The Hanging Drop Method is the process used for observing the living and unstained microorganisms in a suspended drop of culture. It is mainly applied to study the true motility of bacteria, yeasts, and fungal spores. In this method a small drop of microbial suspension is placed on a clean coverslip and it is inverted over the concave depression of the hollow-ground slide. The margin of the depression is usually sealed with petroleum jelly which prevents drying of the drop and it is the reason why the organisms can be examined for a longer period. The sealed chamber slows the evaporation and keeps the cells in their natural environment.
It is the modified wet mount technique and it is referred to as the method that helps in distinguishing the actual motility from Brownian movement. This process occurs when the liquid drop hangs freely in the cavity and the organisms move actively without being compressed by the coverslip. It is considered an important technique as it was first introduced by Robert Koch (1878) and it allowed clear visualization of motile forms especially the spiral bacteria.
Objective of Hanging Drop Method
- To study the motility of microbial cell.
Principle of Hanging Drop Method (Motility test)
The principle of the Hanging Drop Method is based on observing the living microorganisms in a free-hanging drop where the cells remain alive and unstained. It is the process in which a drop of microbial suspension is kept on a coverslip and then inverted over the concave depression of the hollow-ground slide, and the drop hangs freely due to the surface tension. The margin of the depression is sealed with petroleum jelly and it forms a closed moist chamber which slows the drying of the sample. It is this enclosed chamber that maintains the natural conditions for the organisms and allows their movement to be seen for a longer period.
The method is applied to distinguish the true motility of a cell from Brownian movement. True motility shows directional and purposeful movement while Brownian movement is the random vibration caused by molecular collision. Since the drop is suspended without pressure, the microorganisms move naturally. It is considered one important principle used in studying motile bacteria and other cells in their living state.
Materials Required for Hanging Drop Method
- Cavity slide (hollow-ground slide) or a normal slide prepared with a paraffin ring
- Paraffin wax or petroleum jelly for sealing
- Inoculating culture loop
- Clean coverslip
- Compound light microscope for observation
- Bunsen burner for sterilizing the loop
- Young culture not older than 24 hours (broth culture of Bacillus, Rhizopus, Penicillium, Aspergillus, Spirogyra, Chlamydomonas, Volvox, etc.)
Quality Control
Validate the technicians’ competency in the hanging-drop method by testing known motile enterococci or Listeria in a broth assay.
Hanging Drop Method Procedure
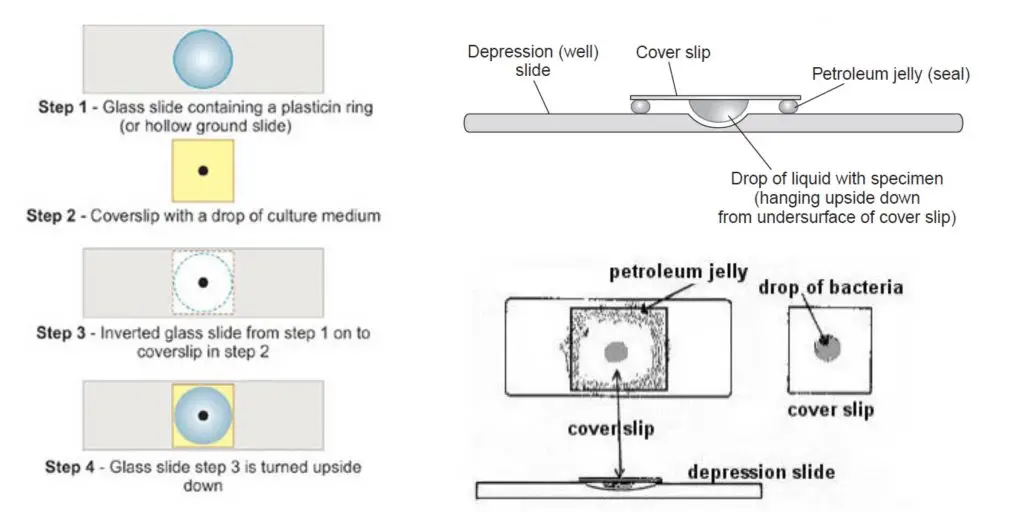
- Arrange materials and sterilize the loop. It is necessary to have a clean cavity slide or a normal slide with a paraffin ring, a clean coverslip, petroleum jelly or paraffin wax, a sterile inoculating loop, a young broth culture (≤24 h), Bunsen burner and compound light microscope ready.
- Clean the coverslip. The coverslip must be thoroughly cleaned and degreased so that the drop will adhere by surface tension.
- Apply sealant. Place a thin ring or four small dabs of petroleum jelly around the margin of the coverslip (or on the rim of the depression of the cavity slide). Avoid excess which may squeeze into the drop or contaminate the objective.
