What is Hektoen Enteric Agar?
- Hektoen Enteric Agar (HE agar) is a selective and differential medium used in clinical microbiology labs to aid in the recovery and identification of Salmonella and Shigella species from clinical specimens, particularly fecal samples. It was introduced in 1968 by Sylvia King and William I. Metzger at the Hektoen Institute in Chicago.
- HE agar was developed to address the need for an effective medium that could enhance the recovery of Shigella species from mixed cultures. The primary goal was to overcome the inhibitory effects of bile salts, which are commonly found in the intestinal tract and can hinder the growth of pathogenic bacteria. To achieve this, the media was enriched with extra amounts of carbohydrates and peptones to counteract the inhibitory effects of bile salts.
- One of the distinguishing features of HE agar is the addition of two dyes: bromthymol blue and acid fuchsin. These dyes were chosen because of their lower toxicity compared to other dyes, resulting in improved recovery of pathogens. HE agar is both selective and differential, allowing for the growth of specific bacteria while facilitating their differentiation based on their metabolic characteristics.
- In terms of selectivity, HE agar contains bile salts, which inhibit the growth of gram-positive and nonpathogenic gram-negative bacteria commonly found in fecal specimens. This selectivity enables the growth of the desired target organisms, Salmonella and Shigella, which are gram-negative rods and pathogenic to humans.
- HE agar is also differential, meaning it allows for the differentiation of bacteria based on their metabolic properties. The carbohydrates present in the medium, such as lactose, sucrose, and salicin, serve as substrates for bacterial fermentation. This fermentation process leads to the production of acid by some bacteria, which causes a color change in the colonies. The acid produced from lactose fermentation results in the formation of green colonies, while non-lactose-fermenting bacteria produce blue-green colonies. This color differentiation aids in the identification of specific bacterial species, as Salmonella and Shigella typically do not ferment lactose and appear as blue-green colonies on HE agar.
- HE agar is widely used in microbiology laboratories for the direct or indirect plating of fecal specimens to enhance the recovery of Salmonella and Shigella species from mixed normal fecal flora. Its formulation and effectiveness have undergone modification and refinement over time, but it remains a valuable tool in the detection and identification of these enteric pathogens. Furthermore, ongoing research and development continue to introduce new selective and differential media for Salmonella and Shigella, expanding the options available to clinical microbiologists in their diagnostic practices.
Principle of Hektoen Enteric Agar
The principle of Hektoen Enteric Agar (HE agar) lies in its selective and differential properties, which allow for the isolation and differentiation of enteric pathogens from clinical specimens.
HE agar contains animal peptones and yeast extract, providing a nutritious base for bacterial growth. The presence of bile salts and dyes in the medium plays a crucial role in selectivity. These components inhibit the growth of most gram-positive organisms, allowing only gram-negative rods to grow on HE agar. The high concentration of bile salts partially or fully inhibits the nonpathogenic coliform flora commonly found in the intestinal tract.
Enteric pathogens, such as Salmonella and Shigella, are more tolerant of the inhibitory substances present in HE agar and can grow faster and larger than coliforms. One of the distinguishing characteristics used to identify coliforms is the fermentation of carbohydrates, including lactose, sucrose, and salicin. Salmonella and Shigella are unable to utilize these specific carbohydrates, while most nonpathogenic coliforms can ferment at least one of them.
When nonpathogenic coliforms grow on HE agar, they produce acid from the fermentation of carbohydrates, causing a color change in the medium. The bromthymol blue indicator changes from its neutral green color to an orange-yellow color in response to the acid production. The presence of colonies with an orange-yellow color indicates the utilization of lactose, sucrose, or salicin by nonpathogenic coliforms.
Salmonella and Shigella, on the other hand, do not produce acid from the utilization of these carbohydrates, resulting in colonies that are translucent, light green, or greenish-blue. This characteristic allows for quick differentiation of these pathogens from nonpathogenic organisms. However, additional testing is necessary to confirm the presence of Salmonella or Shigella.
