What is glycolysis?
- Glycolysis is a fundamental metabolic pathway responsible for the breakdown of glucose, a six-carbon sugar, into two three-carbon molecules of pyruvate. This process takes place in the cytosol of cells and is a critical energy-yielding mechanism across almost all forms of life, from microorganisms to plants and animals.
- The glycolytic pathway consists of ten enzymatic reactions, organized into two main phases: the energy investment phase and the energy payoff phase. In the investment phase, two ATP molecules are consumed to phosphorylate glucose and prepare it for further breakdown. In the payoff phase, four ATP molecules and two molecules of NADH are produced, resulting in a net gain of two ATP molecules per glucose molecule. The overall reaction can be summarized as:
- Glucose + 2 NAD⁺ + 2 ADP + 2 Pi → 2 Pyruvate + 2 NADH + 2 H⁺ + 2 ATP + 2 H₂O.
- Glycolysis is an anaerobic process, meaning it does not require oxygen. Under aerobic conditions, the pyruvate generated enters the mitochondria to undergo further oxidation via the citric acid cycle and oxidative phosphorylation, producing additional ATP. In contrast, under anaerobic conditions, pyruvate is reduced to lactate in animals or ethanol and carbon dioxide in certain microorganisms, ensuring the regeneration of NAD⁺ for continued glycolytic activity.
- The pathway’s regulation is tightly controlled, with key enzymes like phosphofructokinase playing a pivotal role in maintaining the flux of glucose metabolism. This enzyme adjusts the rate of glycolysis based on cellular energy needs and the availability of substrates.
- Historically, glycolysis is also referred to as the Embden-Meyerhof-Parnas (EMP) pathway, named after the scientists who contributed to its discovery and characterization. Its universality and evolutionary conservation suggest that it is one of the earliest metabolic pathways to have evolved, providing a reliable source of energy even under primitive, oxygen-poor conditions.
- In addition to its role in energy production, glycolysis provides intermediates for other metabolic pathways, such as the pentose phosphate pathway and the synthesis of amino acids and lipids. These multifunctional aspects underscore its importance in cellular metabolism and its relevance as a foundational concept in biology and biochemistry.
Definition of glycolysis
Glycolysis is a metabolic process in which glucose, a six-carbon sugar, is broken down into two three-carbon pyruvate molecules, producing a net gain of two ATP and two NADH molecules. This anaerobic pathway occurs in the cytosol and is a key step in cellular energy production.
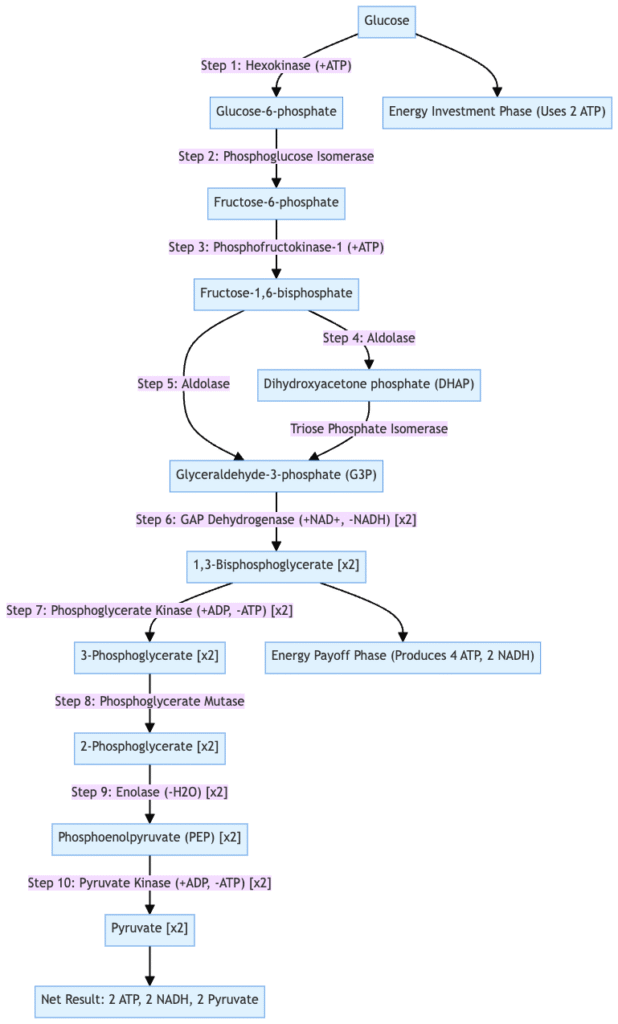
Major Features of Glycolysis
Glycolysis is a fundamental metabolic pathway involved in energy production. Its key features are outlined below:
- ATP Generation in Anaerobic Conditions
Glycolysis is the sole pathway for ATP production under anaerobic conditions. It does not require oxygen and occurs in the cytoplasm of cells. This is crucial for cells lacking mitochondria, such as red blood cells, where pyruvate is converted into lactate instead of entering aerobic pathways. - Pyruvate Production and Oxygen Dependence
When oxygen is available, glycolysis produces pyruvate. This pyruvate is then transported into mitochondria, where it enters the TCA cycle (also known as the citric acid cycle or Krebs cycle), contributing to further ATP production. - Connection to Anabolic Pathways
Beyond energy production, intermediates of glycolysis are utilized in anabolic pathways. These pathways help synthesize essential biomolecules, including glycogen, lipids, nucleotides, and proteins. Metabolites from glycolysis can enter various biosynthetic routes such as the pentose phosphate pathway, hexosamine pathway, and the serine and glycerol biosynthetic pathways, supporting cellular growth and function.
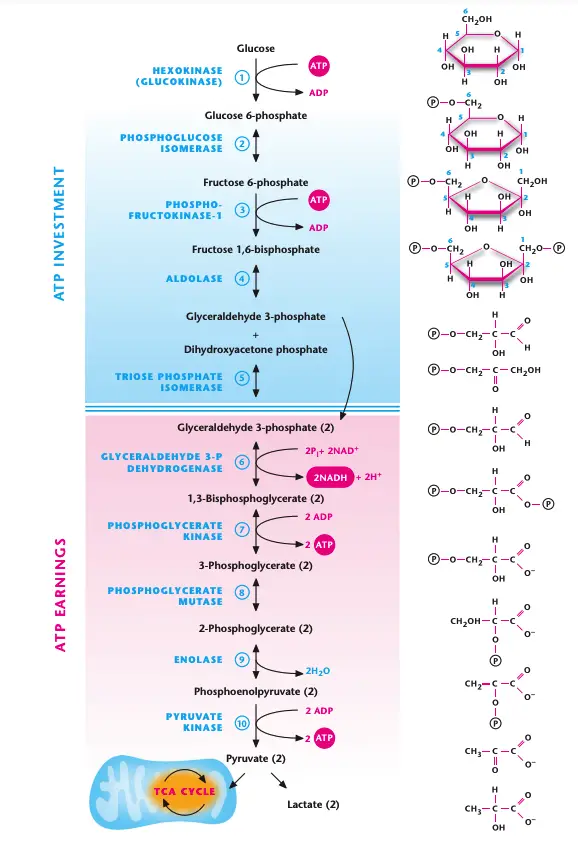
Glycolysis is a critical metabolic pathway that generates ATP without the need for oxygen. This pathway operates in the cytosol, and its primary function is to break down glucose and produce energy. The process can be broken down into two phases: the ATP investment phase and the ATP payoff phase.
- Location and Oxygen Requirements:
Glycolysis occurs in the cytosol and does not require oxygen to generate ATP. There is no net loss of carbon or oxygen atoms during the process, making it an efficient way to produce energy in anaerobic conditions. - Phases of Glycolysis:
Glycolysis is divided into two distinct phases:- ATP Investment Phase (Reactions 1–5):
- This phase requires the input of 2 ATP molecules to convert one molecule of glucose into two molecules of glyceraldehyde 3-phosphate.
- ATP Payoff Phase (Reactions 6–10):
- In this phase, the two molecules of glyceraldehyde 3-phosphate are metabolized into pyruvate, generating 4 ATP molecules via substrate-level phosphorylation.
- ATP Investment Phase (Reactions 1–5):
- ATP Balance:
Glycolysis results in a net production of 2 ATP molecules per glucose molecule. This is calculated by subtracting the 2 ATP molecules used in the investment phase from the 4 ATP molecules generated in the payoff phase. - NADH Production:
Two molecules of NAD+ are reduced to NADH during the glycolytic pathway, which is important for cellular processes like oxidative phosphorylation. - Phosphorylation of Intermediates:
Nine out of the 10 glycolytic intermediates are phosphorylated metabolites, meaning they cannot easily diffuse out of the cell due to their negative charge. This keeps the intermediates within the cell for further metabolism. - Key Regulatory Steps:
Glycolysis features three key regulatory steps that are essential for the pathway’s efficiency and direction. These steps are catalyzed by the enzymes:- Hexokinase (Step 1)
- Phosphofructokinase (Step 3)
- Pyruvate kinase (Step 10)
These steps have large negative ΔG values, meaning they are essentially irreversible and drive the flow of the pathway toward pyruvate.
- End Products and Fate of Pyruvate:
At the end of glycolysis, pyruvate is produced. Pyruvate can then be converted into lactate under anaerobic conditions, or it can enter the mitochondria to fuel the TCA cycle for further energy production in the presence of oxygen.
Glycolysis Equation
The glycolysis equation represents the overall chemical transformation that occurs during the breakdown of one glucose molecule into two pyruvate molecules, along with the generation of energy and reducing equivalents. The process can be summarized as:
C₆H₁₂O₆ + 2 ADP + 2 Pi + 2 NAD⁺ → 2 C₃H₄O₃ + 2 H₂O + 2 ATP + 2 NADH + 2 H⁺
In this equation:
- C₆H₁₂O₆ represents glucose, the six-carbon sugar that serves as the substrate for glycolysis.
- ADP (adenosine diphosphate) and Pi (inorganic phosphate) are used to generate ATP.
- NAD⁺ (nicotinamide adenine dinucleotide) is a coenzyme that accepts electrons during the oxidation steps, forming NADH.
The products include:
- C₃H₄O₃ (pyruvate), a three-carbon molecule that serves as the end product of glycolysis.
- H₂O (water), released during one of the reactions.
- ATP (adenosine triphosphate), the energy currency of the cell, with a net gain of two molecules.
- NADH, which carries electrons to later stages of cellular respiration.
- H⁺ (hydrogen ions), which contribute to maintaining cellular pH balance.