- Flame the loop. The inoculating loop is sterilized over the Bunsen burner until red hot and then cooled briefly before sampling.
- Place the drop of culture on the coverslip. Using sterile loop place one small drop (about 20–50 μL) of the young broth culture at the centre of the prepared coverslip. The drop is held by surface tension.
- Invert and mount. Invert the cavity slide (concave side down) and lower it gently onto the coverslip so that the petroleum jelly contacts the slide rim and forms a seal. The drop will now hang into the cavity.
- Inspect the seal. Confirm that a closed humid chamber is formed and no air bubbles are trapped over the drop. This chamber slows evaporation and it allows prolonged observation.
- Place slide on microscope. The slide assembly is placed on the microscope stage with the coverslip uppermost and the cavity centred.
- Start with low power. Focus the edge of the hanging drop at low magnification (10X objective) to locate the droplet (edge appears as a dark line). This is used as the reference plane.
- Reduce illumination. The condenser is lowered or diaphragm partly closed to reduce light and enhance contrast for unstained living cells.
- Move to higher magnification. Rotate to the high-dry objective (40X) and use fine focus to bring organisms into sharp view.
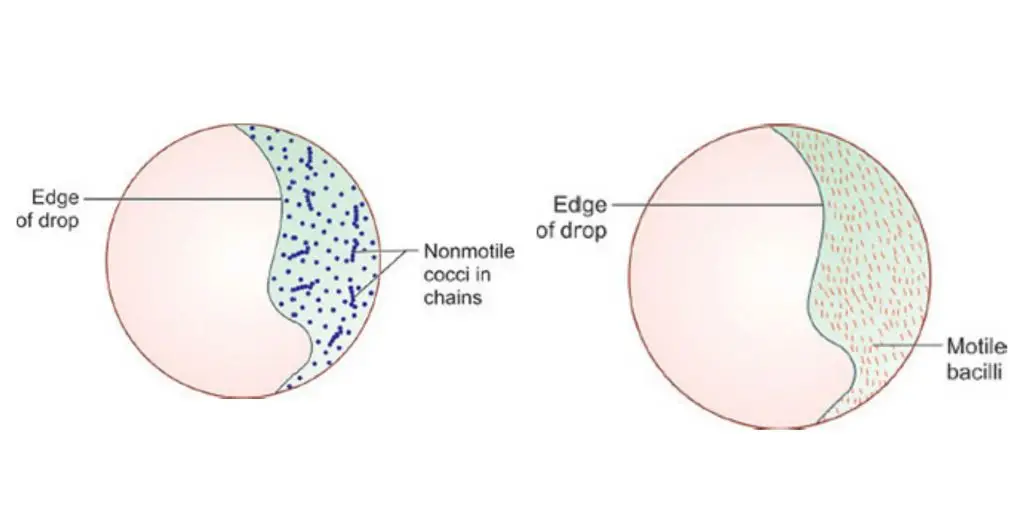
- Distinguish movements. Observe carefully to separate true motility (directional, purposeful translation) from Brownian movement (random jiggling in place caused by molecular bombardment). True motility is directional; Brownian movement is non-directional.
- Use oil immersion only if needed. If 100X oil objective is required, place a tiny drop of immersion oil on the coverslip and take extreme care not to crack the coverslip when lowering the objective.
- Allow short settling time. Give about one minute for organisms to settle after mounting before final observations are recorded.
- Record observations. Note cell shape, arrangement, and type of movement (if any). It is often useful to observe several fields to confirm motility patterns.
- Decontaminate and dispose. After observation the slide assembly is either cleaned thoroughly or immersed in disinfectant and treated as biohazardous sharps for disposal.
- Clean microscope. Remove any oil from objectives and stage using lens tissue as per microscope precautions.
- Safety note. All handling of cultures and contaminated glassware is to be done using appropriate laboratory safety practices and PPE.

Result and Interpretations of Hanging Drop Method
The Hanging Drop Method is used to observe the true motility of living microorganisms. It is the process where the organisms are examined in an unstained and natural condition. The main purpose is to differentiate the true movement of cells from the passive movements that occur in the fluid.
A. Positive Motility (True Motility)
It is the self-directed movement shown by the organism because of the expenditure of energy. This is referred to as true motility, and in most bacteria it is caused by flagella.
Some of the main features are–
- Movement is directional and the organism changes its position in the field.
- It appears as a rapid and purposeful translation from one point to another.
In this step, some special patterns are also seen:
- Darting motility shown by Vibrio cholerae, where the movement is very fast and jerky.
- Run and tumble movement where the organism runs in a straight path and then performs a tumbling action.
- Gliding or bending type motion may also be visible in some species.