HE agar also contains thiosulfate and ferric ammonium citrate, allowing for the detection of hydrogen sulfide (H2S) production by certain enteric gram-negative rods, such as Salmonella. Salmonella produces bacterial enzymes that release a sulfide molecule from the thiosulfate present in the medium. The released sulfide molecule reacts with a hydrogen ion to form H2S gas, which further reacts with ferric ammonium citrate, forming a black precipitate. This results in colonies that are black or have a black center.
Although other nonpathogenic enteric organisms like Proteus sp. and Citrobacter freundii can also produce H2S, they are usually inhibited by the bile salts in HE agar. If these organisms manage to grow despite the inhibitory effects, they can usually be differentiated from pathogens because they can utilize at least one of the carbohydrates present in the medium. An orange-yellow colony with a black center is most likely not an intestinal pathogen, although rare strains of Salmonella capable of lactose fermentation may appear this way.
It’s important to note that HE agar is primarily a screening medium, and additional confirmatory testing is required to confirm or rule out the presence of Salmonella or Shigella. Various options for confirmatory testing include commercial identification kits, tubed biochemical tests, and serological typing of somatic and capsular antigens.
Why Hektoen Enteric agar is called as a selective and differential medium?
Hektoen Enteric agar is referred to as a selective and differential medium due to its unique properties that enable the isolation and differentiation of pathogens from clinical samples.
The medium is selective because it contains bile salts and dyes that inhibit the growth of most Gram-positive organisms. This selective action prevents the growth of these bacteria, allowing only Gram-negative bacilli to develop on the agar. The high concentration of bile salts in Hektoen Enteric agar partially or completely inhibits the non-pathogenic coliform flora typically found in the intestinal tract. However, pathogenic bacteria such as Salmonella and Shigella have the ability to tolerate and overcome the inhibitory substances in the medium. As a result, these pathogens tend to grow faster and larger compared to coliforms.
Hektoen Enteric agar is also differential, as it allows for the differentiation of bacteria based on their ability to ferment specific carbohydrates. Carbohydrate fermentation is a key characteristic used to identify coliforms. The medium contains various carbohydrates such as lactose, sucrose, and salicin. When non-pathogenic coliforms ferment these carbohydrates, they produce acid. The presence of acid causes a color change in the medium. The bromothymol blue indicator turns yellow in the presence of acid, while the dye fuchsin turns red in the presence of aldehyde. Non-pathogenic coliforms that can utilize at least one of these carbohydrates produce orange, yellow, or salmon-colored colonies. In contrast, Salmonella and Shigella species are unable to ferment these specific carbohydrates, resulting in colonies that are translucent, light green, or greenish blue.
In addition to carbohydrate fermentation, Hektoen Enteric agar allows for differentiation based on hydrogen sulfide (H2S) production. The medium contains sodium thiosulfate and iron citrate, which enable the detection of H2S production. Certain enteric gram-negative rods, including Salmonella, have the ability to produce H2S. The released H2S gas reacts with the iron citrate, resulting in the formation of black colonies or a black center. This characteristic staining is due to the formation of iron sulfide.
It’s important to note that the high peptone content in Hektoen Enteric agar compensates for the inhibitory effect of bile salts on Shigella species, ensuring their growth and facilitating their differentiation from other bacteria.
Overall, Hektoen Enteric agar is called a selective and differential medium because it selectively inhibits certain bacteria while promoting the growth of others, and it allows for differentiation based on carbohydrate fermentation and H2S production. These properties make it a valuable tool for the isolation and identification of pathogens from clinical samples.