This equation captures the essence of glycolysis as a process that converts glucose into pyruvate, releasing a modest amount of energy and reducing equivalents for cellular use. It provides the foundational steps for both aerobic and anaerobic metabolic pathways.
Site of Glycolysis
Glycolysis is a metabolic process that predominantly occurs in the cytosol, the liquid matrix within cells. This intracellular compartment houses the necessary enzymes and substrates that facilitate the glycolytic reactions. Notably, certain tissues, such as erythrocytes, the cornea, and the lens, lack mitochondria, the primary site for energy production in many cells. In these mitochondria-devoid tissues, the cytosol becomes the principal site for ATP synthesis via glycolysis. Thus, the cytosol plays a pivotal role in ensuring energy production, especially in cells where alternative energy-generating pathways are absent.
Types of Glycolysis
Glycolysis, a fundamental metabolic pathway, can function under varying oxygen conditions, leading to its classification into two primary types:
- Aerobic Glycolysis: This form of glycolysis transpires in the presence of ample oxygen. The end product of this pathway is pyruvate. Concurrently, there is the synthesis of adenosine triphosphate (ATP) molecules, which serve as the primary energy currency of the cell.
- Anaerobic Glycolysis: In conditions where oxygen is limited or absent, glycolysis proceeds anaerobically. Under such circumstances, lactate is produced as the terminal product. Similar to its aerobic counterpart, ATP molecules are generated during this process.
In essence, the adaptability of glycolysis to function in both oxygen-rich and oxygen-deprived environments underscores its versatility and central role in cellular energy metabolism.
Enzymes involve in Glycolysis
Glycolysis is a ten-step biochemical process catalyzed by a series of enzymes, all of which are located in the cytosol. A common feature among these enzymes is their dependence on magnesium ions (Mg²⁺) to function effectively. Below is a breakdown of the enzymes and their roles in each step of the pathway:
- Hexokinase:
Initiates glycolysis by catalyzing the phosphorylation of glucose into glucose-6-phosphate using ATP. - Phosphoglucoisomerase:
Converts glucose-6-phosphate into fructose-6-phosphate through an isomerization reaction. - Phosphofructokinase (PFK):
Facilitates the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate using ATP. This is a key regulatory step in glycolysis. - Aldolase:
Cleaves fructose-1,6-bisphosphate into two three-carbon molecules: glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. - Phosphotriose Isomerase:
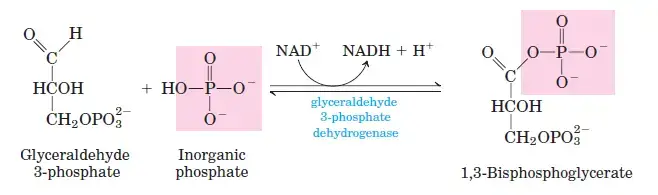
Converts dihydroxyacetone phosphate into glyceraldehyde-3-phosphate, ensuring a single type of three-carbon molecule continues through glycolysis. - Glyceraldehyde 3-Phosphate Dehydrogenase:
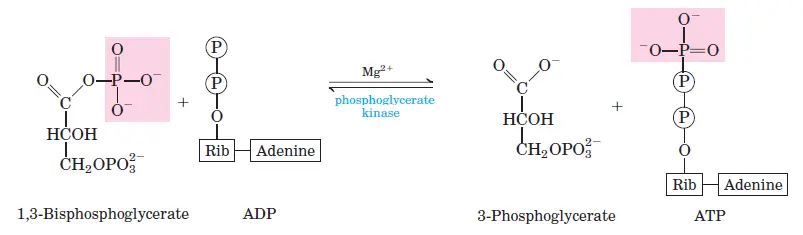
Oxidizes glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate, producing NADH in the process. - Phosphoglycerate Kinase:
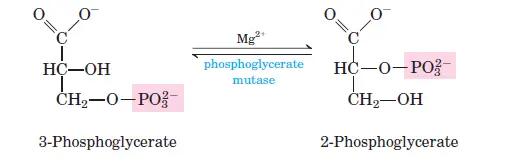
Transfers a phosphate group from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate. - Phosphoglycerate Mutase:
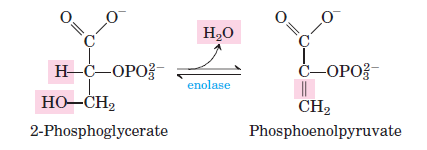
Rearranges 3-phosphoglycerate into 2-phosphoglycerate by transferring the phosphate group to a different position on the molecule. - Enolase:
Catalyzes the dehydration of 2-phosphoglycerate to phosphoenolpyruvate (PEP), forming a high-energy intermediate. - Pyruvate Kinase:
Facilitates the transfer of a phosphate group from phosphoenolpyruvate to ADP, producing ATP and pyruvate, the final product of glycolysis.
Summary Table of Glycolysis Enzymes
| Step | Enzyme | Reaction Catalyzed |
|---|---|---|
| 1 | Hexokinase | Phosphorylation of glucose to glucose-6-phosphate using ATP. |
| 2 | Phosphoglucoisomerase | Isomerization of glucose-6-phosphate to fructose-6-phosphate. |
| 3 | Phosphofructokinase (PFK) | Phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate using ATP. |
| 4 | Aldolase | Cleavage of fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. |
| 5 | Phosphotriose Isomerase | Conversion of dihydroxyacetone phosphate to glyceraldehyde-3-phosphate. |
| 6 | Glyceraldehyde 3-Phosphate Dehydrogenase | Oxidation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate, producing NADH. |
| 7 | Phosphoglycerate Kinase | Transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP, forming ATP. |
| 8 | Phosphoglycerate Mutase | Rearrangement of 3-phosphoglycerate to 2-phosphoglycerate. |
| 9 | Enolase | Dehydration of 2-phosphoglycerate to phosphoenolpyruvate (PEP). |
| 10 | Pyruvate Kinase | Transfer of a phosphate group from phosphoenolpyruvate to ADP, forming ATP and pyruvate. |
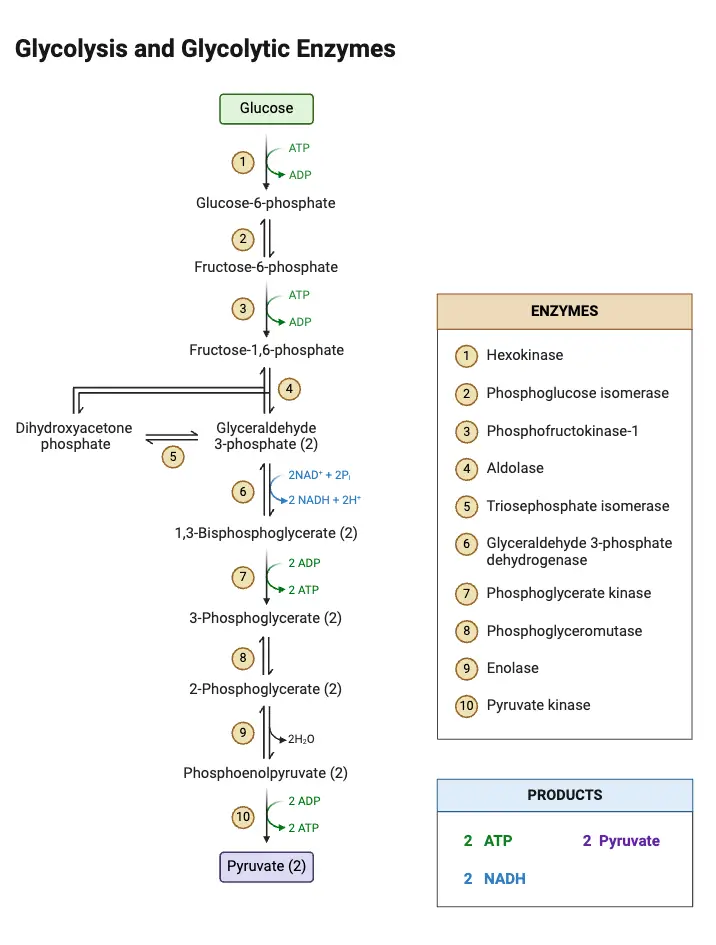
Glycolysis steps
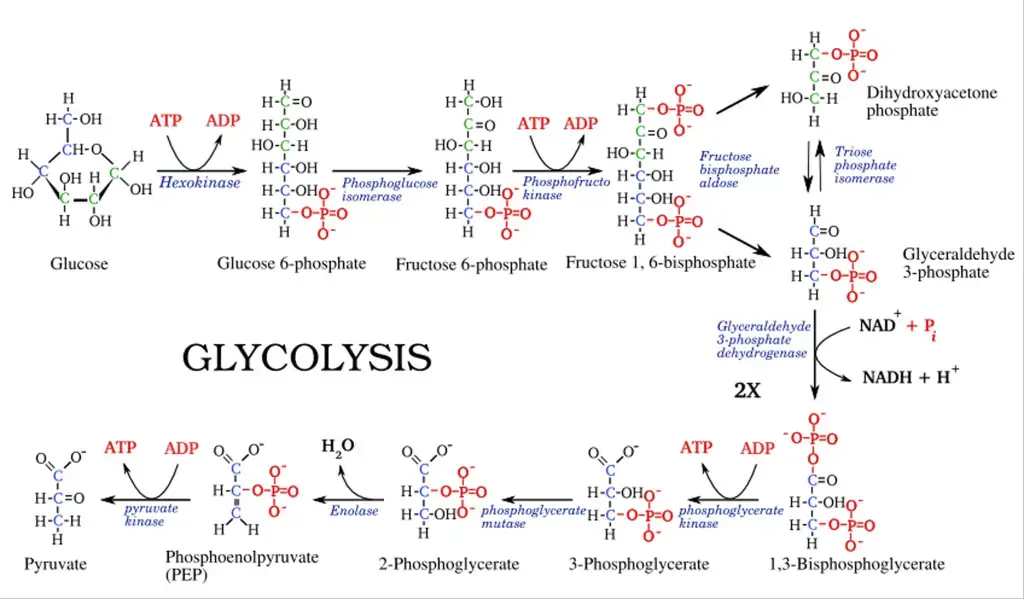
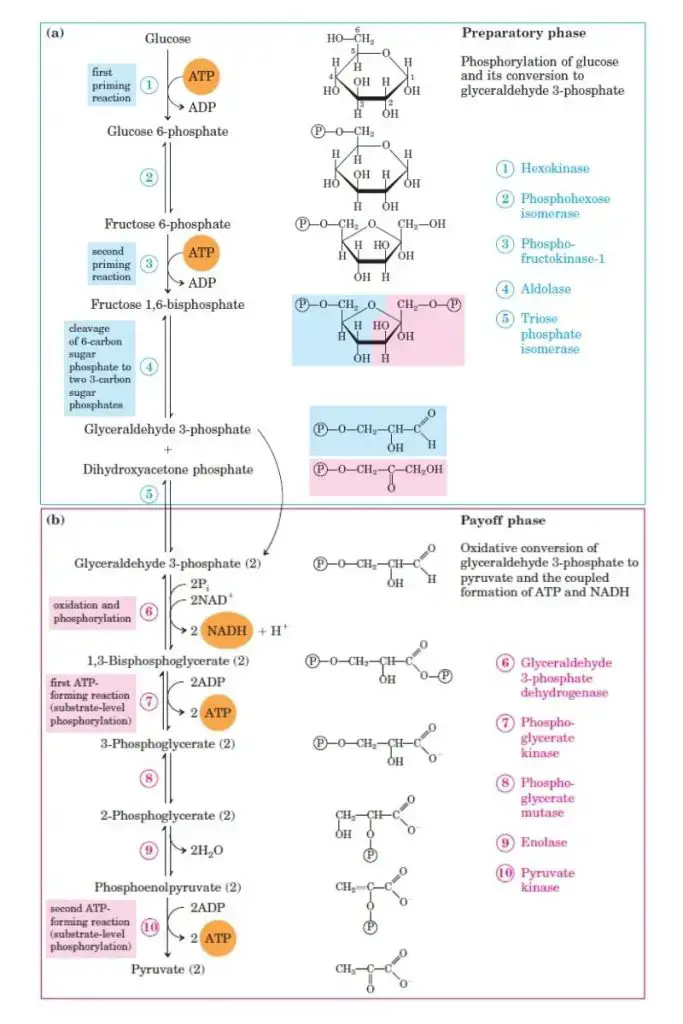
Glycolysis is a metabolic pathway that breaks down glucose into two pyruvate molecules through a series of 10 enzyme-catalyzed steps. These steps occur in two phases: a preparatory phase and an energy-yielding phase.
