What are G Protein Coupled Receptors?
- G protein-coupled receptors (GPCRs), also recognized under various names such as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), represent a significant group of proteins that play a crucial role in cellular communication and signal transduction. These receptors are notable for their structure, which consists of seven transmembrane α-helical domains, an extracellular amino terminus, and an intracellular carboxy terminus. The term “seven-transmembrane receptors” is derived from this characteristic structure, featuring six loops—three extracellular and three intracellular—that facilitate interaction with ligand molecules and G proteins, respectively.
- These receptors are unique to eukaryotes, including organisms ranging from yeast to choanoflagellates. GPCRs are capable of detecting and responding to a diverse array of ligands, such as light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters. This versatility in ligand recognition underlies their involvement in numerous physiological processes and diseases.
- The primary mechanism of GPCRs involves ligand binding, which triggers a conformational change in the receptor. This change allows the GPCR to function as a guanine nucleotide exchange factor (GEF), facilitating the activation of an associated G protein by exchanging GDP for GTP on the G protein. The G protein’s α subunit, bound to GTP, then dissociates from the β and γ subunits, influencing intracellular signaling pathways or directly interacting with target proteins. This mechanism is critical in two principal signal transduction pathways: the cAMP signal pathway and the phosphatidylinositol signal pathway.
- GPCRs are of paramount importance in pharmacology, with approximately 34% of all FDA-approved drugs targeting members of this receptor family. The significance of GPCRs in drug discovery and therapeutic development is highlighted by their involvement in a wide array of diseases, including mental and metabolic disorders, immunological and cardiovascular diseases, inflammatory conditions, sensory disorders, and cancer. Their role in drug action, such as inducing analgesia, is a rapidly evolving area of pharmaceutical research.
- The structural understanding of GPCRs has advanced significantly over the years. Key milestones include the determination of the crystal structure of rhodopsin in 2000, revealing the arrangement of the seven transmembrane helices, and the structure of the β2-adrenergic receptor with a diffusible ligand in 2007, which provided insights into ligand binding and receptor activation. The determination of the structure of a GPCR-G-protein complex in 2011 further opened new avenues for structural investigations of these receptors.
- The Nobel Prize in Chemistry in 2012 was awarded to Brian Kobilka and Robert Lefkowitz for their groundbreaking work on GPCRs, underscoring the receptors’ significance in biochemistry and medicine. Additionally, GPCRs have been linked to several Nobel Prizes awarded for research on G protein–mediated signaling.
- In the human genome, nearly 800 GPCRs have been identified, accounting for over 3% of human genes. These receptors are classified into at least five structurally distinct subfamilies: Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin receptor families. The rhodopsin family constitutes about 90% of all GPCRs.
- GPCRs are implicated in a myriad of pathophysiological conditions, including disorders of the central nervous system, cardiovascular and metabolic diseases, respiratory malfunctions, gastrointestinal disorders, immune diseases, cancer, musculoskeletal pathologies, and eye diseases. Consequently, targeting GPCRs remains a central strategy in drug development, reflecting their role in diverse physiological processes and their potential as therapeutic targets.
Structure of G Protein Coupled Receptors
- General Structure and Components of GPCRs: G protein-coupled receptors (GPCRs) are integral membrane proteins characterized by seven membrane-spanning domains, commonly referred to as transmembrane helices. These structures traverse the lipid bilayer seven times, earning them the designation as 7-transmembrane receptors. Notably, GPCRs lack integral enzyme activity or ion channel function, with all downstream effects mediated via their associated G-proteins. These G-proteins are heterotrimeric, composed of three distinct subunits: alpha (α), beta (β), and gamma (γ). In their inactive state, GDP is bound to the α-subunit.
- GPCR Activation and Signal Diversity: GPCRs can be activated by a wide range of signals including neurotransmitters, hormones, ions, peptides, and even photons in the retina. This versatility in activation allows for significant diversity in cellular responses. Examples of GPCRs include adrenoreceptors, muscarinic acetylcholine receptors, and opioid receptors. The variety in α-, β-, and γ-subunits facilitates numerous combinations of GPCRs and G proteins, enabling a single signal to trigger multiple downstream cellular reactions.
- Membrane Topology and Structural Features: All GPCRs share a common membrane topology, with seven α-helical transmembrane segments, the N-terminus located outside the cell, and the C-terminus inside. Many GPCRs exhibit palmitoylation near the C-terminus, further anchoring them in the membrane. Crystal structures of several GPCRs reveal that these transmembrane segments are broken helices crossing the membrane at various angles. Common structural features have been identified across different GPCRs.
- Ligand Binding and G-Protein Interaction: The binding sites for small agonists are typically nestled between helices, partially across the membrane, while protein ligands bind to an elongated extracellular N-terminus. In class-C receptors, such as mGluR and GABABR, the small ligand binds within a large extracellular “clamshell” formed by the long N-terminus. Biochemical experiments and crystal structures demonstrate that heterotrimeric G-proteins interact with the second and third intracellular loops and the cytoplasmic C-terminus of GPCRs. These interactions dictate the coupling of specific G-proteins to each receptor and transmit the signal from the activated receptor to the G-protein.