Composition of Hektoen Enteric Agar
| Ingredients | Gms/liter |
| Protease peptone | 12.00 |
| Yeast extract | 3.000 |
| Lactose | 12.00 |
| Sucrose | 2.000 |
| Salicin | 9.000 |
| Bile Salts mixture | 9.000 |
| Sodium chloride | 5.000 |
| Sodium thiosulfate | 5.000 |
| Ferric ammonium citrate | 1.500 |
| Acid fuchsin | 0.100 |
| Bromothymol blue | 0.065 |
| Agar | 14.00 |
Final pH (at 25°C): 7.5±0.2
Preparation of Use of Hektoen Enteric Agar
To prepare and use Hektoen Enteric Agar, the following steps can be followed:
- Weigh 72.66 grams of Hektoen Enteric Agar and suspend it in 1000 ml of purified or distilled water. Ensure thorough mixing.
- Heat the suspension to boiling to completely dissolve the medium. It is important to note that Hektoen Enteric Agar should not be autoclaved.
- Allow the medium to cool down to a temperature of 45-50°C.
- Mix the cooled medium well to ensure uniformity, and pour it into sterile Petri plates.
- To inoculate the medium, prepare a fresh fecal sample suspended in Ringer’s solution, or directly use rectal swabs containing the specimen. Ensure that the inoculum is evenly spread over the agar surface to obtain well-separated colonies.
- Incubate the inoculated plates at a temperature of 37°C for 18-24 hours.
- It is recommended to further incubate the plates for a longer duration as it improves the differentiation between Salmonella and Shigella species.
Following these steps will prepare the Hektoen Enteric Agar plates for use in the isolation and differentiation of enteric pathogens from clinical samples.
Quality assurance procedures
Quality assurance procedures are essential to ensure the reliability and consistency of Hektoen Enteric Agar. Here are some key aspects of quality assurance for this medium:
- Appearance: The dehydrated powder of Hektoen Enteric Agar should have a light purplish beige color, be homogeneous, and flow freely. Once the medium is prepared, it should appear brown with a greenish cast and slightly opalescent before pouring it into plates. The prepared plates should have a green color with a yellowish cast and slight opalescence. The agar surface should be smooth and moist, without excessive moisture. It is important not to use plates that show signs of drying, cracking of the agar, or evidence of microbial contamination.
- pH: The pH of the prepared medium should be 7.5 ± 0.2 at 25°C for optimal results. This pH range is crucial for the growth and differentiation of target organisms.
- Performance Testing: Once the Hektoen Enteric Agar plates have solidified, it is recommended to remove several plates from each batch and perform performance testing. Known organisms with specific characteristics should be streaked onto the plates using a sterilized inoculating loop. The plates should then be incubated at 35°C for 18 to 24 hours. After incubation, the plates should be examined for the expected growth and reactions of the organisms. If the results do not align with the expected outcomes, the media should not be used.
- Quality Control Organisms: Recommended quality control organisms for Hektoen Enteric Agar include:
- Enterobacter aerogenes ATCC 13048: Expected growth as orange-yellow colonies.
- Escherichia coli ATCC 25922: Expected growth as orange-yellow colonies (may have bile precipitate).
- Salmonella enterica ATCC 13076: Expected growth as greenish-blue colonies.
- Salmonella typhimurium ATCC 14028: Expected growth as greenish-blue colonies.
- Shigella flexneri ATCC 12022: Expected growth as greenish-blue colonies.
- Streptococcus faecalis ATCC 29212: No growth.
It is important to note that some organisms, such as Salmonella typhimurium and Shigella flexneri, may have a black center due to hydrogen sulfide (H2S) production.
By following these quality assurance procedures, laboratories can ensure the reliability and consistency of Hektoen Enteric Agar, thereby enhancing the accuracy of bacterial identification and differentiation in clinical specimens.