Phase I: Preparatory Phase (Steps 1–5)
This phase does not release energy but prepares glucose for subsequent reactions.
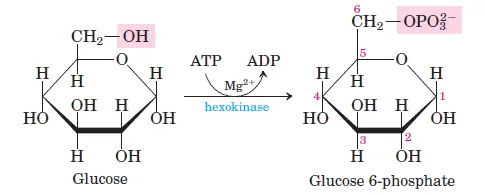
- Step 1: Phosphorylation of Glucose
Glucose is phosphorylated at the C6 carbon by hexokinase or glucokinase (in animals and microbes), forming glucose-6-phosphate. ATP donates the phosphate group, and some energy is lost as heat. - Step 2: Isomerization of Glucose-6-Phosphate
Glucose-6-phosphate is converted into fructose-6-phosphate by phosphohexose isomerase. This shifts the carbonyl oxygen from C1 to C2, transforming the aldose into a ketose. - Step 3: Phosphorylation of Fructose-6-Phosphate
Fructose-6-phosphate undergoes a second phosphorylation to form fructose-1,6-bisphosphate. This reaction, catalyzed by phosphofructokinase, uses ATP and releases heat energy. - Step 4: Cleavage of Fructose-1,6-Bisphosphate
The enzyme aldolase cleaves fructose-1,6-bisphosphate into two three-carbon sugars: glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). - Step 5: Isomerization of Dihydroxyacetone Phosphate
Dihydroxyacetone phosphate is converted into glyceraldehyde-3-phosphate by triose phosphate isomerase, completing the preparatory phase.
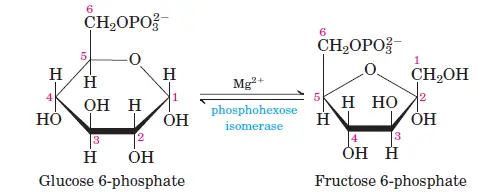
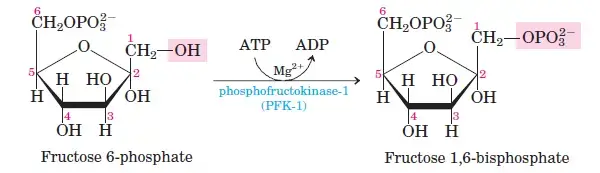
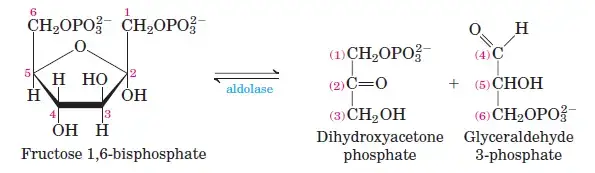
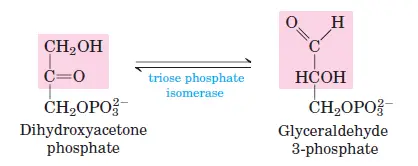
Phase II: Energy-Yielding Phase (Steps 6–10)
This phase conserves energy in the form of ATP and produces pyruvate molecules.
- Step 6: Oxidation of Glyceraldehyde-3-Phosphate
G3P is oxidized to 1,3-bisphosphoglycerate by glyceraldehyde-3-phosphate dehydrogenase. This step generates NADH by reducing NAD+. - Step 7: ATP Formation from 1,3-Bisphosphoglycerate
Phosphoglycerate kinase transfers a phosphate group from 1,3-bisphosphoglycerate to ADP, producing ATP and 3-phosphoglycerate. Two ATP molecules are generated per glucose molecule. - Step 8: Isomerization of 3-Phosphoglycerate
Phosphoglycerate mutase converts 3-phosphoglycerate into 2-phosphoglycerate by shifting the phosphate group from the third to the second carbon. - Step 9: Dehydration of 2-Phosphoglycerate
Enolase catalyzes the dehydration of 2-phosphoglycerate to phosphoenolpyruvate (PEP), resulting in the loss of water molecules. - Step 10: ATP Formation from Phosphoenolpyruvate
Pyruvate kinase transfers a phosphate from PEP to ADP, forming ATP and pyruvate. The pyruvate initially forms in the enol form, which rapidly converts to its keto form.
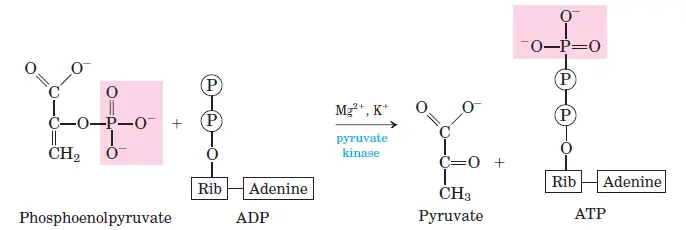
Glycolytic Reaction Steps
Glycolysis is a sequence of ten enzyme-catalyzed reactions that break down glucose into pyruvate, generating ATP and NADH in the process. Each step involves specific enzymatic actions that contribute to the overall energy yield. Below is a breakdown of the glycolytic reactions with their corresponding standard free energy changes (ΔG°0) and actual free energy changes (ΔG) under physiological conditions.
- Step 1: Glucose to Glucose-6-Phosphate
- Reaction: Glucose + ATP → Glucose-6-phosphate + ADP
- ΔG°0: −16.7 kJ/mol
- ΔG: −33.4 kJ/mol
- This step is an irreversible phosphorylation of glucose, driven by ATP hydrolysis.
- Step 2: Glucose-6-Phosphate to Fructose-6-Phosphate
- Reaction: Glucose-6-phosphate ⇌ Fructose-6-phosphate
- ΔG°0: 1.7 kJ/mol
- ΔG: 0 to 25 kJ/mol
- This is an isomerization reaction, which allows the further breakdown of glucose.
- Step 3: Fructose-6-Phosphate to Fructose-1,6-Bisphosphate
- Reaction: Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP
- ΔG°0: −14.2 kJ/mol
- ΔG: −22.2 kJ/mol
- This step involves a second phosphorylation, committing the molecule to glycolysis.
- Step 4: Fructose-1,6-Bisphosphate to Dihydroxyacetone Phosphate and Glyceraldehyde-3-Phosphate
- Reaction: Fructose-1,6-bisphosphate ⇌ Dihydroxyacetone phosphate + Glyceraldehyde-3-phosphate
- ΔG°0: 23.8 kJ/mol
- ΔG: 0 to −6 kJ/mol
- This cleavage reaction splits the six-carbon molecule into two three-carbon intermediates.
- Step 5: Dihydroxyacetone Phosphate to Glyceraldehyde-3-Phosphate
- Reaction: Dihydroxyacetone phosphate ⇌ Glyceraldehyde-3-phosphate
- ΔG°0: 7.5 kJ/mol
- ΔG: 0 to 4 kJ/mol
- An isomerization step that converts the triose phosphate into two molecules of glyceraldehyde-3-phosphate.
- Step 6: Glyceraldehyde-3-Phosphate to 1,3-Bisphosphoglycerate
- Reaction: Glyceraldehyde-3-phosphate + Pi + NAD+ ⇌ 1,3-bisphosphoglycerate + NADH + H+
- ΔG°0: 6.3 kJ/mol
- ΔG: −2 to 2 kJ/mol
- This step includes the reduction of NAD+ to NADH and the addition of an inorganic phosphate.
- Step 7: 1,3-Bisphosphoglycerate to 3-Phosphoglycerate
- Reaction: 1,3-bisphosphoglycerate + ADP ⇌ 3-phosphoglycerate + ATP
- ΔG°0: −18.8 kJ/mol
- ΔG: 0 to 2 kJ/mol
- The substrate-level phosphorylation of ADP to ATP occurs here.
- Step 8: 3-Phosphoglycerate to 2-Phosphoglycerate
- Reaction: 3-phosphoglycerate ⇌ 2-phosphoglycerate
- ΔG°0: 4.4 kJ/mol
- ΔG: 0 to 0.8 kJ/mol
- This step is an isomerization that prepares the molecule for the next step.
- Step 9: 2-Phosphoglycerate to Phosphoenolpyruvate
- Reaction: 2-phosphoglycerate ⇌ Phosphoenolpyruvate + H2O
- ΔG°0: 7.5 kJ/mol
- ΔG: 0 to 3.3 kJ/mol
- Dehydration occurs, generating the high-energy molecule phosphoenolpyruvate.
