What is Hematopoiesis?
Hematopoiesis, derived from the Greek words “haima” meaning blood and “poiein” meaning to make, is the process through which blood cells are formed from hematopoietic stem cells. It is a crucial and continuous process necessary for maintaining the appropriate levels of circulating blood cells within the body.
All cellular components of blood, including red blood cells (erythrocytes), white blood cells (leukocytes), and platelets, originate from hematopoietic stem cells. These stem cells possess the unique ability to self-renew, ensuring a constant supply of new blood cells throughout a person’s life.
The process of hematopoiesis involves the differentiation and maturation of hematopoietic stem cells into three distinct lineages of blood cells:
- Erythroid Lineage: This lineage gives rise to reticulocytes and erythrocytes, commonly known as red blood cells. Red blood cells are responsible for transporting oxygen to tissues and removing carbon dioxide.
- Lymphoid Lineage: This lineage gives rise to lymphocytes, including B cells, T cells, and natural killer (NK) cells. Lymphocytes play a critical role in the immune system, defending the body against infections and diseases.
- Myeloid Lineage: This lineage gives rise to various types of cells, including macrophages, dendritic cells, granulocytes, and megakaryocytes. Macrophages and dendritic cells are involved in immune responses and antigen presentation, while granulocytes help in combating infections. Megakaryocytes are responsible for producing platelets, which are essential for blood clotting.
Hematopoiesis is a highly conserved process across evolution, meaning it has remained largely unchanged throughout species. The continuous production of blood cells is necessary to replace aged or damaged cells, respond to infections, and maintain homeostasis within the body.
The study of hematopoiesis provides valuable insights into cellular differentiation, the development of cancer, and the role of stem cells in the aging process. Understanding the mechanisms underlying hematopoiesis has important implications for treating blood disorders, such as anemia, leukemia, and immunodeficiency diseases.
Definition of Hematopoiesis
Hematopoiesis is the process of blood cell formation from hematopoietic stem cells.
Hematopoiesis Process
- Hematopoiesis is a dynamic process that occurs at different locations in the body depending on the life stage. In the developing embryo, it begins in the yolk sac and then transitions to the spleen, liver, and lymph nodes. This initial phase, known as the primitive wave, is characterized by the production of erythroid progenitors, which differentiate into red blood cells to supply oxygen to the growing fetus. The bone marrow becomes the primary site of hematopoiesis as bones develop.
- During childhood, long bones such as the femur, tibia, and fibula are the main sites of hematopoiesis. In adulthood, hematopoiesis primarily occurs in the pelvis, sternum, cranium, and vertebrae. Although initiated in the bone marrow, further maturation takes place in other lymphoid organs like the spleen, thymus, and lymph nodes.
- Hematopoiesis undergoes a transition from primitive to definitive as the organism progresses beyond the embryonic stage. Definitive hematopoiesis relies on multipotential hematopoietic stem cells derived from the aorta-gonad-mesonephros region of the embryo.
- The process of hematopoiesis is regulated by cytokines, which activate transcription factors and guide the differentiation of multipotential hematopoietic stem cells into specific cell types. Different cytokines induce the differentiation of specific cell lineages. For instance, granulocyte macrophage-colony stimulating factor promotes the myeloid lineage, leading to the development of granulocytes and macrophages. Cytokines, also known as growth factors, are crucial for hematopoiesis and their dysregulation can result in immunocompromised states or cancer.