Protocol on Hektoen Enteric Agar
Protocol on Hektoen Enteric Agar:
- Prepare a plate of Hektoen Enteric (HE) agar using the quadrant streak plate method to obtain isolated colonies. Well-isolated colonies will yield better results for biochemical differentiation. The inoculum can be obtained from different sources, depending on the laboratory activity: a. If using a previously inoculated and incubated culture plate, take an isolated colony from the source plate using a sterile inoculating loop and transfer it to the HE agar plate. Use the quadrant streak plate method to obtain isolated colonies. b. If using feces from human or animal sources, insert a sterile swab into the fecal specimen, roll the swab across one-third of the HE agar plate, and discard the swab. c. If using an enrichment broth, such as selenite broth or gram-negative broth, insert a sterile swab into the broth, roll the swab across one-third of the HE agar plate, and discard the swab. d. If testing environmental samples or food sources, refer to appropriate guidelines, such as the FDA’s Bacteriological Analytical Manual, for instructions.
- Incubate the HE agar plate aerobically at a temperature of 35 to 37°C for 18 to 24 hours. Avoid incubating in a CO2 atmosphere to prevent pH alteration due to acid production.
- Examine the isolated colonies on the HE agar plate for color reactions and the presence of a black precipitate. Avoid examining areas of confluent growth as false negative fermentation reactions may occur, and mixed organisms may be present. Refer to interpretative guidelines for color reactions (Table 2) and associated images for further understanding.
- Perform follow-up testing as required based on the specific laboratory activity.
- Discard used plates into an appropriate waste container.
Table provides interpretative guidelines for HE agar reactions. Observations, such as growth on the plate, the presence of a yellow or orange precipitate around colonies, and the color of the colonies, can help interpret the results. Further tests may be necessary to confirm the presence of specific enteric pathogens.
| Observation | Interpretation |
|---|---|
| Growth on the HE agar plate | The organism is not inhibited by bile |
| Yellow or orange precipitate around the colonies | Bile salts have been precipitated by organism |
| Yellow | Fermentation of lactose, sucrose, or salicin |
| Salmon to orange | Fermentation of salicin |
| Yellow, salmon to orange with black centers | Fermentation of one of the carbohydrates plus H2S production |
| Greenish-blue, light green, or transparent | No fermentation present, suspect Shigella |
| Greenish-blue, light green, or transparent with black centers | No fermentation present, H2S production present, suspect Salmonella |
By following this protocol, laboratories can effectively utilize Hektoen Enteric Agar for the isolation and differentiation of bacteria in clinical or environmental samples.
Result Interpretation on Hektoen Enteric Agar
Interpreting the results on Hektoen Enteric Agar involves observing the colony colors and characteristics after incubation at 37°C aerobically for 24 hours. Here is a breakdown of the result interpretation:
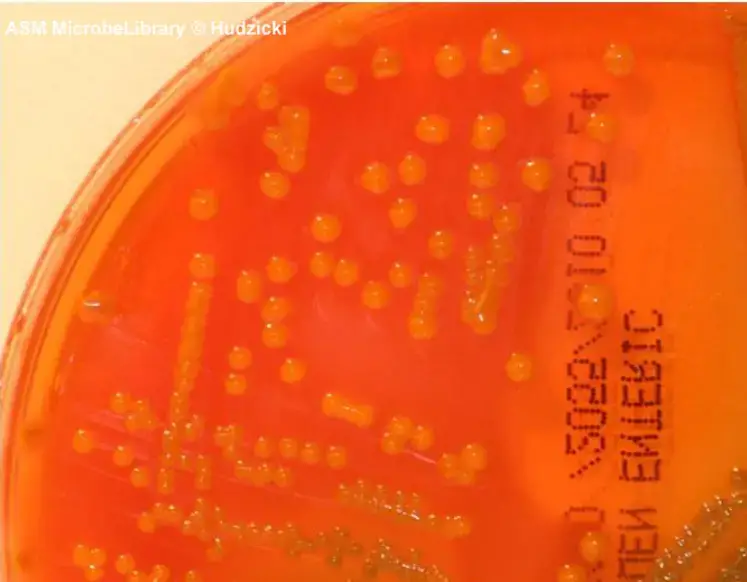
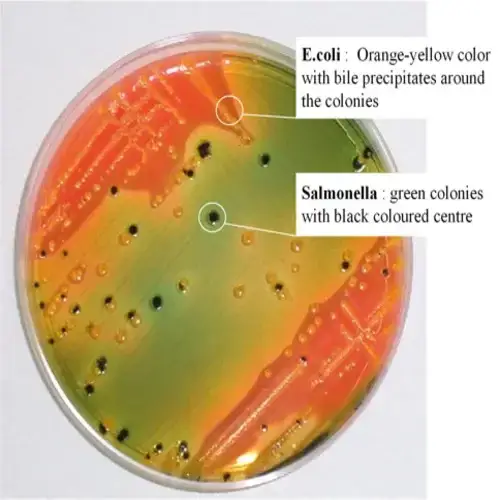
- Rapid lactose fermenters (such as Escherichia coli) are moderately inhibited and produce bright-orange to salmon-pink colonies. There may be the presence of bile precipitate.