- Step 10: Phosphoenolpyruvate to Pyruvate
- Reaction: Phosphoenolpyruvate + ADP → Pyruvate + ATP
- ΔG°0: −31.4 kJ/mol
- ΔG: −16.7 kJ/mol
- The final step generates ATP and pyruvate, completing glycolysis. This reaction is also irreversible.
| Step | Reaction | ΔG°₀ (kJ/mol) | ΔG (kJ/mol) |
|---|---|---|---|
| 1 | Glucose + ATP → Glucose-6-phosphate + ADP | −16.7 | −33.4 |
| 2 | Glucose-6-phosphate ⇌ Fructose-6-phosphate | 1.7 | 0 to 25 |
| 3 | Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP | −14.2 | −22.2 |
| 4 | Fructose-1,6-bisphosphate ⇌ Dihydroxyacetone phosphate + Glyceraldehyde-3-phosphate | 23.8 | 0 to −6 |
| 5 | Dihydroxyacetone phosphate ⇌ Glyceraldehyde-3-phosphate | 7.5 | 0 to 4 |
| 6 | Glyceraldehyde-3-phosphate + Pi + NAD⁺ ⇌ 1,3-bisphosphoglycerate + NADH + H⁺ | 6.3 | −2 to 2 |
| 7 | 1,3-Bisphosphoglycerate + ADP ⇌ 3-phosphoglycerate + ATP | −18.8 | 0 to 2 |
| 8 | 3-Phosphoglycerate ⇌ 2-phosphoglycerate | 4.4 | 0 to 0.8 |
| 9 | 2-Phosphoglycerate ⇌ Phosphoenolpyruvate + H₂O | 7.5 | 0 to 3.3 |
| 10 | Phosphoenolpyruvate + ADP → Pyruvate + ATP | −31.4 | −16.7 |
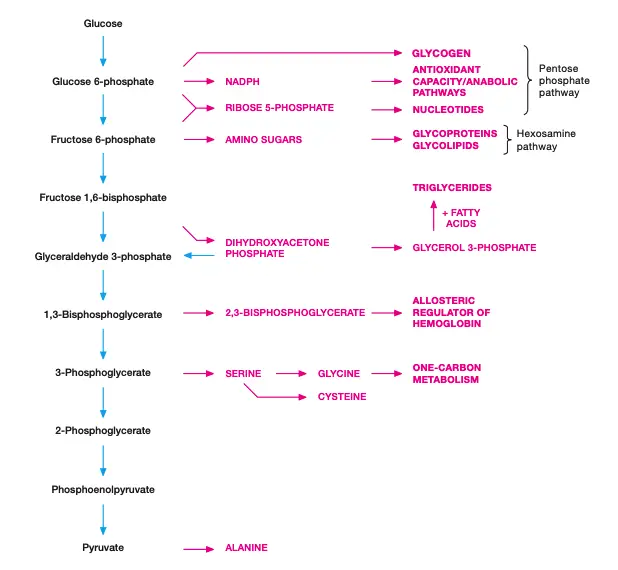
Biosynthetic Capacity of Glycolytic Intermediates
Glycolysis serves more than just ATP production. Its intermediates play vital roles in various biosynthetic pathways. These pathways are critical for cell survival and function, especially when oxygen levels are low.
- Glucose-6-phosphate can enter the pentose phosphate pathway. This provides NADPH, which is crucial for maintaining antioxidant capacity in the cell, and ribose, the building block for nucleotides used in DNA and RNA synthesis.
- Glucose-6-phosphate also contributes to glycogen synthesis, storing energy in cells for later use.
- Fructose-6-phosphate feeds into the hexosamine biosynthetic pathway, generating amino sugars. These are essential for synthesizing glycoproteins, glycolipids, and proteoglycans, all important for cell structure and signaling.
- Dihydroxyacetone phosphate can be converted into glycerol-3-phosphate, a precursor for the synthesis of lipids.
- 1,3-Bisphosphoglycerate produces 2,3-bisphosphoglycerate in red blood cells. This molecule is an allosteric regulator of hemoglobin, affecting its oxygen affinity.
- 3-Phosphoglycerate can be converted into serine, an amino acid that serves as a precursor for cysteine and glycine, both important in protein synthesis and cellular function.
- Pyruvate, the end product of glycolysis, can also be converted into alanine, an amino acid involved in protein synthesis.
Result of Glycolysis/Product of Glycolysis
Glycolysis is a crucial metabolic pathway that processes glucose into pyruvate, while generating essential energy molecules in the process. The end result of glycolysis includes several key transformations that occur during the 10-step pathway.
- Glucose is converted to pyruvate
A single molecule of glucose (6 carbons) is split and oxidized into two molecules of pyruvate (3 carbons each). This step is essential for cells to extract energy from glucose. - NAD+ is reduced to NADH
During glycolysis, NAD+ accepts electrons and is reduced to NADH. This happens particularly during the oxidation of glyceraldehyde 3-phosphate (G3P) to 1,3-bisphosphoglycerate in Step 6. The resulting NADH can later be used in other metabolic processes, like oxidative phosphorylation. - ATP is synthesized
ATP is generated through substrate-level phosphorylation in two distinct steps of glycolysis. First, during Step 7, 1,3-bisphosphoglycerate donates a phosphate group to ADP, forming ATP. Then, in Step 10, phosphoenolpyruvate donates a phosphate group to ADP, forming more ATP. These ATP molecules are directly available for cellular activities.
In the end, glycolysis results in:
- Two molecules of pyruvate
- Two molecules of NADH
- A net gain of two ATP molecules
Result of Glycolysis – Summary Table
| Event | Description | Outcome |
|---|---|---|
| Glucose Conversion | Glucose (6 carbons) is converted into pyruvate (3 carbons). | Two molecules of pyruvate. |
| NAD+ Reduction | NAD+ is reduced to NADH during the oxidation of G3P. | Two molecules of NADH. |
| ATP Generation | ATP is generated through substrate-level phosphorylation. | Net gain of two ATP molecules. |
Fates of Pyruvate
Pyruvate, the end product of glycolysis, can follow different paths based on the organism and the availability of oxygen. The three primary fates of pyruvate are as follows:
- Oxidation of Pyruvate (Aerobic Respiration)
- In aerobic organisms, pyruvate moves into the mitochondria.
- It is oxidized to form acetyl-CoA, releasing one molecule of CO₂ in the process.
- Acetyl-CoA then enters the citric acid cycle where it is completely oxidized into CO₂ and H₂O.
- This pathway occurs after glycolysis in aerobic conditions, including in plants.
- Lactic Acid Fermentation (Anaerobic Conditions)
- In the absence of oxygen, such as in muscle cells during strenuous activity, pyruvate cannot undergo oxidation.
- Instead, pyruvate is reduced to lactate through anaerobic glycolysis.
- This process allows cells to continue generating ATP when oxygen supply is limited.
- Other anaerobic organisms also produce lactate through lactic acid fermentation.
- Alcoholic Fermentation (Anaerobic Fermentation in Microbes)
- In certain microbes like yeast, pyruvate is converted anaerobically into ethanol and CO₂.
- This pathway is a fundamental metabolic process in environments with low or no oxygen.
- Alcoholic fermentation is widely observed in microorganisms and is the basis of brewing and fermentation processes.
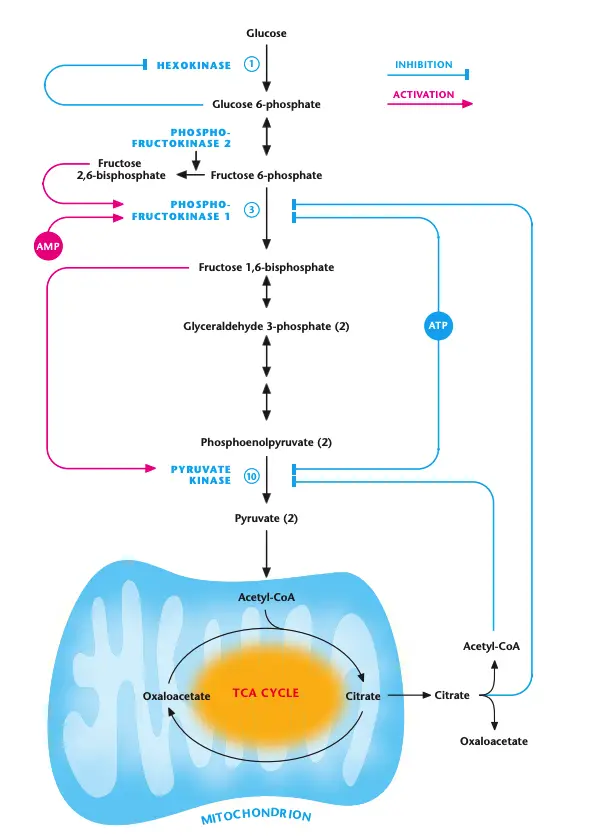
Regulation of Glycolysis
Glycolysis, the primary metabolic pathway for glucose breakdown, is tightly regulated at key enzymes to ensure that the cell can balance energy production with biosynthetic needs. The main control points of glycolysis involve the irreversible reactions catalyzed by hexokinase, phosphofructokinase-1 (PFK-1), and pyruvate kinase. These regulatory steps are influenced by several mechanisms, including allosteric modulation, phosphorylation, and product inhibition.
- Hexokinase Regulation:
- The first step of glycolysis is catalyzed by hexokinase, with four isoforms (HKI-IV) present in various tissues.
- HKI, HKII, and HKIII have a low Km for glucose (<0.5 mM) and are inhibited by glucose 6-phosphate. This feedback inhibition ensures that glucose is not wasted on glycolysis when it is already abundant.
- Hexokinase IV (also known as glucokinase) has a higher Km (6 mM) and is not inhibited by glucose 6-phosphate. This allows the liver, where glucokinase is abundant, to remove excess glucose from the bloodstream when blood glucose levels rise after meals.
- Glucokinase also has a higher Vmax, allowing it to process larger amounts of glucose as needed, helping regulate blood glucose levels.
- Phosphofructokinase-1 (PFK-1) Regulation:
- PFK-1, the enzyme catalyzing one of the key steps in glycolysis, is regulated by several factors.
- High ATP levels allosterically inhibit PFK-1, signaling that the cell has enough energy and doesn’t need to continue glycolysis. Conversely, when ATP is used up, the resulting AMP can overcome this inhibition, activating PFK-1 to continue glycolysis.
- Another crucial modulator is fructose 2,6-bisphosphate, which activates PFK-1. The production of fructose 2,6-bisphosphate is controlled by phosphofructokinase-2 (PFK-2). Insulin dephosphorylates PFK-2, activating it and increasing the levels of fructose 2,6-bisphosphate, which in turn activates PFK-1. In contrast, glucagon phosphorylates PFK-2, reducing fructose 2,6-bisphosphate and decreasing PFK-1 activity.
