas.
- These squares can be subdivided into 16 smaller squares, each measuring 1/400 sq.mm.
- The improved neubauer chamber has the same depth, i.e. 0.1mm.
Burker Counting Chamber
- This is similar to the neubauer counting chamber and has a ruled surface of 9 sq.mm.
- It has a depth of 0.1mm. It is divided by double lining, and not one.
Fuch’s Rosenthal Chamber
- Although this chamber was originally intended for counting cells in CSF, some workers prefer it for counting blood cells.
- This chamber measures 0.2mm in depth.
- The ruled area is divided into 16mm squares and divided by triple lines.
- These squares can be subdivided into 16 smaller squares, each measuring 1/16th of an sq.mm.
Clinical significance
- Anemia is a decrease in the number circulating erythrocytes.
- A higher number of erythrocytes could indicate polycythemia.
- A temporary increase in WBC counts indicates that there is a bacterial infection.
- A progressive increase in abnormal WBC counts indicates leukemia.
FAQ
What is Counting Chamber?
It is a thick, glass slide with a double ruling area. The troughs separate the two areas. These four troughs extend across the slide parallel to one another. The fifth is used to separate the two ruling areas.
What is The differences between Old neubauer counting chamber and New neubauer counting chamber?
- The triple lines of the old neubauer chamber are used to create extra large squares.
- The gap between triple lines in the old neubauer chamber was large and the rectangle space between them looks almost identical to the squares in which the cells are to count. This made it very difficult to count and the chances of making an error were high.
- The lines in the old Neubauer chamber were extremely dull, making it difficult at times to identify them.
- However, these faults can be eliminated in an improved neubauer chamber.
- Its triple lining is very close to one another, these are dark and easy to recognize.
- It is easy to divide the central square into 25 squares for the RBC or Platelet count.
References
- emsdiasum.com/microscopy/technical/datasheet/68052-14.aspx
- https://www.microbehunter.com/the-hemocytometer-counting-chamber/
- https://www.abcam.com/protocols/counting-cells-using-a-haemocytometer
- https://en.wikipedia.org/wiki/Hemocytometer
- https://www.dlsweb.rmit.edu.au/Toolbox/Laboratory/laboratory/studynotes/SNHaemo.htm#:~:text=A%20Haemocytometer%20is%20a%20specialised,surface%20separated%20by%20a%20trough.
- https://www.brainkart.com/article/Haemocytometer_819/