B. Negative Motility (Passive Movement)
This occurs when the organism do not show directional movement. These are not true movements and must be separated from motility.
These are–
- Brownian movement: It is the process where the cells show a vibrating or shaking motion in the same position. It is caused by the collision of water molecules and not by any locomotive organ.
- Drifting movement: All the particles appear to move in the same direction. It occurs due to evaporation or pressure in the fluid. This movement is not accepted for interpretation because it can hide the actual motility.
Uses of Hanging Drop Method
- It is used to determine the true motility of microorganisms in a living condition.
- It helps in differentiating true motility from Brownian movement and fluid drifting.
- It is used for observing special motility patterns such as darting motility of Vibrio cholerae.
- It is used for studying the natural morphology and arrangement because the cells are not distorted by staining or fixation.
- It is useful for examining the spiral forms like spirochetes which must be observed in live state.
- It allows monitoring of growth and cell division for a longer time because the drop remains moist for several hours or days.
- It is used to study cytological changes like spore formation, germination, and other physiological processes in living cells.
- Cytoplasmic inclusions such as vacuoles and granules are clearly observed in this method.
- It helps in observing microbial interactions such as symbiosis, parasitism, and predation inside the same drop.
- It is used for presumptive diagnosis of Vibrio cholerae by identifying its darting motility directly from clinical samples.
- A modified form is used in honey bee disease diagnosis to differentiate spores and bacterial clusters in foulbrood infections.
Advantages of Hanging Drop Method
- It allows prolonged observation because the petroleum jelly seal prevents rapid drying of the drop.
- It forms a humid chamber inside the concavity which helps in keeping the microorganisms alive for several hours or even 2–3 days.
- It reduces the chances of fluid drift, so the interpretation of motility becomes more reliable.
- It preserves the natural morphology of microorganisms since no staining or heat fixing is required.
- It is useful for studying the true shape and arrangement of delicate organisms like spirochetes.
- It gives a clearer view of motility compared to the simple wet mount method.
- It helps in differentiating true motility from Brownian movement and other passive movements.
- It confines the sample in a small area making the microorganisms easy to observe under the microscope.
- It is suitable for studying cytological changes like spore formation, germination, and cell division in living cells.
- Cytoplasmic inclusions such as granules and vacuoles are easily observed in the hanging drop preparation.
Limitations of Hanging Drop Method
- It is not safe for examining highly pathogenic organisms because breakage of the coverslip can release infectious material.
- It requires a fragile coverslip and cavity slide, and these are expensive and difficult to handle.
- The coverslips cannot be reused because of contamination risk.
- Only one sample can be observed at a time which limits the use of this method in routine work.
- The preparation of the hanging drop slide takes more time than a simple wet mount.
- The sample needs to be diluted to an appropriate density for proper visualization.
- There is a chance of contamination while preparing the drop on the coverslip.
- Focusing on living unstained cells requires a skilled operator because the cells are transparent.
- The field of view is limited due to the structure of the cavity slide.
- Motility may not be observed in old cultures, causing false negative results.
- Motile cells may be missed if most of the population is non-motile.
- Excess petroleum jelly may squeeze into the drop and interfere with observation.
- The sealing material can also stick to the objective lens, disturbing the image.
- If the seal is not airtight, fluid drift occurs which can be misinterpreted as true motility.
Precautions
- The method is not used when the organisms are highly pathogenic.
- All steps is done carefully to avoid aerosol or splash formation.
- Any spill or accidental exposure is reported at once to the laboratory authority.
- The whole slide assembly is discarded immediately after observation.
- The slide, coverslip and suspension is placed directly into a strong disinfectant solution (Lysol).
- Contaminated coverslips and slides are treated as biohazardous sharps.
- These are placed inside puncture-proof sharps containers.
- Final decontamination is done by autoclaving or by approved medical waste disposal service.
- Coverslips cannot be reused because contamination risk is high.
- The coverslip must be clean and free from grease.
- If grease is present the drop does not adhere properly and the preparation collapses.
- Young broth culture (less than 24 hours) is used for observing true motility.
- Only a small drop is taken and the drop is not spread over the coverslip.
- Petroleum jelly is applied as a thin layer at the edges or in small dabs.
- Excess jelly may squeeze toward the centre and interfere with the view.
- Excess jelly may also stick to the objective lens.
- The inverted depression slide is lowered gently so the jelly adheres properly.
- Light intensity is reduced during observation for better contrast of unstained cells.
- Focusing starts with low power objective and at the margin of the drop.
- High-dry or oil immersion objective is used with only fine adjustment.
- The coverslip is very fragile and care is taken not to break it with the objective.
- Coarse adjustment is not used with high-dry or oil immersion objectives.