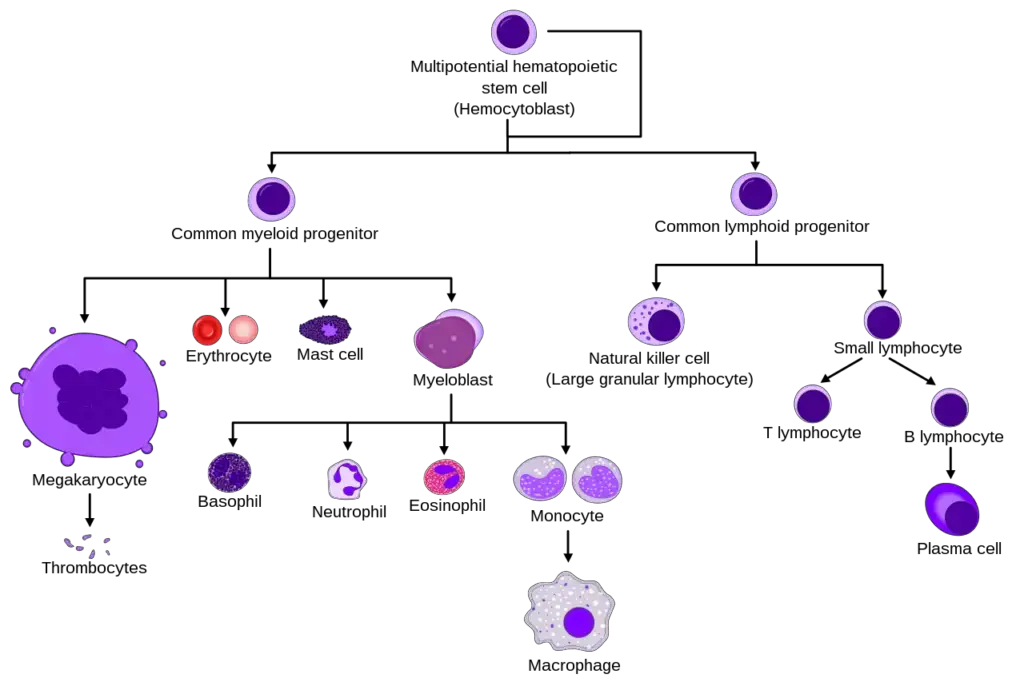
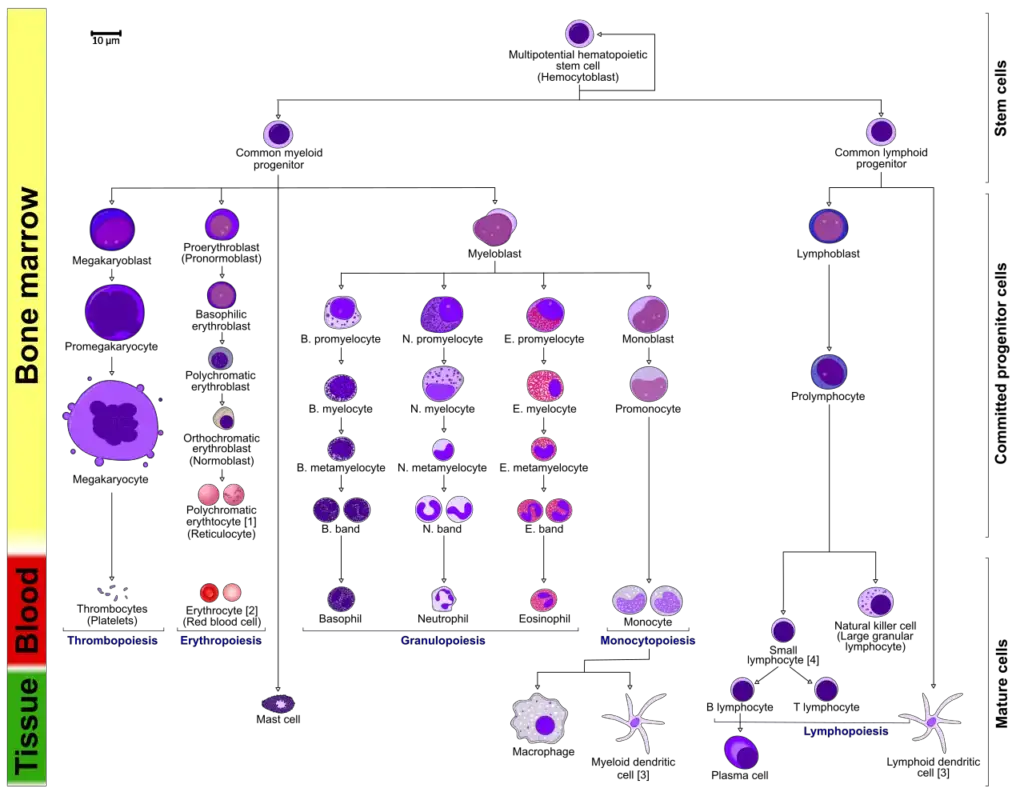
- The typical process of hematopoiesis involves the differentiation of multipotential hematopoietic stem cells into common myeloid or lymphoid progenitors. Depending on the activation of specific cytokines and transcription factors, myeloid progenitors can differentiate into myeloblasts, which give rise to granulocytes (basophils, neutrophils, or eosinophils) or monocytes (macrophages and dendritic cells). Megakaryocytes differentiate into platelets, while erythroblasts develop into erythrocytes. Lymphoid dendritic cells can form directly from the common lymphoid progenitor, and lymphoblasts lead to the further development of natural killer cells or lymphocytes (T and B cells). B cells, when activated in secondary lymphoid organs, further differentiate into plasma cells that produce antibodies.
- Overall, hematopoiesis is a complex and highly regulated process involving the differentiation of stem cells into various blood cell lineages, with cytokines and transcription factors playing critical roles in its control and progression.