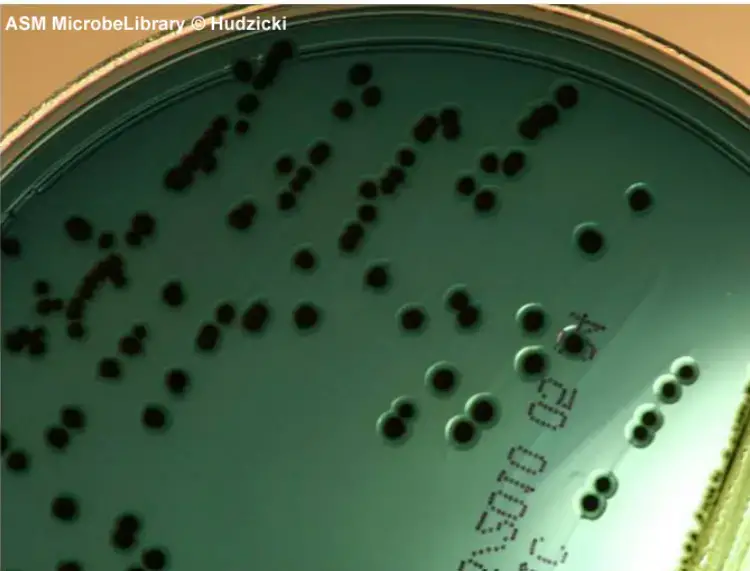
- Salmonella colonies appear blue-green, typically with black centers due to the production of hydrogen sulfide gas. Salmonella Typhimurium, Salmonella Abony, Salmonella Enteritidis, and Salmonella Typhi show this characteristic coloration.
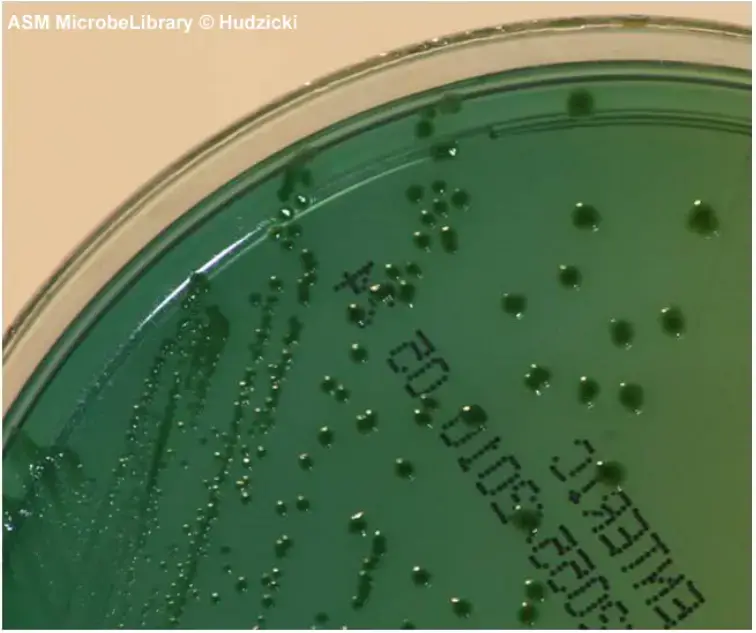
- Shigella colonies appear greener than Salmonella, with the color fading towards the periphery of the colony. Shigella flexneri colonies are greenish blue in color, while Shigella sonnei colonies have good to excellent growth with light green color.