- True motility is differentiated from Brownian movement and fluid drift.
- Fluid drift can mislead the observation and give false interpretation of motility.
Hanging Drop Method Video
Hanging Drop Method Images
FAQ
Q1. What is the Hanging Drop Test?
It is a microscopic method used to observe living bacterial cells in an unstained condition. It is mainly used to study true motility inside a suspended drop.
Q2. How is hanging drop preparation done?
It is prepared by placing a small drop of bacterial suspension on a clean coverslip and applying petroleum jelly around it. A depression slide is inverted over the coverslip and then turned upright so the drop hangs freely in the hollow cavity.
Q3. Why is the hanging drop method used?
It is used because the method allows the bacteria to move freely in a liquid environment, so true motility can be seen clearly. It also keeps the cells alive.
Q4. What is the principle of the hanging drop method?
The principle is that unstained living bacteria suspended in a fluid show movement when observed under reduced light. True motility is seen when the organism moves in a definite direction, and this is different from random Brownian motion.
Q5. What are the uses of the hanging drop method?
• To detect true motility of bacteria.
• To study the general shape and arrangement of cells.
• To observe living organisms without staining.
Q6. How can bacterial cells be observed to determine motility?
It is done by focusing on the edge of the drop under low power first, then shifting to high power. Actively motile bacteria move in a directional manner across the field.
Q7. What are the materials required for the hanging drop method?
• Clean coverslip
• Depression slide
• Petroleum jelly
• Inoculating loop
• Bacterial suspension (young culture)
• Microscope
Q8. How do you interpret the results of a hanging drop test?
If the cells show directional movement, the test is positive for motility. If the cells only vibrate in place without directional movement, it is negative because this is Brownian motion.
Q9. What is the difference between true motility and Brownian movement?
True motility is the purposeful directional movement of the cell. Brownian movement is a random shaking caused by collision with water molecules and does not show direction.
Q10. What are the advantages of the hanging drop method?
• It allows clear observation of living cells.
• Motility is seen accurately because the drop does not dry quickly.
• No staining is required.
Q11. What are the limitations of the hanging drop method?
• Not suitable for highly pathogenic organisms.
• Requires skill to differentiate motility from drifting.
• Coverslip is fragile and can break easily.
Q12. What is darting motility in bacteria?
It is the fast, jerky, rapid movement seen in some motile bacteria, often due to polar flagella.
Q13. What is a stool hanging drop test?
It is a hanging drop preparation made from fresh stool to detect motile organisms like Vibrio cholerae. The motility pattern helps in quick presumptive diagnosis.
Q14. Which is the best method for determining bacterial motility among wet mount, hanging drop and semi-solid media inoculation?
The hanging drop method gives the clearest view of true motility, but semisolid media inoculation is safer and preferred for pathogenic organisms.
Q15. What specific bacteria can be identified using the hanging drop method?
It is commonly used for organisms showing characteristic motility such as Vibrio cholerae, Proteus species, Pseudomonas species, and other motile rods.
- American Society for Microbiology (ASM). (2011). Motility test medium protocol.
- Burke, K. C. (2020). 3.4: Materials and procedures. In Bio 221Lab: Introduction to microbiology (Burke). Biology LibreTexts.
- Centers for Disease Control and Prevention (CDC). (n.d.). How to perform a motility test.
- Courtenay, B. (2023, November 4). An overview of hanging drop technique for analysing bacterial cultures. Rubicon Science.
- Crawford, K. (n.d.). Microorganisms – a world in a hanging drop. Science & Plants for Schools.
- KRIPA RAGHUNATHAN. (n.d.). Hanging drop method_microbiology_KripaRaghunathan.pptx [PowerPoint presentation]. SlideShare.
- (n.d.). Lab – 4 bacterial motility (hanging drop technique).
- (n.d.). Preparing a slide with the modified hanging drop technique.
- Reynolds, J. (2021). 15: Hanging drop wet mount. In Microbiology labs I. Biology LibreTexts.
- (n.d.). Single concavity microscope slides, Box/12. The Science Company.
- (n.d.). The hanging drop method: A comprehensive monograph on technique, interpretation, and clinical utility.
- Siny Medical. (2024, September 3). How to dispose of microscope slides safely.
- Supriya, N. (n.d.). What is hanging drop method? Definition, procedure & applications. Biology Reader.
- The University of Texas Rio Grande Valley (UTRGV). (2022). Biological safety manual (BSL-2) (Revised July 2022). Environmental Health and Safety and Risk Management.
- UCLA Environment, Health & Safety. (n.d.). Tissues or specimens in chemical fixatives.
- University of Michigan Pollinator Initiative. (n.d.). Modified hanging drop.
- Wikipedia. (n.d.). Flagellum.