Location of Hematopoiesis
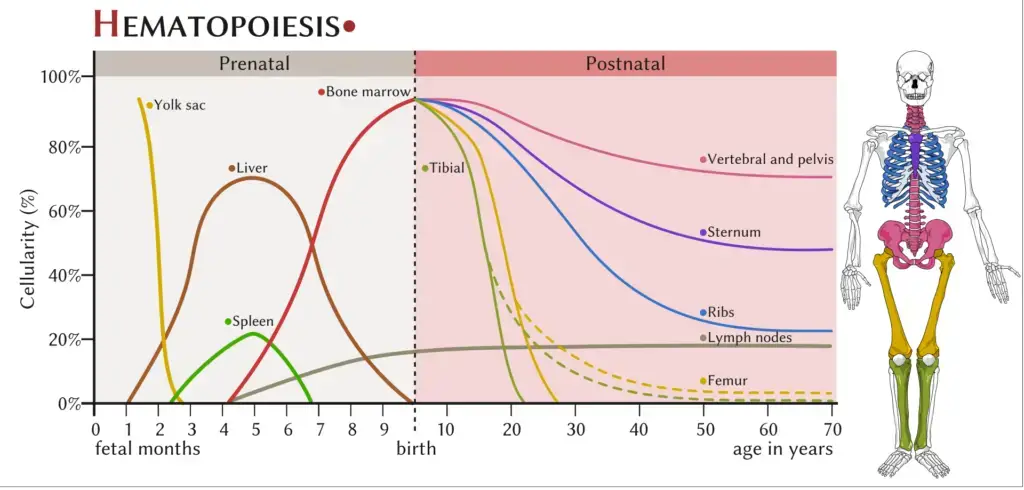
- Hematopoiesis, the process of blood cell formation, occurs in different locations depending on the stage of development. In developing embryos, blood formation initially takes place in structures called blood islands within the yolk sac. As development progresses, hematopoiesis also occurs in the spleen, liver, and lymph nodes.
- However, once bone marrow develops, it becomes the primary site for blood cell production in the entire organism. Most of the blood cells, including red blood cells, white blood cells, and platelets, are formed in the bone marrow. The long bones, such as the femur and tibia, serve as the main site of hematopoiesis in children.
- In adults, hematopoiesis primarily occurs in specific bones, including the pelvis, cranium (skull), vertebrae, and sternum (breastbone). These bones provide a supportive environment for the production and maturation of blood cells.