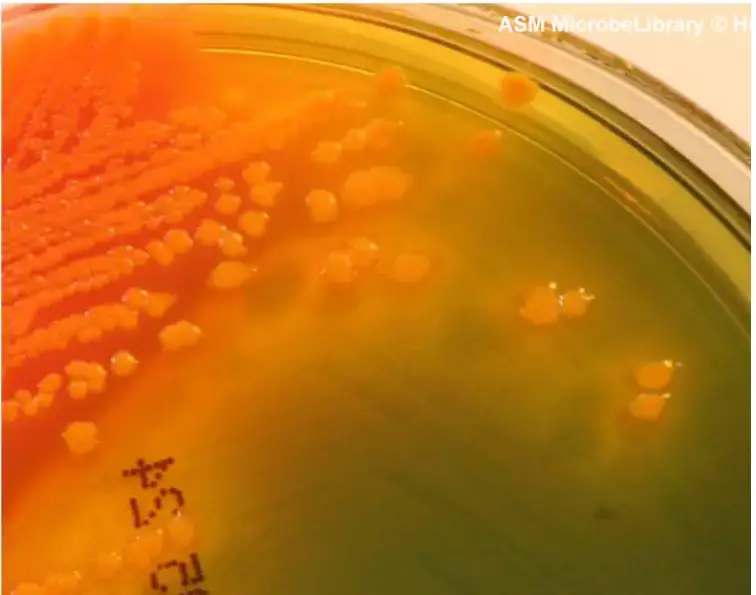
- Proteus strains are somewhat inhibited, and the colonies that do develop are small, transparent, and may have a glistening or watery appearance. They can vary in color from blue-green to blue or salmon, with most strains having a black center or being completely black.
- Enterobacter and Klebsiella colonies are large in size and display a yellow to salmon color.
Overall, the interpretation of results is based on the following observations:
- Fermentation of at least one of the sugars (lactose, sucrose, or salicin) results in salmon, yellow, or orange coloration of the colonies.
- The absence of fermentation leads to blue or green coloration of the colonies.
- The production of hydrogen sulfide (H2S) is characterized by black or black-centered colonies.
Gram-positive bacteria generally show no or low growth on Hektoen Enteric Agar.
By observing these characteristic colony colors and features, microbiologists can make preliminary identifications and differentiations of bacterial species present in the samples. Further confirmatory tests and additional analysis are typically required for definitive identification.
| Organisms | Growth |
| Salmonella Typhimurium | Blue-green with or without black centers |
| Salmonella Abony | Blue-green with or without black centers |
| Salmonella Enteritidis | Blue-green with or without black centers |
| Salmonella Typhi | Blue-green with or without black centers |
| Escherichia coli | Orange (may have bile precipitate) |
| Shigella flexneri | Greenish blue |
| Shigella sonnei | Growth good to excellent; colonies light green |
| Proteus | Variable, blue-green to blue or salmon, most strains with black center or completely black |
| Enterobacter/Klebsiella | Large, yellow to salmon color |
Uses of Hektoen Enteric Agar
Hektoen Enteric Agar has various uses in the field of microbiology. Here are some key applications:
- Selective Isolation of Salmonella and Shigella: Hektoen Enteric Agar is recommended for the differential and selective isolation of Salmonella and Shigella species from enteric pathological specimens. It helps in distinguishing these pathogens from other normal enteric flora present in the samples.
