What is Frank-Starling Law?
- The Frank-Starling Law of the heart, also referred to as the Frank-Starling mechanism, elucidates a fundamental physiological principle governing cardiac function. This law describes the relationship between the volume of blood in the ventricles at the end of diastole (end-diastolic volume) and the subsequent stroke volume—the amount of blood ejected with each heartbeat. Specifically, it posits that an increase in end-diastolic volume results in a proportionate increase in stroke volume, assuming all other physiological conditions remain constant.
- At the heart of this relationship lies the stretching of myocardial fibers. When the ventricles fill with a greater volume of blood during diastole, the myocardial fibers experience elongation. This stretch optimally aligns the sarcomeres—the basic contractile units of the heart muscle—enabling a stronger force of contraction. Research indicates that there exists an optimal length for sarcomeres where tension and contraction strength peak. Deviations from this ideal length, whether through increased or decreased spacing between sarcomeres, result in diminished contractile force. This highlights the importance of maintaining an appropriate preload—the initial stretching of cardiac muscle fibers—ensuring effective cardiac output.
- The physiological relevance of the Frank-Starling mechanism is particularly evident in its role in matching cardiac output with venous return and arterial blood supply. As blood volume increases, the heart adapts by enhancing the force of contraction to expel this additional volume. This self-regulating feature ensures that the left and right ventricles maintain equal output, thereby preventing discrepancies in systemic and pulmonary circulation.
- Historically, the Frank-Starling Law is attributed to physiologists Otto Frank and Ernest Henry Starling, although its conceptual roots can be traced back to earlier work by Italian physiologist Dario Maestrini. In 1914, Maestrini’s experiments laid the groundwork for the understanding of cardiac output regulation in relation to end-diastolic volume. Otto Frank’s pivotal experiments with frog hearts in 1895 further elucidated the relationship between diastolic pressure and ventricular volume. By employing pressure-volume diagrams, Frank established key insights into peak isovolumic pressure and its influence on cardiac performance.
- Ernest Starling, through his studies of intact mammalian hearts, sought to comprehend why variations in factors such as arterial pressure, heart rate, and temperature did not significantly disrupt cardiac output. His pioneering hypothesis in 1914 suggested that the mechanical energy produced in transitioning from a resting to an active state is intrinsically linked to the length of the cardiac fibers. This insight, along with Starling’s use of volume-pressure diagrams to formulate length-tension relationships, provided substantial evidence supporting the impact of myocardial fiber length on systolic pressure.
- In summary, the Frank-Starling Law of the heart stands as a critical principle in cardiovascular physiology, linking the mechanical properties of cardiac muscle to the regulation of stroke volume. This intrinsic mechanism enables the heart to adapt effectively to varying volumes of blood, thereby ensuring optimal performance and maintaining the delicate balance required for efficient circulatory function.
Mechanism of the Frank-Starling mechanism
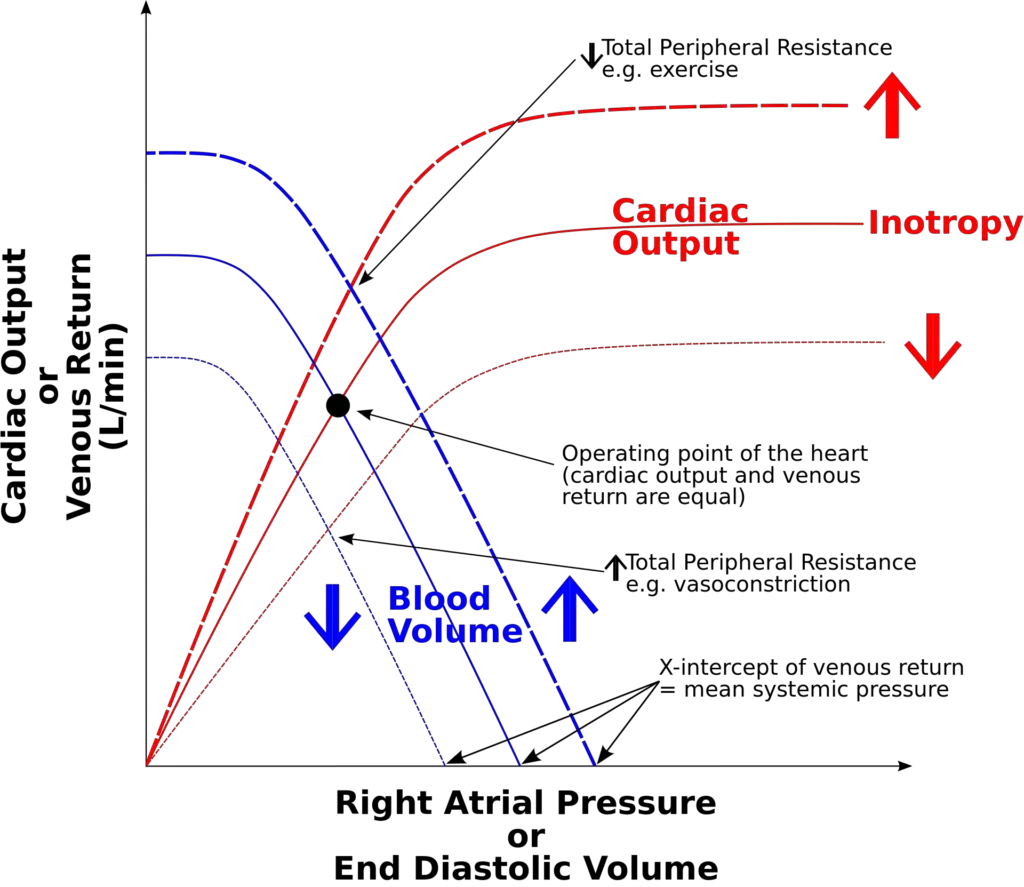
The mechanism underlying left ventricular performance is intricately linked to the Frank-Starling Law, which establishes the relationship between preload and cardiac output. This relationship can be observed through the Frank-Starling curves, which depict how variations in left ventricular end-diastolic volume (EDV) or pressure influence stroke volume and overall cardiac output. The following points outline this mechanism in a structured format:
- Preload and Cardiac Performance:
- Preload refers to the initial stretching of the cardiac muscle fibers prior to contraction, determined by the volume of blood filling the ventricles during diastole.
- The Frank-Starling curve illustrates that, in a normally functioning heart, an increase in preload results in a corresponding increase in stroke volume and cardiac output.
- Effects of Contractility:
- When there is an increase in left ventricular contractility, such as through norepinephrine infusion, cardiac performance for a given preload enhances.
- This phenomenon is represented by an upward shift of the Frank-Starling curve, indicating improved cardiac output at each preload level.
- Conversely, a decrease in left ventricular contractility, often observed in conditions like systolic heart failure, leads to diminished cardiac performance at a given preload. This scenario is depicted by a downward shift of the curve, illustrating the impaired ability of the heart to generate adequate stroke volume.
- Impact of Myocardial Changes:
- Decreased contractility can occur due to various factors, including myocardial infarction, which results in the loss of functional myocardium, and pharmacological agents like beta-blockers and non-dihydropyridine calcium channel blockers.
- Conditions such as dilated cardiomyopathy also lead to reduced myocardial contractility, further shifting the curve downward.
- Afterload Considerations:
- Afterload is defined as the resistance that the ventricle must overcome to eject blood during systole.
- Changes in afterload can also influence the Frank-Starling curve:
- A decrease in afterload leads to an upward shift in the curve, similar to the effects seen with increased contractility.
- Conversely, an increase in afterload results in a downward shift, mirroring the effects of decreased contractility.
- Role of Catecholamines:
- The influence of catecholamines, such as norepinephrine, becomes particularly prominent during exercise.
- Catecholamines bind to beta1-adrenergic receptors on myocardial cells, which are G protein-coupled receptors. This binding triggers a series of intracellular events that result in enhanced release of calcium ions (Ca++) from the sarcoplasmic reticulum.
- The increased availability of Ca++ amplifies the force of contraction, thus causing an upward shift of the Frank-Starling curve and improving cardiac performance.
The physiology of the Frank-Starling mechanism
The physiology of the Frank-Starling mechanism is central to understanding the functioning of cardiac muscle and its response to changes in preload. This mechanism describes how the length-tension relationship within striated muscle influences cardiac performance, specifically in the heart’s ventricles. The following points elucidate the physiological processes involved in this mechanism:
- Length-Tension Relationship:
- The Frank-Starling mechanism is fundamentally rooted in the length-tension relationship observed in striated muscles, which include skeletal, arthropod, and cardiac muscles.
- In striated muscle, active tension arises from the interaction between thick and thin filaments. The optimal length of the muscle fibers allows for maximal overlap between these filaments, producing the greatest isometric active tension.
- Muscle Fiber Length and Optimal Performance:
- In relaxed skeletal muscles, passive elastic properties generally maintain fiber length near optimal values. This length is determined by the fixed distance between tendon attachment points to the bone or exoskeleton.
- However, in cardiac muscle, particularly in a resting ventricle, the relaxed sarcomere length is typically less than the optimal length for effective contraction. Unlike skeletal muscle, cardiac muscle does not have fixed structures, allowing sarcomere length to vary significantly based on ventricular filling and chamber expansion.