- Receptor Dimerization and Signaling: Since 1998, evidence suggests many GPCRs can form dimers—complexes of two receptors. Both homodimers and heterodimers are observed, with notable examples being mgluR5 and GABAB receptors. Some receptor dimers are active in signaling, and in certain cases, dimerization is obligatory for signaling. Dimerization potentially alters agonist and antagonist specificity, G-protein coupling, and membrane trafficking, endowing dimeric receptors with new properties distinct from monomeric forms.
- Crystal Structure and Conformational Changes: The first crystal structure of a mammalian GPCR, bovine rhodopsin, was solved in 2000, followed by the human β2-adrenergic receptor. These structures show similarity and indicate how ligand binding leads to conformational changes in the receptor. The most significant change is the outward movement of the 5th and 6th transmembrane helices’ cytoplasmic part. This movement creates a cavity for Gα binding, as confirmed in the structure of the activated beta-2 adrenergic receptor in complex with Gs.
- Comparison with Other 7TMH Proteins: GPCRs exhibit structural similarities to other proteins with seven transmembrane domains, such as microbial rhodopsins and adiponectin receptors. However, these receptors do not associate with G proteins. Notably, ADIPOR1 and ADIPOR2, unlike GPCRs, are oriented oppositely in the membrane, with an extracellular C-terminus and cytoplasmic N-terminus.
Classification of G Protein Coupled Receptors
The G protein-coupled receptor (GPCR) superfamily encompasses a vast array of receptors, with at least 831 human genes, approximately 4% of the protein-coding genome, predicted to code for GPCRs. Despite the lack of detectable shared sequence homology between classes, all GPCRs share a common structure and signal transduction mechanism.
- Classical Classification System: Historically, the GPCR superfamily has been divided into three main classes based on sequence homology and functional characteristics:
- Class A (Rhodopsin-like): This is the largest class, accounting for nearly 85% of GPCR genes, with over half predicted to encode olfactory receptors. The remaining receptors in this class are either liganded by known endogenous compounds or classified as orphan receptors.
- Class B (Secretin receptor family): Includes receptors such as secretin, VIP, and PACAP.
- Class C (Metabotropic glutamate/pheromone): Comprises receptors like mGluR and GABAB.
- Class D (Fungal mating pheromone receptors).
- Class E (Cyclic AMP receptors).
- Class F (Frizzled/Smoothened).
- GRAFS Classification System: An alternative classification system, known as GRAFS, has been proposed for vertebrate GPCRs. This system aligns with the classical classes as follows:
- Glutamate (Class C)
- Rhodopsin (Class A)
- Adhesion (Class B2)
- Frizzled/Taste2 (Class F)
- Secretin (Class B)
- GPCR Functions and Ligands in the Nervous System: GPCRs in the central nervous system include receptors for various neurotransmitters and hormones, and for sensory input transduction. Common ligands in the nervous system include:
- Monoamines: Adrenaline, noradrenaline, serotonin, dopamine, histamine.
- Other small neurotransmitters: Acetylcholine (mACh), gamma-aminobutyric acid (GABAB), glutamate (mGluR), ATP (P2Y), adenosine, cannabinoids.
- Peptide neurotransmitters and hormones: Opioids, somatostatin, NPY, oxytocin, vasopressin, neurotensins, VIP, galanin, kinins, releasing hormones, and more.
- Sensory modalities: Light (rhodopsin), odorants, taste (sweet, bitter, umami).
- GPCR Subtypes and Signal Diversity: The diversity in GPCR subtypes allows the same extracellular signal to elicit different intracellular responses based on the receptor subtypes and splice variants expressed. For example, the adrenergic receptors, which have nine subtypes, couple to different G-proteins (Gq, Gi, Gs) leading to varied cellular responses.
- GPCR Classes Specific to the Nervous System:
- Class 1 (Rhodopsin family): Encompasses most neurotransmitter and hormone receptors except those in the following classes.
- Class 2 (Secretin family): Includes receptors for secretin, VIP, PACAP, GHRH.
- Class 3 (Glutamate family): Includes mGluR, GABAB, CaSR, and some taste receptors.
- Class F (Frizzled family): Comprises the wnt receptor (frizzled).
- GPCR Evolution and Specialization: GPCR signaling systems specialized for different ligands are found across various eukaryotes, indicating their evolutionary significance. The diversification of GPCRs through gene duplications has led to an extensive repertoire of G-protein coupling and signal transduction pathways.
Mechanism of G Protein Coupled Receptors
- Ligand Binding and Activation:
- G protein-coupled receptors (GPCRs) are activated by external signals such as ligands or signal mediators. These ligands include a wide range of molecules, including sensory signal mediators (e.g., light and olfactory molecules), various neurotransmitters (e.g., dopamine, serotonin), and hormones (e.g., glucagon, oxytocin).
- Upon ligand binding, GPCRs undergo a conformational change. This change is crucial for the activation of the receptor and the subsequent signaling pathway.