- TCA cycle intermediates like citrate and acetyl-CoA can also inhibit PFK-1 and pyruvate kinase when the TCA cycle is saturated. This prevents excessive accumulation of pyruvate when the cell has enough energy.
- Pyruvate Kinase Regulation:
- Pyruvate kinase is another key enzyme in glycolysis and is regulated by AMP and ATP levels. High ATP levels inhibit pyruvate kinase, while AMP can activate it during periods of low ATP.
- Fructose 1,6-bisphosphate, the product of the reaction catalyzed by PFK-1, activates pyruvate kinase in a feed-forward activation mechanism. This coordination ensures that glycolysis moves forward efficiently, as pyruvate kinase activity helps maintain a low concentration of intermediates in the pathway.
- Glucose Availability and Transport:
- The availability of glucose is controlled by transporters called GLUTs. There are five types of GLUTs, each located in different tissues, such as GLUT1 in red blood cells, GLUT2 in the liver, and GLUT4 in muscle and fat cells. The expression of GLUTs can be regulated to control the amount of glucose entering the cell.
- Additionally, cells can store glucose as glycogen. When glucose levels drop, glycogen phosphorylase breaks down glycogen into glucose, while glycogen synthase controls glycogen storage. These processes are regulated by feedback loops involving glucose and its metabolites, as well as phosphorylation mechanisms.
- Oxygen and the Pasteur Effect:
- The availability of oxygen also affects glycolysis. In aerobic conditions, cells primarily use oxidative phosphorylation, which diminishes glycolysis. However, in anaerobic conditions, glycolysis accelerates. This is known as the Pasteur effect.
- The Pasteur effect occurs because oxygen, by increasing mitochondrial activity, reduces the need for glycolysis. In tissues with high mitochondrial capacity, such as muscle cells or hepatocytes, this effect is more prominent. However, in other tissues like pancreatic cells, the effect is less noticeable.
- Enzyme Induction:
- The transcriptional control of glycolytic enzymes allows cells to adjust glycolysis rates in response to hormonal changes. For instance, insulin increases the activity of enzymes like hexokinase and pyruvate kinase, boosting glycolysis in response to elevated glucose levels.
| Regulated Enzyme | Regulation Mechanisms | Key Points |
|---|---|---|
| Hexokinase | – Feedback inhibition by glucose-6-phosphate (G6P) | – Hexokinase has four isoforms (HKI-IV) with different Km values for glucose. – HKI, HKII, HKIII are inhibited by high G6P levels. – Glucokinase (Hexokinase IV) is found in the liver, with a higher Km and no G6P inhibition, allowing glucose removal when blood levels rise. |
| Phosphofructokinase-1 (PFK-1) | – Allosteric inhibition by ATP – Activation by AMP and fructose 2,6-bisphosphate – Inhibition by citrate and acetyl-CoA | – ATP inhibition signals sufficient energy, while AMP activates PFK-1 when ATP is low. – Fructose 2,6-bisphosphate (F2,6BP) activates PFK-1; its production is controlled by PFK-2. – Insulin activates PFK-2, increasing F2,6BP, while glucagon reduces F2,6BP levels, decreasing PFK-1 activity. – High citrate and acetyl-CoA from the TCA cycle inhibit PFK-1 and pyruvate kinase when the cycle is saturated. |
| Pyruvate Kinase | – Allosteric regulation by ATP and AMP – Activation by fructose 1,6-bisphosphate (feed-forward activation) | – AMP activates pyruvate kinase during low ATP levels, while ATP inhibits it. – Fructose 1,6-bisphosphate, a product of PFK-1, activates pyruvate kinase to maintain glycolysis flow. |
| Glucose Transport (GLUTs) | – Regulated transporter expression | – GLUTs are transporters for glucose. Different types are expressed in various tissues (e.g., GLUT1 in RBCs, GLUT2 in liver, GLUT4 in muscle/fat cells). – GLUT expression is regulated based on glucose availability. |
| Glycogen Metabolism | – Glycogen phosphorylase and glycogen synthase regulation | – Glycogen phosphorylase breaks down glycogen to release glucose. – Glycogen synthase promotes glycogen storage. – Feedback loops involving glucose and metabolites regulate these enzymes. |
| Oxygen (Pasteur Effect) | – Effect of oxygen on glycolysis | – Aerobic conditions: Oxidative phosphorylation reduces glycolysis. – Anaerobic conditions: Glycolysis accelerates to produce ATP without oxygen. – The Pasteur effect is more pronounced in tissues with high mitochondrial capacity (e.g., muscles, hepatocytes). |
| Enzyme Induction | – Transcriptional control of glycolytic enzymes | – Insulin increases glycolytic enzyme activity (e.g., hexokinase, pyruvate kinase) in response to elevated glucose levels, boosting glycolysis. |
Regulated Enzymes in Glycolysis
The regulation of glycolysis is a complex process that ensures the pathway operates efficiently based on the cell’s energy demands and external conditions. Key enzymes—hexokinase (or glucokinase in the liver), phosphofructokinase (PFK), and pyruvate kinase—are crucial in managing the flux through glycolysis, adjusting it according to both internal and external factors.
- Hexokinase and Glucokinase
- Hexokinase catalyzes the conversion of glucose to glucose-6-phosphate (G6P) once glucose enters the cell. High levels of G6P inhibit hexokinase, thereby regulating glucose entry based on cellular needs.
- Glucokinase, found specifically in the liver, works similarly to hexokinase but operates differently. It is not inhibited by G6P and is active only when blood glucose levels are high. This allows the liver to regulate glycolysis based on blood sugar concentrations.
- Phosphofructokinase (PFK)
- PFK is a major regulatory step in glycolysis due to its irreversible nature. It is activated by AMP and fructose-2,6-bisphosphate (F2,6BP), which enhances glycolytic flow.
- The liver regulates PFK activity through the levels of F2,6BP. When blood glucose levels are low, hormones like glucagon elevate cAMP, activating protein kinase A (PKA). This leads to the phosphorylation of PFK2, converting F2,6BP back into fructose-6-phosphate, reducing PFK activity, and promoting gluconeogenesis instead of glycolysis.
- The competition between ATP and AMP for binding to PFK is critical. Although ATP is more abundant in cells, a drop in ATP increases AMP, which activates PFK and stimulates glycolysis.
- Citrate, though it inhibits PFK in vitro, is less significant in vivo, as it is primarily used for fatty acid and cholesterol synthesis rather than directly regulating glycolysis.
- TIGAR, a p53-induced protein, modulates F2,6BP levels. It can either convert F6P to F2,6BP (activating glycolysis) or reverse this process, helping to adjust glycolysis in response to oxidative stress.
- Pyruvate Kinase
- The final step in glycolysis is catalyzed by pyruvate kinase, which converts phosphoenolpyruvate to pyruvate, generating ATP in the process. This enzyme is tightly regulated by several mechanisms:
- Covalent modification: In the liver, glucagon activates PKA, which phosphorylates and inhibits pyruvate kinase during fasting, preventing unnecessary glycolysis when glucose is scarce.
- Insulin response: When blood glucose levels rise, insulin stimulates protein phosphatase 1, dephosphorylating and activating pyruvate kinase.
- Tissue-specific regulation: In muscle tissue, pyruvate kinase is less sensitive to PKA, allowing glycolysis to continue even during fasting, ensuring energy availability during physical activity.
- The final step in glycolysis is catalyzed by pyruvate kinase, which converts phosphoenolpyruvate to pyruvate, generating ATP in the process. This enzyme is tightly regulated by several mechanisms:
| Enzyme | Regulation Mechanisms | Key Points |
|---|---|---|
| Hexokinase & Glucokinase | – Feedback inhibition by glucose-6-phosphate (G6P) – Glucokinase in the liver is not inhibited by G6P | – Hexokinase converts glucose to glucose-6-phosphate (G6P), which inhibits it at high G6P levels, regulating glucose entry. – Glucokinase in the liver is active when blood glucose is high, helping regulate blood sugar. |
| Phosphofructokinase (PFK) | – Activation by AMP and fructose-2,6-bisphosphate (F2,6BP) – Inhibition by ATP – Regulation by hormones (glucagon) | – AMP activates PFK to stimulate glycolysis when ATP is low. – F2,6BP enhances PFK activity; glucagon reduces F2,6BP, slowing glycolysis. – Citrate inhibits PFK in vitro but has less impact in vivo. |
| Pyruvate Kinase | – Covalent modification (phosphorylation by glucagon/PKA) – Activation by insulin (dephosphorylation) – Tissue-specific regulation | – Glucagon inhibits pyruvate kinase in the liver during fasting by phosphorylation. – Insulin activates pyruvate kinase when glucose is abundant. – Muscle tissue maintains pyruvate kinase activity even during fasting. |
Post-Glycolysis Processes
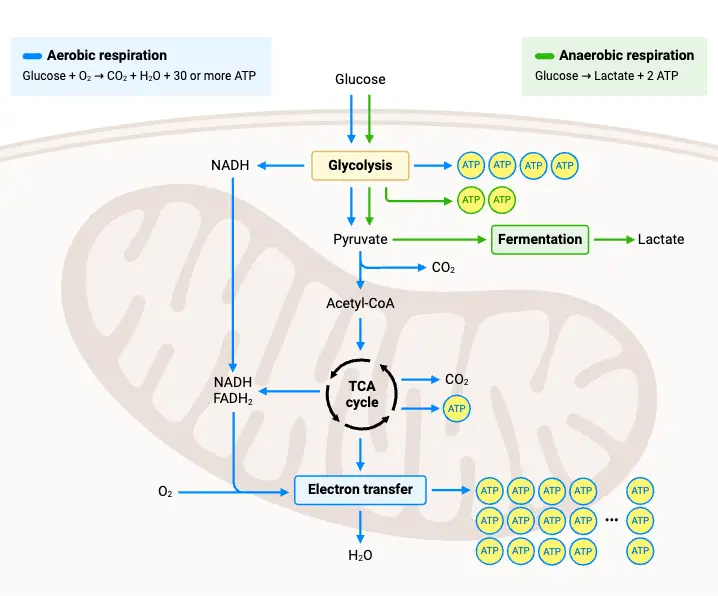
Once glycolysis has broken down glucose into pyruvate, several processes are required to handle the pyruvate and regenerate the necessary cofactors like NAD+ to keep glycolysis running. These processes vary based on the presence or absence of oxygen, leading to either anaerobic or aerobic pathways. Below are key steps that follow glycolysis, dictating how energy is further processed.