- Testing for Salmonella in Dietary Supplements: The United States Pharmacopoeia recommends the use of Hektoen Enteric Agar for testing the presence of Salmonella in dietary supplements. It serves as a reliable medium to detect the presence of this pathogen in dietary supplement samples.
- Testing for Salmonella in Food Samples: Hektoen Enteric Agar is also recommended for testing Salmonella in food samples according to various standards. It aids in the recovery and isolation of Salmonella from food matrices suspected of contamination.
- Recovery of Salmonella and Shigella from Fecal Specimens: HE agar is used as both a direct and indirect plating medium for fecal specimens. It enhances the recovery of Salmonella and Shigella species from fecal samples with heavy numbers of mixed normal fecal flora. This helps in isolating and identifying these enteric pathogens.
- Recovery of Gastrointestinal Pathogens from Various Samples: Hektoen Enteric Agar serves as a plating medium for the recovery of gastrointestinal pathogens, particularly Salmonella and Shigella, from food, water, and fecal samples. It is a valuable tool in detecting and isolating these organisms in suspected contaminated samples.
The use of Hektoen Enteric Agar contributes to the accurate identification and differentiation of Salmonella and Shigella, assisting in the diagnosis, surveillance, and prevention of enteric diseases caused by these pathogens.
Limitations of Hektoen Enteric Agar
Hektoen Enteric Agar has certain limitations that should be considered when using this medium. These limitations include:
- Incomplete Recovery of Pathogens: Hektoen Enteric Agar may not be able to recover all pathogens present in a specimen. Additional media specifically designed for the isolation of Salmonella, Shigella, and other enteric pathogens should be used alongside HE agar to increase the chances of detecting these organisms.
- Similarity of Proteus mirabilis Colonies to Salmonella: Proteus mirabilis colonies on HE agar can resemble Salmonella colonies, making it difficult to differentiate between the two solely based on colony appearance. Further biochemical and serological testing is necessary for accurate identification.
- Extended Incubation for Certain Shigella Strains: Some strains of Shigella may require longer incubation periods, up to 42 to 48 hours, to develop characteristic growth and color reactions on HE agar. It is important to consider this when interpreting the results.
- Additional Testing for Complete Identification: While certain diagnostic tests can be performed directly on Hektoen Enteric Agar, complete identification of suspected Salmonella or Shigella colonies requires biochemical and, if necessary, immunological testing using pure cultures. These tests provide more accurate and definitive identification.
- Confirmation of Suspected Pathogens: Colonies suspected to be Salmonella or Shigella on HE agar must be confirmed and identified through additional biochemical and serological tests. This confirms the presence of the pathogens and helps differentiate them from other organisms.
- Interpretation of Carbohydrate Utilization: Interpretation of carbohydrate utilization reactions should be performed within 18 to 24 hours of incubation. If the incubation period exceeds 24 hours, the carbohydrates in the medium may be exhausted, leading to a loss of color reactions. This can result in the misinterpretation of the organism’s ability to utilize carbohydrates.
- Screening Medium: Hektoen Enteric Agar should be used as a screening medium rather than a standalone method for the recovery of intestinal pathogens. It is recommended to use a combination of selective and nonselective media when attempting to recover intestinal pathogens from fecal specimens.
Considering these limitations and following proper testing protocols can help ensure accurate and reliable results when using Hektoen Enteric Agar in the laboratory.
Important Informations
Here are some important pieces of information to consider when using Hektoen Enteric Agar:
- Crystallization of Bile Salts: During storage, the bile salts in HE agar plates may crystallize and precipitate out into the media. This crystallization does not affect the performance of the agar and can be disregarded.
- Liquid Medium Appearance: The liquid medium should have a hunter green color before pouring it into plates. Ensure that the medium has cooled down adequately before pouring to avoid any heat-related issues.