- GPCRs that lack known ligands are referred to as orphan receptors. Unlike other receptor types where ligands bind externally, GPCR ligands typically bind within the transmembrane domain.
- Conformational Change and G Protein Interaction:
- The activated GPCR is bound to a heterotrimeric G protein in its inactive state.
- Binding of an agonist to the GPCR results in a conformational change transmitted to the G protein’s α subunit.
- The Gα subunit then exchanges GDP for GTP, leading to its activation and dissociation from the Gβγ dimer and the receptor.
- Both the dissociated Gα and Gβγ subunits interact with other intracellular proteins to propagate the signal.
- G-Protein Activation/Deactivation Cycle:
- G-proteins, consisting of Gα, Gβ, and Gγ subunits, are inactive when bound to GDP and active when bound to GTP.
- Upon receptor activation, the GPCR facilitates the exchange of GDP for GTP on the Gα subunit.
- This activation leads to the dissociation of the G protein into a Gα-GTP monomer and a Gβγ dimer, both of which can interact with other intracellular proteins.
- The Gα subunit has intrinsic GTPase activity and eventually hydrolyzes GTP to GDP, returning to its inactive form and allowing reassociation with the Gβγ dimer.
- This cycle is regulated by Regulators of G-protein Signaling (RGS proteins), which accelerate the GTP hydrolysis rate.
- Signal Transduction and Crosstalk:
- GPCRs can interact with various types of G proteins, each specific for certain receptors and targets.
- Upon activation, the G protein subunits interact with enzymes or ion channels in the plasma membrane, relaying the signal onward.
- The duration of the signal is controlled by the Gα subunit’s GTPase activity, often enhanced by other proteins or specific RGS proteins.
- Crosstalk with other signaling pathways, such as integrin signaling, is also possible. For example, integrin signaling can modulate GPCR activity through phosphorylation mechanisms.
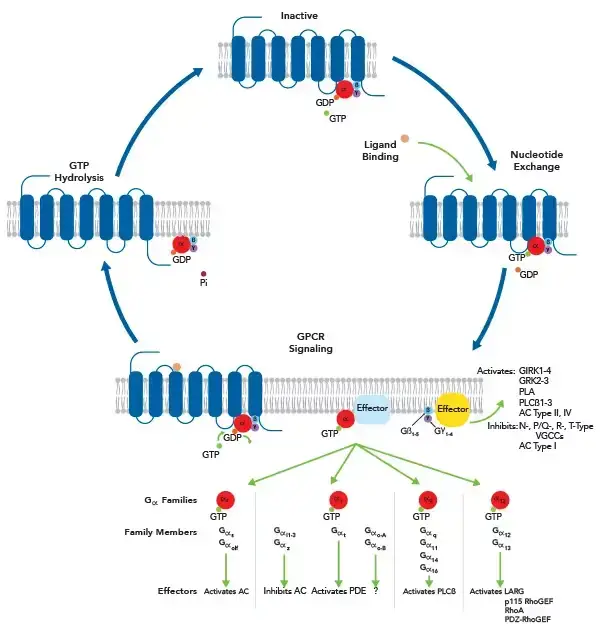
Signaling Pathway of G Protein Coupled Receptors
The signaling pathway of G Protein-Coupled Receptors (GPCRs) involves several key steps. GPCRs are a large family of cell surface receptors that respond to a variety of external signals and are crucial in many physiological processes. Here’s a simplified overview of the steps involved in GPCR signaling:
- Ligand Binding: The process begins when an external signaling molecule (ligand), such as a hormone, neurotransmitter, or sensory signal, binds to the GPCR. This binding occurs on the extracellular side of the cell membrane.
- Receptor Activation: Ligand binding causes a conformational change in the GPCR. This change in structure activates the receptor.
- G Protein Activation: The activated GPCR then interacts with a nearby G protein, which is a complex of three subunits: alpha (α), beta (β), and gamma (γ). The G protein is inactive when GDP (guanosine diphosphate) is bound to the α subunit.
- GDP-GTP Exchange: The activated GPCR acts as a guanine nucleotide exchange factor (GEF), promoting the exchange of GDP for GTP (guanosine triphosphate) on the α subunit. This exchange activates the G protein.
- G Protein Subunits Dissociation: Upon GTP binding, the G protein undergoes a conformational change, leading to the dissociation of the α subunit (with GTP bound) from the βγ dimer.
- Effector Activation: The free α subunit and the βγ dimer can then interact with and regulate various downstream effector proteins. These effectors include enzymes like adenylyl cyclase and phospholipase C, and ion channels. The type of effector activated depends on the type of G protein and the specific GPCR.
- Second Messenger Generation: The activation of effectors leads to the production of second messengers such as cAMP (cyclic AMP), IP3 (inositol trisphosphate), and DAG (diacylglycerol). These second messengers amplify the signal inside the cell and lead to various cellular responses.
- Signal Termination: The signal is terminated when the GTP bound to the α subunit is hydrolyzed to GDP, turning the G protein inactive. This hydrolysis is catalyzed by the intrinsic GTPase activity of the α subunit. The inactive α subunit reassociates with the βγ dimer, returning the G protein to its resting state.