- Regeneration of NAD+ (Anaerobic Conditions)
- Lactic Acid Fermentation: In oxygen-deprived environments, cells regenerate NAD+ by converting pyruvate into lactate. This reaction occurs in the cytoplasm and involves the reduction of pyruvate by NADH, yielding lactate and regenerating NAD+. This pathway is especially important in muscle cells during intense exercise when oxygen availability is limited.
- Pyruvate + NADH + H⁺ → Lactate + NAD⁺
- Ethanol Fermentation: In yeast and certain bacteria, NAD+ is regenerated through ethanol fermentation. Pyruvate is first converted into acetaldehyde and CO₂, which is then reduced to ethanol. This process also operates without oxygen and provides an alternative energy pathway under anaerobic conditions.
- Lactic Acid Fermentation: In oxygen-deprived environments, cells regenerate NAD+ by converting pyruvate into lactate. This reaction occurs in the cytoplasm and involves the reduction of pyruvate by NADH, yielding lactate and regenerating NAD+. This pathway is especially important in muscle cells during intense exercise when oxygen availability is limited.
- Aerobic Conditions and the Mitochondrial Pathway
- Transport of Pyruvate into Mitochondria: In the presence of oxygen, pyruvate is transported into the mitochondria where it is further metabolized. However, the inner mitochondrial membrane is impermeable to NADH and NAD+, so special shuttles are used to transfer electrons across the membrane.
- The malate-aspartate shuttle transfers electrons from NADH to oxaloacetate, forming malate, which can pass into the mitochondria.
- The glycerol-phosphate shuttle transfers electrons from NADH to dihydroxyacetone phosphate, forming glycerol-3-phosphate, which is oxidized back to dihydroxyacetone, donating electrons to FAD instead of NAD+.
- Transport of Pyruvate into Mitochondria: In the presence of oxygen, pyruvate is transported into the mitochondria where it is further metabolized. However, the inner mitochondrial membrane is impermeable to NADH and NAD+, so special shuttles are used to transfer electrons across the membrane.
- Pyruvate Decarboxylation
- Once in the mitochondrion, pyruvate undergoes decarboxylation, forming acetyl-CoA, CO₂, and NADH. Acetyl-CoA is then ready to enter the citric acid cycle, where it undergoes further transformations to generate additional energy carriers, such as NADH and FADH₂, which are used in the electron transport chain for ATP production.
- Citric Acid Cycle (Krebs Cycle)
- Acetyl-CoA combines with oxaloacetate to form citric acid, beginning the citric acid cycle. Through a series of enzyme-catalyzed steps, citric acid is decarboxylated and rearranged, eventually regenerating oxaloacetate. This cycle produces high-energy molecules like NADH, FADH₂, and GTP (or ATP) while releasing CO₂ as a byproduct.
- Oxidative Phosphorylation
- The NADH and FADH₂ generated in the citric acid cycle donate their electrons to the electron transport chain (ETC), which is located in the inner mitochondrial membrane. The energy from electron transfer is used to pump protons across the membrane, creating an electrochemical gradient.
- This gradient drives ATP synthase, a protein complex that produces ATP as protons flow back into the mitochondrial matrix.
- Fatty Acid and Cholesterol Synthesis
- The acetyl-CoA produced from pyruvate also serves as a building block for the synthesis of fatty acids and cholesterol. Acetyl-CoA cannot directly cross the mitochondrial membrane, so it is converted into citrate, which is transported into the cytosol. There, it is cleaved back into acetyl-CoA, which can be used for the biosynthesis of lipids like fatty acids and cholesterol.
- Conversion of Pyruvate into Oxaloacetate
- In certain cells, pyruvate can be converted into oxaloacetate by the enzyme pyruvate carboxylase. This is an anaplerotic reaction, meaning it replenishes intermediates in the citric acid cycle, helping to maintain the cycle’s function when the body’s energy demand increases, such as during exercise.
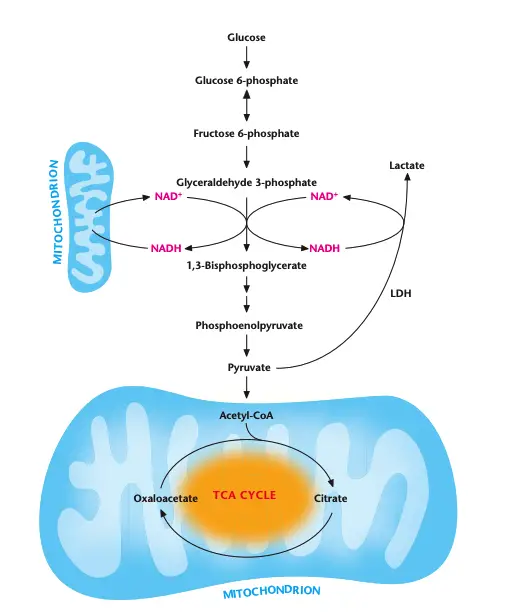
What happens to the nadh and pyruvate generated during glycolysis?
During glycolysis, NADH and pyruvate are key metabolites that undergo distinct fates depending on the availability of oxygen and cellular energy demands.
- Oxygen Present (Aerobic Conditions):
- Pyruvate enters the mitochondria.
- Inside the mitochondria, pyruvate is converted into acetyl-CoA and enters the TCA cycle for further energy production.
- NADH generated in glycolysis is transported into the mitochondria.
- Electron Transport Chain (ETC) oxidizes NADH to NAD+, which is then shuttled back into the cytosol to support glycolysis, maintaining a steady NAD+ pool for continued glycolysis activity.
- The transport of NADH and NAD+ in and out of the mitochondria involves complex shuttling mechanisms, resulting in a slower regeneration of NAD+ compared to anaerobic conditions.
- Oxygen Absent (Anaerobic Conditions):
- In the absence of oxygen, the electron transport chain cannot function, which prevents NADH oxidation to NAD+.
- Lactate Dehydrogenase (LDH) converts pyruvate into lactate, coupled with the oxidation of NADH to NAD+.
- This reaction is essential to regenerate NAD+ and sustain glycolysis under anaerobic conditions, a process known as anaerobic glycolysis or homolactic fermentation.
- Lactate buildup occurs when oxygen cannot reach muscles fast enough during vigorous exercise, inhibiting the mitochondrial processes.
- Fermentation in Yeast:
- Yeast also uses pyruvate for fermentation when oxygen is unavailable.
- Pyruvate is converted to ethanol and CO2 in a two-step process that also regenerates NAD+.
- Under anaerobic conditions, yeast consumes glucose to produce ethanol in higher quantities than under aerobic conditions, a phenomenon discovered by Louis Pasteur.
- Pasteur Effect:
- The Pasteur effect refers to the inhibition of fermentation when oxygen is present. Oxygen allows mitochondrial ATP generation, reducing the need for anaerobic glycolysis and fermentation.
- Crabtree Effect:
- The Crabtree effect occurs when high glucose levels suppress mitochondrial ATP production under aerobic conditions, promoting glycolysis and fermentation instead.
- Regulation Mechanisms:
- Metabolites regulate glycolytic enzymes in the short term.
- Transcription factors control the long-term expression of glycolytic enzymes to adapt to different metabolic conditions.
Glycolysis and Disease
Glycolysis plays a crucial role in various diseases, affecting everything from metabolism to cell function. The way the pathway operates can be altered by different conditions, leading to several health problems, including diabetes, genetic disorders, and cancer.
- Diabetes and Glycolysis:
Glycolysis is a key player in regulating blood glucose levels. In healthy individuals, insulin stimulates the cellular uptake of glucose, which is then metabolized through glycolysis, lowering blood sugar. However, in diabetes, insulin resistance or insufficient insulin production causes glucose to remain in the bloodstream, leading to hyperglycemia. The liver exacerbates this condition by producing even more glucose via gluconeogenesis. When glycolysis in hepatocytes fails to properly regulate glucose metabolism, it contributes to sustained high blood glucose levels, worsening the diabetic state. - Genetic Disorders and Glycolysis Mutations:
Mutations in the enzymes of glycolysis are rare, given the pathway’s importance for cell survival. When they do occur, they typically result in cellular respiration failure, leading to cell death at an early stage. One well-known example is pyruvate kinase deficiency, which causes chronic hemolytic anemia. In some genetic disorders like combined malonic and methylmalonic aciduria (CMAMMA), caused by ACSF3 deficiency, glycolysis can be reduced by about 50%. This happens due to reduced lipoylation of key mitochondrial enzymes, such as the pyruvate dehydrogenase complex. The resulting defect hinders energy production and contributes to metabolic disturbances. - Cancer and Altered Glycolysis:
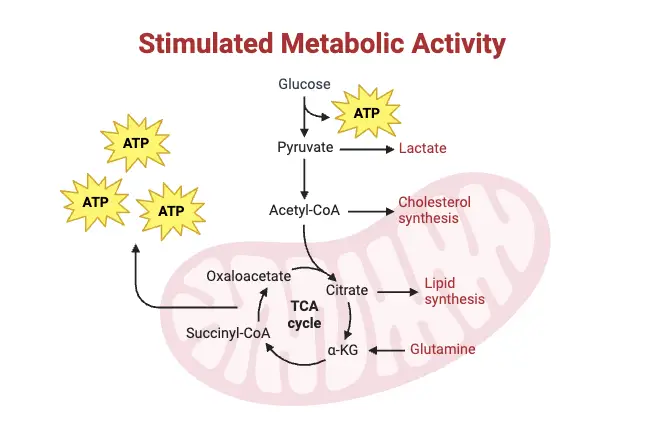
In cancer, glycolysis is often upregulated to support the energy demands of rapidly dividing tumor cells. These cells perform glycolysis up to ten times faster than normal tissue. The Warburg effect, first observed by Otto Warburg in the 1930s, explains how tumors rely on anaerobic glycolysis for ATP production, even in the presence of oxygen. This phenomenon occurs because tumor cells often experience hypoxia (a lack of oxygen) due to inadequate capillary supply. To adapt, they increase glycolysis to produce sufficient energy, even in low-oxygen conditions. Tumor cells often overexpress specific glycolytic enzymes or use isoenzymes that are less sensitive to feedback inhibition, further promoting the glycolytic flux. This heightened glycolysis helps bypass the constraints of hypoxia and ensures ATP generation.- This accelerated glycolytic activity is not just a metabolic oddity; it has important clinical applications. The Warburg effect is leveraged in cancer diagnosis and treatment monitoring, where positron emission tomography (PET) scans use FDG (fluorodeoxyglucose), a modified glucose molecule, to detect high glycolytic activity in tumors.