- In certain situations, such as during certain diseases or conditions, the liver, thymus, and spleen may resume their hematopoietic function. This phenomenon is known as extramedullary hematopoiesis. When extramedullary hematopoiesis occurs, these organs may increase in size significantly. During fetal development, before the bone marrow develops, the liver plays a crucial role as the primary site of hematopoiesis.
- Extramedullary hematopoiesis and myelopoiesis, the formation of white blood cells, can occur in response to cardiovascular disease and inflammation in adulthood. The spleen’s macrophages and adhesion molecules are believed to play a role in regulating the generation of myeloid cells in these conditions.
Hematopoietic Stem Cell Lineages and Growth Factors
- Hematopoietic stem cells (HSCs) play a vital role in the formation and development of red and white blood cells in humans. During embryonic development, hematopoiesis begins in the yolk sac, where yolk-sac stem cells differentiate into primitive erythroid cells that contain embryonic hemoglobin.
- Around the third month of gestation, HSCs migrate from the yolk sac to the fetal liver and spleen, which become the major organs for hematopoiesis from the third to the seventh month. Afterward, hematopoiesis primarily occurs in the bone marrow until birth, with little or no hematopoiesis in the liver and spleen.
- Hematopoietic stem cells are multipotent or pluripotent, meaning they can differentiate into various cell types, including erythrocytes, granulocytes, monocytes, mast cells, lymphocytes, and megakaryocytes. However, their numbers are relatively low, with only about one hematopoietic stem cell per every 5,104 cells in the bone marrow. Despite their scarcity, hematopoietic stem cells have an enormous capacity for proliferation.
- During hematopoiesis, multipotent stem cells differentiate along either the common lymphoid progenitor or common myeloid progenitor pathways, depending on their microenvironment. Common lymphoid progenitor cells give rise to B-cells, T-cells, natural killer (NK) cells, and some dendritic cells.
- On the other hand, myeloid stem cells generate progenitors of red blood cells (erythrocytes), various white blood cells (neutrophils, eosinophils, basophils, monocytes, mast cells, dendritic cells), and platelets.
- The differentiation of progenitor cells into specific cell lineages is regulated by growth factors and cytokines. These soluble agents and membrane-bound molecules interact with the progenitor cells, promoting their proliferation and differentiation into mature erythrocytes, specific types of leukocytes, or platelet-generating cells (megakaryocytes). Hematopoietic growth factors, such as those produced by activated macrophages and T-cells during infection, stimulate hematopoiesis.
- Within the bone marrow, hematopoietic cells grow and mature on a mesh of stromal cells, which include fat cells, endothelial cells, fibroblasts, and macrophages. These non-hematopoietic stromal cells provide a hematopoietic-inducing microenvironment (HIM) by creating a cellular matrix and releasing factors that support growth and differentiation.
- At the genetic level, hematopoiesis involves the action of transcription factors. One such factor is GATA-2, which recognizes the tetranucleotide sequence GATA and plays a role in the development of lymphoid, erythroid, and myeloid lineages. GATA-2 is an essential transcription factor that influences multiple hematopoietic lineages.
Regulation of Hematopoiesis and Programmed Cell death
Hematopoiesis, the process of blood cell production, is regulated to maintain a steady and constant supply of mature blood cells while balancing cell loss due to aging. Red blood cells (erythrocytes) have an average lifespan of 120 days before being phagocytosed and digested by spleen macrophages. White blood cells, including neutrophils, have shorter lifespans ranging from a few days to over 20 years for certain T-lymphocytes. To maintain a steady-state level, the human body needs to produce a significant number of white blood cells daily.
The regulation of hematopoiesis involves complex mechanisms that affect individual cell types. These regulatory mechanisms ensure the steady production of blood cells while allowing for flexibility to rapidly increase production in response to situations like hemorrhage or infection. Several strategies contribute to steady-state regulation of hematopoiesis:
- Control of cytokine levels and types produced by bone marrow stromal cells.
- Production of hematopoietically active cytokines by other cell types, such as activated T cells and macrophages.