- Receptor Desensitization: Prolonged activation of the GPCR can lead to its phosphorylation and binding of arrestin proteins, which prevent further G protein activation and target the receptor for internalization and recycling or degradation.
This pathway is a fundamental mechanism through which cells respond to external signals and is involved in numerous physiological processes and pharmacological responses.
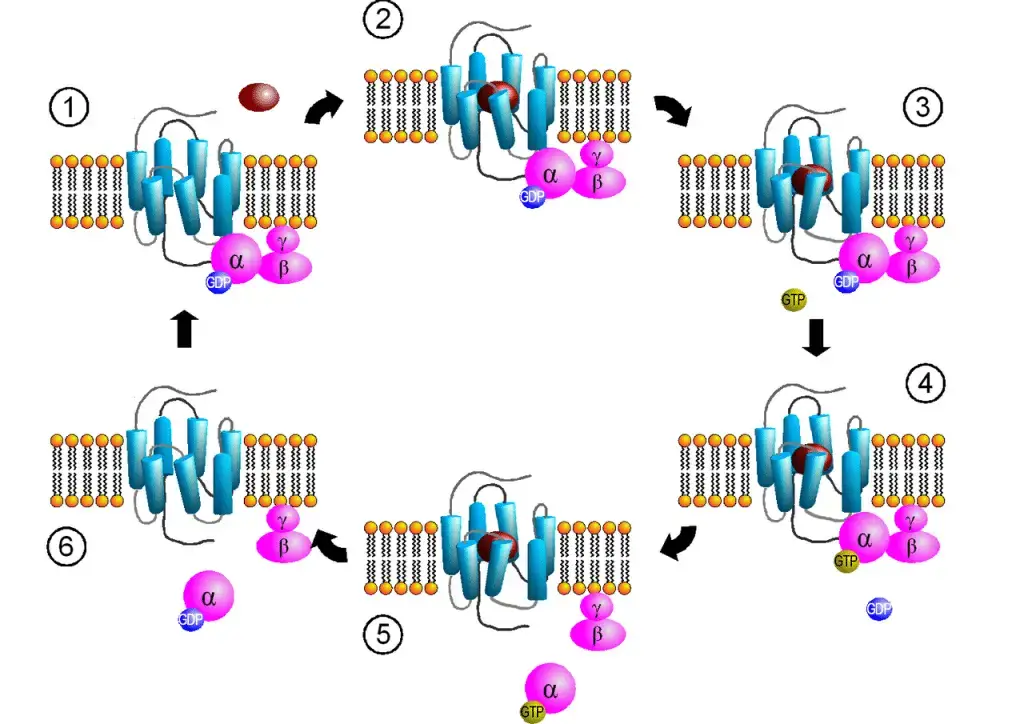
Ligand Binding to GPCRs
Ligand binding to GPCRs initiates a series of events that ultimately lead to cellular responses. This process can be broken down into several key steps:
1. Ligand Binding to the Extracellular Portion
- Agonists, which are substances that trigger cellular responses, bind to GPCRs at the extracellular portion of the receptor. Ligand binding can occur either at the N-terminus or within specific binding sites within the transmembrane region of the receptor.
2. Conformational Change in the GPCR
- Upon ligand binding, the GPCR undergoes a conformational change. This change in shape is critical for the receptor’s activation.
3. Release of GDP from the α-Subunit
- The conformational change induced by ligand binding results in the release of GDP (guanosine diphosphate) that was bound to the α-subunit of the associated G-protein.
4. Replacement of GDP with GTP
- The released GDP is then replaced with GTP (guanosine triphosphate), leading to the activation of the G-protein.
5. Dissociation of α-Subunit and βγ-Subunit
- The binding of GTP causes the α-subunit to dissociate from the transmembrane portion of the GPCR, along with the βγ-subunit. These subunits play crucial roles in transmitting the signal to downstream effectors.
6. Interaction with Downstream Effectors
- The α-subunit, once dissociated, interacts with specific downstream effectors inside the cell. These effectors can include ion channels or enzymes, among others. The binding of the α-subunit to effectors initiates downstream cellular responses, such as ion channel opening or regulation of enzyme activity.
7. Signal Amplification
- Importantly, although one GPCR typically has only one α-subunit, it can interact with multiple secondary messengers. These secondary messengers, in turn, can activate various enzymes and catalyze multiple reactions. This cascade of events amplifies the cellular response, allowing a single agonist binding event to initiate multiple downstream reactions.
8. Switching Off GPCR Activity
- To prevent excessive signaling, GPCR activity must be terminated. GTPase, an enzyme associated with the α-subunit of the G-protein, catalyzes the hydrolysis of GTP into GDP and inorganic phosphate (Pi). This hydrolysis reduces the α-subunit’s affinity for downstream effectors.
- As GDP increases the α-subunit’s affinity for the βγ-subunit, the heterotrimeric complex of the G-protein reforms, and the G-protein reassociates with the transmembrane receptor. This process effectively switches off the GPCR, making it available for the binding of another ligand.