- Stability of Plates: The stability of plates prepared from dehydrated powder may vary. While the suggested stability time frame is 70 days, some reviewers have reported shorter stability periods. It is recommended to check the expiration date or assess the stability of plates prepared from dehydrated powder in your specific laboratory setting.
- Inhibition of Growth: Although HE agar inhibits the growth of certain organisms, it does not guarantee the prevention of all growth. Some organisms may still grow on HE agar but with delayed appearance and smaller colony sizes compared to other media used for enteric gram-negative rods.
- Subculturing Techniques: When transferring growth from a previously inoculated plate for subculture, it is advisable to make a light suspension of the organism in sterile saline or sterile water before inoculating the HE agar. This helps prevent the transfer of an excessive amount of organism, which could potentially overcome the inhibitory properties of HE agar.
By being aware of these important considerations, laboratory technicians can ensure the optimal performance and reliability of Hektoen Enteric Agar in their microbiological work.
FAQ
What is Hektoen Enteric Agar?
Hektoen Enteric Agar is a selective and differential medium used for the isolation and differentiation of enteric pathogens, particularly Salmonella and Shigella, from clinical specimens.
What are the main components of Hektoen Enteric Agar?
Hektoen Enteric Agar contains bile salts, animal peptones, yeast extract, carbohydrates (lactose, sucrose, salicin), and indicators such as bromothymol blue and acid fuchsin.
How does Hektoen Enteric Agar selectively inhibit Gram-positive bacteria?
The presence of bile salts in Hektoen Enteric Agar inhibits the growth of Gram-positive bacteria, allowing only Gram-negative rods to grow on the medium.
How does Hektoen Enteric Agar differentiate between different enteric organisms?
Hektoen Enteric Agar differentiates between organisms based on their ability to ferment specific carbohydrates (lactose, sucrose, salicin) and produce characteristic color reactions. This helps in distinguishing pathogenic organisms like Salmonella and Shigella from nonpathogenic coliforms.
What color reactions indicate the presence of Salmonella on Hektoen Enteric Agar?
Salmonella colonies on Hektoen Enteric Agar typically appear blue-green with or without black centers due to the production of hydrogen sulfide gas.
How do Shigella colonies appear on Hektoen Enteric Agar?
Shigella colonies on Hektoen Enteric Agar appear greenish blue, and the color may fade toward the periphery of the colony.
Can Hektoen Enteric Agar be used as a standalone medium for the identification of pathogens?
Hektoen Enteric Agar is primarily used as a screening medium. Additional biochemical and serological testing is necessary for complete identification and confirmation of suspected pathogens.
What is the purpose of using Hektoen Enteric Agar?
Hektoen Enteric Agar allows for the selective growth of enteric pathogens while inhibiting the growth of other bacteria. It also provides a means of differentiating between various enteric organisms based on their ability to ferment carbohydrates and produce characteristic color reactions.
Can Hektoen Enteric Agar be used for environmental or food samples?
Yes, Hektoen Enteric Agar is commonly used for the recovery of gastrointestinal pathogens, such as Salmonella and Shigella, from environmental samples and food samples suspected of contamination.
Are there any limitations to the use of Hektoen Enteric Agar?
Some limitations include the incomplete recovery of all pathogens, similarity between Proteus mirabilis colonies and Salmonella colonies, longer incubation requirements for certain Shigella strains, and the need for additional testing and confirmation of suspected pathogens. Additionally, Hektoen Enteric Agar should be used in conjunction with other media when attempting to recover intestinal pathogens from fecal specimens.
References
- https://asm.org/ASM/media/Protocol-Images/Hektoen-Enteric-Agar-Protocol.pdf?ext=.pdf
- https://microbiologie-clinique.com/hektoen-enteric-agar.html
- https://microbeonline.com/hektoen-enteric-agar-composition-principle-uses/#google_vignette
- https://www.austincc.edu/microbugz/hektoen_enteric_agar.php
- https://www.bd.com/resource.aspx?IDX=8970
- https://hardydiagnostics.com/g63