- Regulation of the expression of receptors for hematopoietically active cytokines in stem cells and progenitor cells.
- Controlled induction of cell death to remove some cells.
Maintaining a balance between the production of hematopoietic lineages and cell death is crucial. Failure in any of these regulatory mechanisms can result in abnormalities, such as uncontrolled cell proliferation and the development of certain types of leukemias.
Programmed cell death, known as apoptosis, is an essential process in hematopoiesis. Each immune cell has a specific lifespan, after which programmed cell death is initiated. For instance, neutrophils, which are present in circulation at a significant number, have a lifespan of a few days before programmed cell death occurs. This regulated cell death, combined with constant neutrophil production, helps maintain a stable number of cells.
However, if programmed cell death is disrupted, it can contribute to the development of a leukemic state, where uncontrolled cell proliferation occurs. Proper regulation of programmed cell death is vital for maintaining a balanced hematopoietic system.
Maturation
During the process of maturation, stem cells undergo changes in gene expression that narrow down their potential and move them closer to a specific cell type. This process is known as cellular differentiation. Changes in gene expression can be tracked by monitoring the presence of specific proteins on the cell surface, which indicate the cell’s progress towards its final cell type.
The fate of a cell, whether it will differentiate into a specific cell type, is determined by two proposed models: determinism and stochastic theory. According to the determinism theory, the microenvironment of the hematopoietic system, including colony-stimulating factors and other factors, determines the path of cell differentiation. This classical view suggests that the fate of undifferentiated blood cells is predetermined. On the other hand, the stochastic theory proposes that cell differentiation occurs randomly. Experimental evidence supporting the stochastic theory shows that within a population of hematopoietic progenitor cells, variability in the distribution of a stem cell factor called Sca-1 leads to variable rates of cellular differentiation. This randomness is reversible, as cells can regain their original subpopulation under certain conditions. Stochasticity may also play a role in apoptosis (programmed cell death) and self-renewal, where the microenvironment determines which cells survive and which undergo apoptosis, maintaining a balance between different cell types.
Growth factors play a crucial role in the proliferation, maturation, and self-renewal of hematopoietic cells. Stem cell factor (SCF) is a key player in self-renewal and development, binding to the c-kit receptor on hematopoietic stem cells (HSCs). Other important growth factors include interleukins (IL-2, IL-3, IL-6, IL-7), which regulate proliferation and maturation, and colony-stimulating factors (CSFs) such as granulocyte-macrophage CSF (GM-CSF), granulocyte CSF (G-CSF), and macrophage CSF (M-CSF), which stimulate the production of committed cells.
Transcription factors are activated by growth factors and play a vital role in determining cell fate during hematopoiesis. The expression of specific transcription factors at different stages of differentiation leads to the formation of different cell lineages. For example, PU.1 is involved in myeloid commitment, while GATA-1 is associated with the lymphoid-primed multipotent progenitor. Transcription factors such as Ikaros, Gfi1, and IRF8 are also involved in regulating the development of specific cell types. Importantly, transcription factors can act as caretakers of differentiation, maintaining the cell’s differentiated state and preventing de-differentiation.
Mutations in transcription factors are closely linked to blood cancers, such as acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). For example, mutations in Ikaros, a regulator of various biological events, are associated with BCR-Abl patients and serve as a poor prognostic marker.
In summary, the process of maturation involves changes in gene expression, cell fate determination influenced by growth factors, and the activation of transcription factors that guide the differentiation of hematopoietic cells into specific lineages. Understanding these processes and their regulation is crucial for studying normal hematopoiesis and identifying the mechanisms underlying blood disorders and cancers.