Specificity of GPCR signaling
- G Protein Coupling Specificity:
- Most G Protein-Coupled Receptors (GPCRs) primarily couple to Gα subunits belonging to one of five signaling families. However, some GPCRs, known as promiscuous receptors, can couple to several Gα subunits. Additionally, certain agonists, termed biased agonists, can alter the G-protein specificity of a response. Despite the apparent potential for loss of specificity with around 1000 types of receptors coupling to only five signaling pathways, specificity is maintained through various mechanisms.
- Cell-Specific Receptor Expression:
- Each cell type expresses a unique subset of the available GPCRs, typically ranging from 40 to 80 different receptors in varying ratios. This selective expression ensures that each agonist interacts specifically with appropriate cells. For example, light affects photoreceptors, while GnRH stimulates pituitary gonadotropes.
- Target Protein Specificity:
- Cells express specific subsets of downstream protein targets responsive to the second messengers regulated by GPCRs. Therefore, the same second messenger can elicit different responses in different cells. For instance, cAMP causes Leydig cells in the testis to produce testosterone, affects electrical coupling in horizontal cells of the retina, accelerates the cardiac beat rate in pacemaker cells, and induces relaxation in vascular smooth muscle.
- Localized GPCR Distribution:
- GPCRs may be localized to specific parts of the plasma membrane, allowing different cell regions (such as dendrites, cell bodies, axons, and nerve terminals in neurons) to respond differently to the same set of GPCR agonists. However, strong evidence for this localization is mostly limited to special cases, like the localization of rhodopsin in vertebrate photoreceptors.
- Convergence and Physiological Responses:
- Significant convergence in GPCR signaling can be beneficial, allowing diverse cells and organs to elicit a common physiological response. For example, an increase in heart rate and blood circulation can be triggered by various agonists like noradrenaline, glucagon, or histamine, each originating from different sources and serving different physiological needs.
- GPCR Agonist Distribution and Action:
- Unlike the localized action of neurotransmitters in fast chemical synapses, GPCR agonists typically have longer extracellular lifetimes (200 ms to several minutes), allowing them to spread by diffusion and affect multiple cells. This broader action contrasts with the point-to-point communication in fast chemical synapses, where neurotransmitters are quickly removed from the synaptic cleft.
- Structural Aspects of GPCRs:
- The β1-adrenergic receptor, for instance, has a crystal structure with transmembrane helical segments and an antagonist binding site, illustrating the complex architecture of GPCRs. This structure facilitates the interaction with G proteins and other intracellular components, contributing to the specificity of GPCR signaling.
- Comparison with Fast Chemical Synapses:
- GPCR signaling differs significantly from fast chemical synaptic transmission. GPCRs are not ion channels and their actions, ranging from 100 ms to minutes, are slower and more complex, involving G proteins, second messengers, and various intracellular targets. In contrast, fast chemical synapses primarily alter membrane potential and sometimes allow calcium ion entry, acting within a fraction of a millisecond.
- GPCR-Mediated Neurotransmission:
- The actions of GPCR-coupled monoamines and peptides, due to their longer extracellular lifetimes, are not confined to single postsynaptic cells but affect larger groups of cells. This mode of action, termed spillover and volume transmission, influences neural circuits and mental states in a hormone-like, paracrine manner, rather than providing specific information to a single neuron.
cAMP and PIP2 pathways
1. cAMP Signal Pathway
- Overview: The cAMP signal transduction pathway involves several key components: stimulative hormone receptor (Rs) or inhibitory hormone receptor (Ri), stimulative regulative G-protein (Gs) or inhibitory regulative G-protein (Gi), adenylyl cyclase, protein kinase A (PKA), and cAMP phosphodiesterase.
- Receptor Function: Rs binds with stimulative signal molecules, while Ri binds with inhibitory signal molecules. These receptors play a pivotal role in determining the pathway’s response to different hormonal signals.
- G-Protein Role: Gs is linked to Rs and activates enzymes or intracellular metabolism upon stimulation. Conversely, Gi, associated with Ri, inhibits these processes upon activation.
- Adenylyl Cyclase: This 12-transmembrane glycoprotein catalyzes ATP conversion to cAMP, using Mg2+ or Mn2+ as cofactors. cAMP acts as a second messenger, influencing various cellular processes.
- Protein Kinase A (PKA): PKA, crucial in cell metabolism, regulates metabolic pathways, gene expression, cellular secretion, and membrane permeability through phosphorylation. It consists of two catalytic and two regulatory subunits, becoming active when cAMP binds to the regulatory subunits.
- Signal Termination: cAMP phosphodiesterase degrades cAMP to 5′-AMP, inactivating PKA and thus terminating the signal.
2. Phosphatidylinositol (PIP2) Signal Pathway
- Pathway Initiation: Binding of an extracellular signal molecule to the G-protein receptor (Gq) activates phospholipase C on the plasma membrane.
- Second Messenger Production: Phospholipase C hydrolyzes PIP2 into IP3 (inositol 1,4,5-trisphosphate) and DAG (diacylglycerol), both acting as second messengers.