- The potential for treating cancer by targeting glycolysis has prompted research into therapies that reduce glycolytic activity. Strategies like the ketogenic diet, which shifts metabolism away from glycolysis, are being explored as possible ways to starve cancer cells of the energy they need to proliferate.
The relationship between glycolysis and cancer metabolism has gained significant attention. In the 1920s, Otto Warburg observed that cancer cells consume glucose and produce lactate at much higher rates than normal tissue, even in the presence of sufficient oxygen. This observation laid the foundation for what is now known as the Warburg effect—a hallmark of cancer metabolism.
- The Warburg Effect:
- Higher glucose uptake: Tumor cells take in glucose at an accelerated rate compared to healthy tissue. This increased glucose uptake supports their high metabolic demands.
- Lactate secretion: Even in oxygen-rich environments, cancer cells convert glucose into lactate, a process typically seen under anaerobic conditions.
- Clinical application: The Warburg effect is leveraged in positron emission tomography (PET), which uses a glucose analog to detect tumors based on their elevated glucose uptake. This allows for non-invasive cancer diagnosis and monitoring.
- Initial Hypothesis and Later Findings:
- Warburg initially speculated that cancer cells had defective mitochondrial function, which would prevent pyruvate from being fully oxidized in the mitochondria. This would push the pyruvate into lactate production, even with oxygen present.
- However, research later showed that most cancer cells have functional mitochondria. The elevated glycolysis in tumors is not necessarily due to mitochondrial dysfunction but rather to changes in cell signaling pathways that enhance glycolytic activity.
- Role of Glycolysis Beyond ATP Production:
- While glycolysis generates ATP, this is not the primary advantage of the Warburg effect in cancer cells. Instead, glycolysis provides a significant biosynthetic advantage.
- The intermediates of glycolysis serve as precursors for important biosynthetic pathways. These include:
- Pentose phosphate pathway: Generates NADPH and ribose 5-phosphate, crucial for nucleotide synthesis and maintaining redox balance.
- Hexosamine pathway: Supplies materials for glycosylation, important for protein modification and signaling.
- Amino acid and lipid synthesis: These are critical for the rapid growth and proliferation of cancer cells, as they require new macromolecules to divide and expand.
- Glycolysis in Normal Proliferating Cells:
- Interestingly, the Warburg effect is not unique to cancer cells. Highly proliferative normal cells, such as T lymphocytes, also exhibit increased glycolysis when activated. This suggests that the Warburg effect is linked to cell proliferation rather than simply to cancerous transformation.
- Both normal and cancer cells increase the expression and activity of glycolytic enzymes through signaling pathways and transcription factors. This allows them to maintain high metabolic flux through glycolysis, supporting their growth and biosynthesis.
By reprogramming their metabolism to favor glycolysis, cancer cells not only meet their energy demands but also ensure the biosynthesis necessary for their rapid proliferation. The Warburg effect is a pivotal feature of cancer cell biology, reflecting the shift from a simple energy production pathway to one that supports the anabolic needs of proliferating cells.
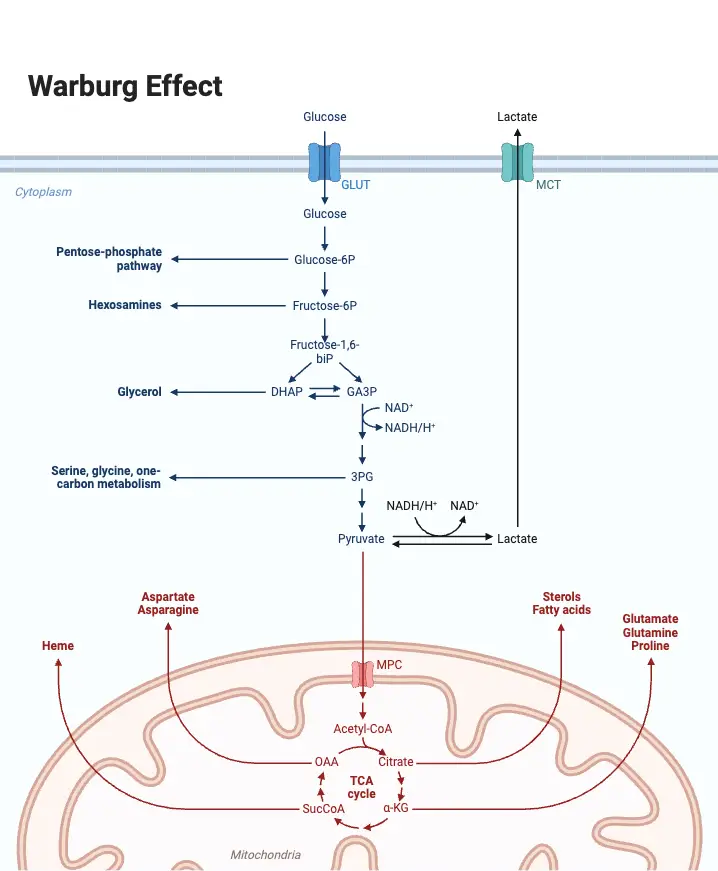
Significance of Glycolysis Pathway
Glycolysis plays a pivotal role in cellular metabolism by converting glucose into pyruvate, generating energy in the form of ATP. This pathway is crucial for cells under both aerobic and anaerobic conditions. Below are the key points detailing its importance:
- Energy Production
- Glycolysis begins the process of glucose breakdown, providing ATP and NADH for cellular activities.
- It occurs in the cytoplasm, does not require oxygen, making it an anaerobic pathway.
- A single glucose molecule yields two ATP molecules (net gain) and two NADH molecules.
- Universality Across Organisms
- Glycolysis is a highly conserved process found in nearly all organisms, from bacteria to humans.
- This universal occurrence highlights its early evolution and its fundamental role in cellular energy production.
- Precursor for Other Metabolic Pathways
- The end product of glycolysis, pyruvate, is a key intermediate for multiple pathways:
- Aerobic Respiration: In the presence of oxygen, pyruvate enters the Krebs cycle for further ATP production.
- Anaerobic Respiration: Under low-oxygen conditions, pyruvate is converted to lactate (in animals) or ethanol (in yeast) through fermentation.
- Gluconeogenesis: Pyruvate is a substrate for the synthesis of glucose when carbohydrates are scarce.
- The end product of glycolysis, pyruvate, is a key intermediate for multiple pathways:
- Critical for Brain and Muscle Activity
- Glucose, through glycolysis, is the main fuel for neurons in the brain.
- During intense physical activity, muscles rely on glycolysis to generate rapid ATP for short bursts of energy.
- Link to Other Metabolic Pathways
- Glycolysis connects to various biosynthetic pathways by providing key intermediates.
- The pathway also regulates metabolic flux through feedback mechanisms, adjusting ATP and NADH levels to meet cellular needs.
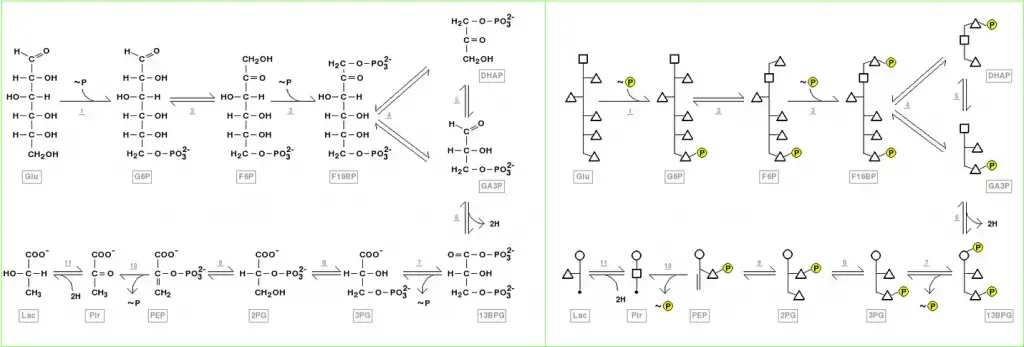
Structure of glycolysis components in Fischer projections and polygonal model
Fischer projections depict the chemical changes that occur in glycolysis intermediates. This image can be used to compare with the polygonal model representation. A video shows another comparison of Fischer projections with Poligonal Models in glycolysis. You can also see video animations on YouTube for another metabolic pathway (Krebs Cycle), and for the representation and application of Polygonal Models in Organic Chemistry.
Interactive pathway map
Change in free energy for each step of glycolysis
| Step | Reaction | ΔG°’ / (kJ/mol) | ΔG / (kJ/mol) |
|---|---|---|---|
| 1 | Glucose + ATP4− → Glucose-6-phosphate2− + ADP3− + H+ | −16.7 | −34 |
| 2 | Glucose-6-phosphate2− → Fructose-6-phosphate2− | 1.67 | −2.9 |
| 3 | Fructose-6-phosphate2− + ATP4− → Fructose-1,6-bisphosphate4− + ADP3− + H+ | −14.2 | −19 |
| 4 | Fructose-1,6-bisphosphate4− → Dihydroxyacetone phosphate2− + Glyceraldehyde-3-phosphate2− | 23.9 | −0.23 |
| 5 | Dihydroxyacetone phosphate2− → Glyceraldehyde-3-phosphate2− | 7.56 | 2.4 |
| 6 | Glyceraldehyde-3-phosphate2− + Pi2− + NAD+ → 1,3-Bisphosphoglycerate4− + NADH + H+ | 6.30 | −1.29 |
| 7 | 1,3-Bisphosphoglycerate4− + ADP3− → 3-Phosphoglycerate3− + ATP4− | −18.9 | 0.09 |
| 8 | 3-Phosphoglycerate3− → 2-Phosphoglycerate3− | 4.4 | 0.83 |
| 9 | 2-Phosphoglycerate3− → Phosphoenolpyruvate3− + H2O | 1.8 | 1.1 |
| 10 | Phosphoenolpyruvate3− + ADP3− + H+ → Pyruvate− + ATP4− | −31.7 | −23.0 |
Concentrations of metabolites in erythrocytes
| Compound | Concentration / mM |
|---|---|
| Glucose | 5.0 |
| Glucose-6-phosphate | 0.083 |
| Fructose-6-phosphate | 0.014 |
| Fructose-1,6-bisphosphate | 0.031 |
| Dihydroxyacetone phosphate | 0.14 |
| Glyceraldehyde-3-phosphate | 0.019 |
| 1,3-Bisphosphoglycerate | 0.001 |
| 2,3-Bisphosphoglycerate | 4.0 |
| 3-Phosphoglycerate | 0.12 |
| 2-Phosphoglycerate | 0.03 |
| Phosphoenolpyruvate | 0.023 |
| Pyruvate | 0.051 |
| ATP | 1.85 |
| ADP | 0.14 |
| Pi | 1.0 |
How to Remember the Enzyme Names?