![Hematopoiesis - Definition, Process, Locations 4 Diagram including some of the important cytokines that determine which type of blood cell will be created.[23] SCF= Stem cell factor; Tpo= Thrombopoietin; IL= Interleukin; GM-CSF= Granulocyte Macrophage-colony stimulating factor; Epo= Erythropoietin; M-CSF= Macrophage-colony stimulating factor; G-CSF= Granulocyte-colony stimulating factor; SDF-1= Stromal cell-derived factor-1; FLT-3 ligand= FMS-like tyrosine kinase 3 ligand; TNF-a = Tumour necrosis factor-alpha; TGFβ = Transforming growth factor beta](https://biologynotesonline.com/wp-content/uploads/2024/04/image-1602.png)
FAQ
What is hematopoiesis?
Hematopoiesis is the process of blood cell formation in the body. It involves the production, maturation, and differentiation of various blood cell types, including red blood cells, white blood cells, and platelets.
Where does hematopoiesis occur in the body?
In developing embryos, blood formation initially occurs in structures called blood islands in the yolk sac. As development progresses, it takes place in organs such as the liver, spleen, and lymph nodes. However, the primary site of hematopoiesis in adults is the bone marrow, specifically in the pelvis, cranium, vertebrae, and sternum.
How is hematopoiesis regulated?
Hematopoiesis is regulated by complex mechanisms involving various factors. The levels and types of cytokines produced by bone marrow stromal cells, as well as other cell types like activated T cells and macrophages, play a role in regulating hematopoiesis. The expression of receptors for these cytokines in stem cells and progenitor cells is also regulated. Additionally, the controlled induction of cell death helps to maintain the balance between the production and removal of blood cells.
What happens if hematopoiesis is not properly regulated?
A failure in the regulatory mechanisms of hematopoiesis can lead to abnormalities in the expression of hematopoietic cytokines or their receptors. This can result in unregulated cell proliferation and contribute to the development of certain types of leukemias or other blood disorders.
Are all blood cells produced at the same rate?
No, different blood cell types have varying lifespans and rates of production. For example, red blood cells (erythrocytes) have an average lifespan of about 120 days before being removed by macrophages. White blood cells, such as neutrophils, have shorter lifespans ranging from a few days to a few weeks. The body needs to produce a large number of white blood cells daily to maintain steady-state levels.
What are growth factors and their role in hematopoiesis?
Growth factors are proteins that regulate the proliferation, maturation, and self-renewal of hematopoietic cells. They bind to specific receptors on the surface of cells and initiate signal transduction pathways, leading to the activation of transcription factors that control gene expression. Examples of growth factors involved in hematopoiesis include stem cell factor (SCF), interleukins, and colony-stimulating factors.
Is hematopoiesis a deterministic or stochastic process?
Both deterministic and stochastic theories have been proposed to explain hematopoiesis. The deterministic theory suggests that the microenvironment and specific factors in the hematopoietic niche determine the path of cell differentiation. In contrast, the stochastic theory suggests that cell fate determination occurs randomly, influenced by factors such as variability in stem cell populations.
Can hematopoiesis occur outside of the bone marrow?
Yes, under certain circumstances, hematopoiesis can occur outside of the bone marrow. This is known as extramedullary hematopoiesis. It can happen in organs such as the liver, thymus, and spleen, particularly during fetal development or in response to certain diseases or conditions. However, the bone marrow is the primary site of hematopoiesis in healthy adults.
What role do transcription factors play in hematopoiesis?
Transcription factors are proteins that bind to specific DNA sequences and regulate gene expression. In hematopoiesis, transcription factors play a crucial role in determining cell fate and lineage commitment. They control the expression of genes involved in the maturation and differentiation of blood cells. Mutations in transcription factors can disrupt normal hematopoiesis and contribute to the development of blood disorders.
How is hematopoiesis related to blood cancers?
Blood cancers, such as leukemia and lymphoma, are often characterized by abnormal hematopoiesis. Mutations in genes encoding transcription factors or other regulatory proteins can lead to uncontrolled cell proliferation, impaired differentiation, and the accumulation of immature or abnormal blood cells. Understanding the molecular mechanisms underlying hematopoiesis is essential for studying and developing treatments for blood cancers.