- IP3 and Calcium Ion Release: IP3 interacts with receptors in the smooth endoplasmic reticulum and mitochondria, opening Ca2+ channels. This release of calcium ions triggers various cellular responses.
- DAG and Protein Kinase C (PKC): DAG activates PKC, which phosphorylates multiple proteins, altering their activities and leading to cellular responses.
- Calcium’s Multifaceted Role: Calcium ions, in conjunction with DAG, activate PKC. They also initiate the CaM kinase pathway, where calmodulin (CaM) binds Ca2+, changes conformation, and activates CaM kinase II. This kinase can autophosphorylate, enhancing its affinity for CaM and phosphorylating target enzymes.
- Interconnection of Pathways: The cAMP and PIP2 pathways intersect through Ca2+-CaM, which regulates adenylyl cyclase and phosphodiesterase in the cAMP pathway.
Receptor regulation
Receptor Regulation in G Protein-Coupled Receptor (GPCR) Signaling
1. Desensitization of GPCRs
- Homologous Desensitization: GPCRs become desensitized when exposed to their ligand for an extended period. This can occur through two recognized forms: homologous desensitization, where the activated GPCR itself is downregulated.
- Heterologous Desensitization: In heterologous desensitization, the activation of one GPCR leads to the downregulation of a different GPCR. The key mechanism underlying this downregulation is the phosphorylation of the intracellular (cytoplasmic) receptor domain by protein kinases.
2. Phosphorylation by cAMP-Dependent Protein Kinases
- Activation: Cyclic AMP-dependent protein kinases (protein kinase A or PKA) are activated by the G protein-mediated signal chain initiated by the GPCR via adenylate cyclase and cyclic AMP (cAMP).
- Phosphorylation of Receptor: In a feedback mechanism, activated PKA phosphorylates the GPCR. Prolonged receptor activity results in increased PKA activation and subsequent receptor phosphorylation.
- Switching of G Protein Coupling: Phosphorylation of the receptor, as observed in β2-adrenoceptors, can lead to the switching of G protein coupling from Gs to Gi class, altering downstream signaling.
3. Phosphorylation by G Protein-Coupled Receptor Kinases (GRKs)
- GRKs Function: G Protein-Coupled Receptor Kinases (GRKs) are serine-threonine protein kinases that phosphorylate only active GPCRs. GRKs-mediated receptor phosphorylation initiates rapid impairment of receptor signaling and desensitization.
- Regulation: GRKs’ activity and subcellular targeting are tightly regulated through interactions with receptor domains, G protein subunits, lipids, anchoring proteins, and calcium-sensitive proteins.
4. Consequences of Phosphorylation
- Translocation: Phosphorylated receptors, along with the membrane they are embedded in, can be translocated inside the cell. Within the acidic vesicular environment, they undergo dephosphorylation and may return to the plasma membrane or be degraded.
- Arrestin Linking: Phosphorylated receptors can be linked to arrestin molecules, temporarily inhibiting G protein binding and activation. This mechanism effectively “switches off” the receptor for a short period.
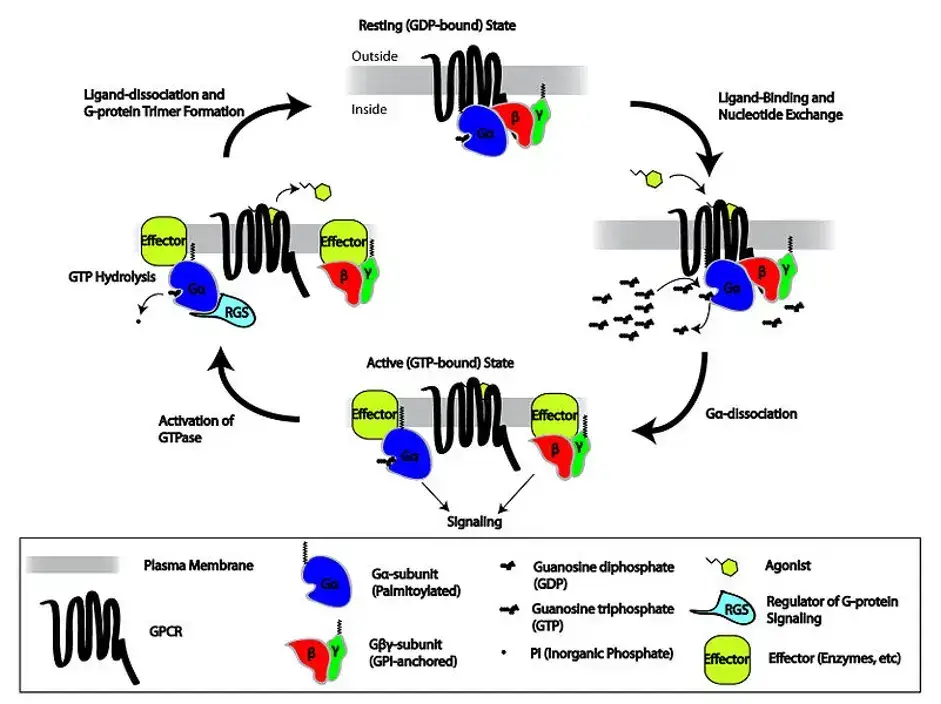
5. Mechanisms of GPCR Signal Termination
- GTP Hydrolysis by G-Proteins: G-proteins can deactivate themselves through intrinsic GTP→GDP hydrolysis. This process is slow but can be accelerated by RGS proteins, enhancing the speed and precision of GPCR signaling.