Remembering enzyme names can be challenging, but using mnemonic devices or associations can make it easier. Here’s a simple mnemonic that you can use to remember the enzymes involved in glycolysis:
“Hannah’s Pretty Pink Apron Turns Gracefully, Making Pies Perfectly.”
Each word in this mnemonic corresponds to the first letter of an enzyme’s name:
- Hannah’s (Hexokinase)
- Pretty (Phosphofructokinase)
- Pink (Phosphoglycerate Kinase)
- Apron (Aldolase)
- Turns (Triose Phosphate Isomerase)
- Gracefully (Glyceraldehyde-3-Phosphate Dehydrogenase)
- Making (Enolase)
- Pies (Pyruvate Kinase)
- Perfectly (Phosphoglycerate Mutase)
You can use this mnemonic to help you recall the enzyme names in the correct order of their appearance in the glycolytic pathway.
Additionally, creating associations or mental images related to the enzyme names or their functions can help reinforce your memory. For instance, you might visualize “Hannah” using a hexagon-shaped cookie cutter (like glucose is a hexagon), “Pretty Pink” could remind you of the pink color of ATP molecules (from ATP production), and so on.
Feel free to create your own associations or mnemonics that make sense to you. Associating enzyme names with memorable images or stories can make learning and recalling them much more enjoyable and effective.
How to Remember Each Step of Glycolysis Serially? Here is 5 Shortcut
here are five mnemonic shortcuts to help you remember the steps of glycolysis in order:
1. “Good People Always Drink Green Tea After Cold Play.”
- Each word corresponds to the first letter of a step in glycolysis:
- Good (Glucose Entry)
- People (Preparation Phase)
- Always (ATP and Pyruvate Formation)
- Drink (Dihydroxyacetone Phosphate Conversion)
- Green (Glyceraldehyde-3-Phosphate Dehydrogenase)
- Tea (Triose Phosphate Isomerase)
- After (ATP Generation – Substrate-Level Phosphorylation)
- Cold (Cleavage Phase)
- Play (Pyruvate Formation – Final Energy-Payoff Phase)
2. “Glorious People Are Also Daring, Diverse, and Generous To People.”
- This mnemonic uses the first letter of each word to represent a step:
- Glorious (Glucose Entry)
- People (Preparation Phase)
- Are (ATP and Pyruvate Formation)
- Also (Dihydroxyacetone Phosphate Conversion)
- Daring (Glyceraldehyde-3-Phosphate Dehydrogenase)
- Diverse (Triose Phosphate Isomerase)
- Generous (ATP Generation – Substrate-Level Phosphorylation)
- To (Cleavage Phase)
- People (Pyruvate Formation – Final Energy-Payoff Phase)
3. “Great Pyramids Allow Tigers Down Ancient Canyons, Proclaiming Perfectly.”
- This mnemonic maps each word to a glycolysis step:
- Great (Glucose Entry)
- Pyramids (Preparation Phase)
- Allow (ATP and Pyruvate Formation)
- Tigers (Dihydroxyacetone Phosphate Conversion)
- Down (Glyceraldehyde-3-Phosphate Dehydrogenase)
- Ancient (ATP Generation – Substrate-Level Phosphorylation)
- Canyons (Cleavage Phase)
- Proclaiming (Phosphoglycerate Mutase)
- Perfectly (Pyruvate Formation – Final Energy-Payoff Phase)
4. “Girls Play All Day, Dance, Dance, Till Friday’s Party.”
- Each word corresponds to a glycolysis step:
- Girls (Glucose Entry)
- Play (Preparation Phase)
- All (ATP and Pyruvate Formation)
- Day (Dihydroxyacetone Phosphate Conversion)
- Dance (Dihydroxyacetone Phosphate Isomerization)
- Dance (ATP Generation – Substrate-Level Phosphorylation)
- Till (Triose Phosphate Isomerase)
- Friday’s (Phosphoglycerate Kinase)
- Party (Pyruvate Formation – Final Energy-Payoff Phase)
5. “Going Places Always Demands Drama, Disguises, and Pretty Performances.”
- Use the first letter of each word to remember the steps:
- Going (Glucose Entry)
- Places (Preparation Phase)
- Always (ATP and Pyruvate Formation)
- Demands (Dihydroxyacetone Phosphate Conversion)
- Drama (Dihydroxyacetone Phosphate Isomerization)
- Disguises (ATP Generation – Substrate-Level Phosphorylation)
- and (Aldolase Cleavage)
- Pretty (Phosphoglycerate Kinase)
- Performances (Pyruvate Formation – Final Energy-Payoff Phase)
Choose the mnemonic that works best for you or create your own using associations that resonate with you. Associating each step with a memorable image or story can help reinforce your memory of the glycolytic pathway.
Quiz Practice
[mcq_display ids=”66551,66541,66540,66538,66539,66544,66542,66547,66548,66556,66550,66552,66571,66555,66546,66543,66553,66549,66545,66537″]
FAQ
Where does glycolysis take place?
Glycolysis takes place in the cytoplasm of cells.
What is aerobic glycolysis?
Aerobic glycolysis is the process of oxidation of glucose into pyruvate followed by the oxidation of pyruvate into CO2 and H2O in the presence of a sufficient amount of oxygen.
What is anaerobic glycolysis?
Anaerobic glycolysis is the process that takes place in the absence of enough oxygen resulting in the reduction of pyruvate into lactate and reoxidation of NADH into NAD+.
does glycolysis require oxygen?
Glycolysis requires no oxygen. It is an anaerobic type of respiration performed by all cells, including anaerobic cells that are killed by oxygen.
is glycolysis aerobic or anaerobic?
Glycolysis is an anaerobic process. None of its nine steps involve the use of oxygen.
how many nadh are produced by glycolysis?
Two NADH molecules are produced by glycolysis.
what does glycolysis produce?
Glucose is the source of almost all energy used by cells. Overall, glycolysis produces two pyruvate molecules, a net gain of two ATP molecules, and two NADH molecules.
in glycolysis, atp molecules are produced by _.a. photophosphorylationb. oxidative phosphorylationc. photosynthesisd. cellular respiratione. substrate-level phosphorylation
Ans: e. substrate-level phosphorylation.
For each glucose that enters glycolysis, _____ acetyl coa enter the citric acid cycle.
For each glucose that enters glycolysis, 2 acetyl CoA enter the citric acid cycle.
why is atp required for glycolysis?
Energy is needed at the start of glycolysis to split the glucose molecule into two pyruvate molecules. These two molecules go on to stage II of cellular respiration. The energy to split glucose is provided by two molecules of ATP.
in addition to atp, what are the end products of glycolysis?
The end products of glycolysis are: pyruvic acid (pyruvate), adenosine triphosphate (ATP), reduced nicotinamide adenine dinucleotide (NADH), protons (hydrogen ions (H2+)), and water (H2O). Glycolysis is the first step of cellular respiration, the process by which a cell converts nutrients into energy.
how many atp are produced in glycolysis?
Glycolysis produces 2 ATP
which kind of metabolic poison would most directly interfere with glycolysis?
A poison that closely mimics the structure of glucose but is not metabolized. If the poison cannot be metabolized, then no NADH, pyruvate or ATP will be produced in glycolysis.
where does glycolysis take place in eukaryotic cells?
Glycolysis takes place in the cytoplasm. Within the mitochondrion, the citric acid cycle occurs in the mitochondrial matrix, and oxidative metabolism occurs at the internal folded mitochondrial membranes (cristae).
why is glycolysis considered to be one of the first metabolic pathways to have evolved?
Photosynthesis is one of the earliest reactions where carbon dioxide and water come together to form glucose. In glucose the energy of the sun is trapped. Glycolysis breaks down glucose molecules in carbon dioxide and water. Breaking down glucose releases energy.
Most of the cells respire anaerobically. All these cells have glycolysis in their metabolic pathway. Therefore it is one of the earliest metabolic pathways
- A. Hassan, Baydaa. (2019). glycolysis Embden–Meyerhof–Parnas (EMP) pathway. 10.13140/RG.2.2.36127.82089.
- Chaudhry R, Varacallo M. Biochemistry, Glycolysis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482303/
- Chandel NS. Glycolysis. Cold Spring Harb Perspect Biol. 2021 May 3;13(5):a040535. doi: 10.1101/cshperspect.a040535. PMID: 33941515; PMCID: PMC8091952.
- Protasoni, Margherita & Zeviani, Massimo. (2021). Mitochondrial Structure and Bioenergetics in Normal and Disease Conditions. International Journal of Molecular Sciences. 22. 586. 10.3390/ijms22020586.
- Fernie, A. R., Carrari, F., & Sweetlove, L. J. (2004). Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport. Current opinion in plant biology, 7(3), 254–261. https://doi.org/10.1016/j.pbi.2004.03.007
- Granner, D. K., & Rodwell, V. W. (2006). Harper’s illustrated biochemistry (27th ed.). New York: Lange Medical Books/McGraw-Hill.
- Rath, L. (2019, February 20). Cancer and Sugar: Is There a Link? WebMD; WebMD. https://www.webmd.com/cancer/features/cancer-sugar-link
- Bailey, R. (2022). Learn About The 10 Steps of Glycolysis. ThoughtCo. Retrieved 2 May 2022, from https://www.thoughtco.com/steps-of-glycolysis-373394.
- Nelson, D., Lehninger, A., Cox, M., & Nelson, D. (2005). Lecture notebook for Lehninger principles of biochemistry, fourth edition (pp. 522-535). W.H. Freeman.
(2022). Retrieved 3 May 2022, from https://www.cliffsnotes.com/study-guides/biology/biochemistry-i/glycolysis/glycolysis-regulation. - https://byjus.com/biology/glycolysis/#:~:text=Glycolysis%20is%20the%20process%20in,both%20aerobic%20and%20anaerobic%20organisms.
- https://en.wikipedia.org/wiki/Glycolysis#Glycolysis_in_disease
- https://www.ttuhsc.edu/medicine/academic-affairs/documents/sakai-files/bct/7_Glycolysis_Notes_Ganapathy.pdf
- https://www.news-medical.net/life-sciences/What-is-Glycolysis.aspx
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.