- Receptor Desensitization: GPCRs can be desensitized themselves, either through ligand occupation or through PKC/PKA-mediated phosphorylation. This can lead to homologous desensitization or increased affinity for β-arrestin, promoting heterologous desensitization.
- Endocytosis and Lysosomal Degradation: Internalized receptors may be trafficked to lysosomes, where low pH and degradative enzymes denature and degrade GPCRs.
6. Gene Transcription Factors
- Regulation of Gene Transcription: Gene transcription factors can upregulate or downregulate the generation of new GPCRs, affecting the number of receptors that travel to the cell membrane. This process contributes to GPCR regulation.
G-proteins
- Basic Structure of G-Proteins:
G-proteins are composed of alpha (α), beta (β), and gamma (γ) subunits, forming a heterotrimeric complex. In their resting state, guanosine diphosphate (GDP) is bound to the α-subunit of the trimer. Upon receptor activation by an agonist, the G-protein is attracted to the receptor, facilitating the displacement of GDP by guanosine triphosphate (GTP) on the α-subunit. This exchange activates the G-protein by causing the dissociation of the α-subunit from the βγ dimer. - Function of the α Subunit:
The α subunit of the G-protein exists in different subtypes, each with specific functions. These functions include:- Inhibiting or stimulating adenylyl cyclase, an enzyme responsible for converting ATP to cyclic AMP (cAMP).
- Activating phospholipase C, which is involved in the hydrolysis of phosphatidylinositol bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG).
- Inhibiting voltage-gated calcium channels, which regulate the influx of calcium ions into the cell.
- Activating GPCR kinases, enzymes that phosphorylate GPCRs and modulate their activity.
- Initiating the mitogen-activated protein kinase (MAPK) cascade, a signaling pathway involved in cell growth and differentiation.
- Role of the βγ Subunit Dimer:
The βγ subunit dimer, while often less emphasized, plays critical roles in cellular signaling. Its functions include:- Activating potassium channels, which are essential for maintaining the cell’s resting membrane potential and modulating electrical signaling in neurons.
- Interacting with and activating specific protein kinases, enzymes that phosphorylate target proteins, thereby altering their function and activity.
- Activation Mechanism of G-Proteins:
The activation of G-proteins is intricately linked with GPCR signaling. Upon the binding of an agonist to a GPCR, a conformational change in the receptor leads to the attraction of the adjacent G-protein. This interaction catalyzes the replacement of GDP with GTP on the α-subunit, a critical step that activates the G-protein. Subsequently, the activated α-subunit, now bound to GTP, dissociates from the βγ dimer, allowing each to interact with various downstream effectors and initiate specific cellular responses. - Inactivation and Cycling of G-Proteins:
Following activation, the intrinsic GTPase activity of the α-subunit hydrolyzes GTP back to GDP, rendering the α-subunit inactive. The α-subunit then reassociates with the βγ dimer, returning the G-protein to its resting state. This cycle of activation and inactivation allows G-proteins to act as molecular switches in cellular signaling pathways.
GPCR Pathways
- Cyclic AMP Pathway:
- Initial Activation: This pathway begins with an agonist stimulating the GPCR, which in turn activates a G-protein (Gαs or Gαi).
- Role of Gαs: Gαs stimulates its target enzyme, adenylyl cyclase (AC), leading to the conversion of ATP to cyclic AMP (cAMP).
- Role of Gαi: Conversely, Gαi inhibits adenylyl cyclase, resulting in a decrease in cAMP levels.
- Effects of cAMP: Once formed, cAMP activates protein kinases, notably protein kinase A (PKA). PKA then induces various cellular responses, such as lipolysis, reduction in glycogen synthesis, and increased glycogenolysis. These processes are vital for regulating homeostasis, particularly in glucose and lipid metabolism.
- Cyclic GMP [cGMP] Pathway:
- NO as a Mediator: Nitric oxide (NO), formed from L-arginine by NO synthase, diffuses between cells to mediate chemical communication.
- Activation of Guanylate Cyclase: NO activates guanylate cyclase (GC), leading to the formation of cGMP from GTP.
- Function of cGMP: cGMP primarily causes vasodilation through the activation of protein kinases. This process is crucial in regulating blood vessel tone and blood flow.
- Inactivation of cGMP: cGMP is hydrolyzed and inactivated by phosphodiesterase enzymes (PDEs), which vary in expression across different tissues.
- Clinical Implications: This pathway is targeted in the treatment of angina and erectile disorders. Organonitrates like nitroglycerin, which release NO, and phosphodiesterase inhibitors like sildenafil (Viagra), are used to induce vasodilation in specific vascular beds.
- Phospholipase C (PLC) Pathway:
- Activation of Gαq: The pathway is initiated by the activation of Gαq, which subsequently activates phospholipase C.
- Formation of IP3 and DAG: Phospholipase C catalyzes the breakdown of PIP2 (phosphatidylinositol 4,5-bisphosphate) into two secondary messengers, IP3 (inositol triphosphate) and DAG (diacylglycerol).
- Role of IP3: IP3 increases cytosolic Ca2+ levels by releasing Ca2+ from intracellular compartments, such as the endoplasmic and sarcoplasmic reticulum. This release of calcium ions influences various cellular functions including muscle contraction, secretion, enzyme activation, and membrane hyperpolarization.
- Function of DAG: DAG activates protein kinase C, which in turn phosphorylates a range of target proteins, thereby controlling numerous cellular functions.
Physiological roles/Functions of G Protein Coupled Receptors
- Visual Sense: G protein-coupled receptors (GPCRs) play a critical role in the visual sense. Opsins, a class of GPCRs, utilize a photoisomerization reaction to convert electromagnetic radiation into cellular signals. A quintessential example is rhodopsin, which employs the transformation of 11-cis-retinal to all-trans-retinal to facilitate this process. This conversion is fundamental in translating light into visual signals.
- Gustatory Sense (Taste): In the gustatory system, GPCRs located in taste cells are responsible for the detection of bitter, umami, and sweet substances. These receptors mediate the release of gustducin, a process crucial for the perception of these taste modalities.
- Olfactory Sense: GPCRs are integral to the sense of smell. Olfactory receptors in the olfactory epithelium bind to odorants, while vomeronasal receptors are specialized for pheromone detection. These interactions are key to the olfactory system’s ability to identify a wide array of scents.
- Behavioral and Mood Regulation: In the mammalian brain, various neurotransmitters such as serotonin, dopamine, histamine, GABA, and glutamate interact with GPCRs. These interactions play significant roles in the regulation of behavior and mood, highlighting the importance of GPCRs in neurological and psychological processes.
- Immune System and Inflammation: GPCRs are crucial in regulating immune system activity and inflammation. Chemokine receptors bind ligands that facilitate communication between immune cells, while other receptors, like histamine receptors, bind inflammatory mediators and activate target cells in the inflammatory response. Additionally, GPCRs modulate immune functions, such as regulating interleukin induction or suppressing T-cell responses.
- Autonomic Nervous System Transmission: The autonomic nervous system, encompassing both the sympathetic and parasympathetic branches, relies heavily on GPCR pathways for regulation. These receptors control various automatic body functions, including blood pressure, heart rate, and digestive processes, underscoring their role in maintaining physiological balance.
- Cell Density Sensing: A novel function of GPCRs is in regulating cell density sensing. This role demonstrates the diverse capabilities of GPCRs in cellular communication and environmental response.
- Homeostasis Modulation: GPCRs are involved in modulating homeostasis, such as in water balance regulation. This function is vital for maintaining internal equilibrium in response to environmental and physiological changes.
- Tumor Growth and Metastasis: Certain types of tumors involve GPCRs in their growth and metastasis processes. This connection between GPCRs and oncological pathways indicates their potential as targets in cancer treatment and research.
- Endocrine System Function: In the endocrine system, GPCRs play a pivotal role in hormone signaling. Peptide and amino-acid derivative hormones bind to GPCRs on target cell membranes, triggering cAMP activation. This cascade activates kinases, leading to various cellular responses, including transcription. This mechanism illustrates the GPCRs’ integral role in hormonal communication and systemic regulation.
References
- Zhao, Juan; Deng, Yulin; Jiang, Zhaotan; Qing, Hong (2016). G Protein-Coupled Receptors (GPCRs) in Alzheimer’s Disease: A Focus on BACE1 Related GPCRs. Frontiers in Aging Neuroscience, 8(), –. doi:10.3389/fnagi.2016.00058
- Rehman S, Rahimi N, Dimri M. Biochemistry, G Protein Coupled Receptors. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK518966/
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009 May 21;459(7245):356-63. doi: 10.1038/nature08144. PMID: 19458711; PMCID: PMC3967846.
- Kennedy, Justine E. (2015). [Progress in Molecular Biology and Translational Science] Trafficking of GPCRs Volume 132 || Regulation of GPCR Trafficking by Ubiquitin. , (), 15–38. doi:10.1016/bs.pmbts.2015.02.005
- Maurice, Pascal (2011). [Advances in Pharmacology] Pharmacology of G Protein Coupled Receptors Volume 62 || GPCR-Interacting Proteins, Major Players of GPCR Function. , (), 349–380. doi:10.1016/b978-0-12-385952-5.00001-4
- https://www.novusbio.com/research-areas/cell-biology/gpcr.html
- https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=694
- https://teachmephysiology.com/biochemistry/molecules-and-signalling/g-protein/
- https://www.khanacademy.org/science/ap-biology/cell-communication-and-cell-cycle/changes-in-signal-transduction-pathways/v/g-protein-coupled-receptors
- http://www.scholarpedia.org/article/G_protein-coupled_receptor#Structure