- Optimal Sarcomere Length in the Human Heart:
- In humans, maximal force generation occurs at an initial sarcomere length of approximately 2.2 micrometers. This length is rarely exceeded under normal physiological conditions.
- When sarcomere lengths deviate from this optimal range, contractile force diminishes. Longer sarcomere lengths result in less overlap between thin and thick filaments, while shorter lengths reduce calcium sensitivity in the myofilaments.
- Effects of Ventricular Filling:
- Increased filling of the ventricle enhances the load on cardiac muscle cells, stretching their sarcomeres closer to the optimal length. This stretching augments contraction by elevating the calcium sensitivity of myofibrils.
- Specifically, the sensitivity of troponin to calcium ions increases, leading to greater formation of actin-myosin cross-bridges. Additionally, stretch promotes the release of calcium from the sarcoplasmic reticulum, enhancing contraction strength.
- Calcium Dynamics:
- As cardiac myocytes are stretched, there is an increase in the rate of calcium sparks, indicating enhanced calcium release from internal stores. This release further contributes to the strength of contraction.
- Stretching the muscle fibers also appears to reduce the spacing between thick and thin filaments, facilitating more cross-bridge formations, which is vital for effective contraction.
- Relationship Between Force and Sarcomere Length:
- The force generated by individual cardiac muscle cells correlates with the sarcomere length at the time of calcium activation. As ventricular filling occurs, it stretches the muscle fibers, thus determining their sarcomere length.
- Consequently, the pressure generated by cardiac muscle fibers is directly related to the end-diastolic volume in both the left and right ventricles, emphasizing the complexities of the force-sarcomere length relationship.
- Intrinsic Properties of the Myocardium:
- The intrinsic property of the myocardium enables the heart to accommodate increases in venous return without external intervention, regardless of heart rate.
- This mechanism is functionally significant, as it ensures that left ventricular output remains aligned with right ventricular output. If this synchronization were absent, discrepancies in outputs could lead to fluid accumulation in either the pulmonary or systemic circulation.
Clinical Significance
The clinical significance of the Frank-Starling mechanism lies in its crucial role in the compensation of heart failure and other cardiovascular disorders. By understanding this mechanism, healthcare professionals can better manage conditions related to impaired cardiac function. The following points summarize its importance and implications in clinical practice:
- Compensation for Systolic Heart Failure:
- The Frank-Starling mechanism helps buffer the decline in cardiac output experienced during systolic heart failure, which is primarily due to impaired contractile function of the left ventricle.
- In this condition, the left ventricular performance curve shifts downward. This shift indicates that, at any given preload, the stroke volume is reduced compared to normal function.
- The consequence of decreased stroke volume is incomplete emptying of the left ventricle, leading to an accumulation of blood during diastole, which increases the residual volume in the ventricle.
- Increased Stroke Volume through Myocardial Stretch:
- The increased blood volume in the left ventricle stretches the myocardial fibers, allowing the Frank-Starling mechanism to enhance stroke volume during subsequent contractions.
- Therefore, this mechanism plays a vital role in improving the emptying of an enlarged left ventricle and helps to preserve overall cardiac output despite the underlying dysfunction.
- Limitations in Severe Heart Failure:
- The compensatory benefits of the Frank-Starling mechanism are limited, especially in severe heart failure cases where contractility is significantly compromised.
- In such instances, the ventricular performance curve may become nearly flat at elevated diastolic volumes, resulting in minimal increases in cardiac output even with further increases in chamber filling.
- This situation can lead to elevated end-diastolic volume (EDV) and left ventricular end-diastolic pressure (EDP), which may result in pulmonary congestion.
- Role in Dilated Cardiomyopathy:
- The Frank-Starling mechanism also compensates for conditions such as dilated cardiomyopathy, characterized by dilation of both ventricles and decreased contractile function.
- Impaired myocyte contractility leads to a reduction in stroke volume and cardiac output; however, increased diastolic volume from the Frank-Starling mechanism facilitates a compensatory rise in stroke volume.
- Additionally, neurohormonal activation, mediated by the sympathetic nervous system, further compensates for decreased output by increasing heart rate and contractility. This dual mechanism can obscure clinical symptoms during early stages of ventricular dysfunction.
- Use of Inotropic Agents:
- In clinical settings, patients with impaired myocardial systolic function may be treated with inotropic drugs to enhance ventricular contraction force.
- Pharmacologic agents such as cardiac glycosides (e.g., digitalis), sympathomimetic amines (e.g., dopamine and epinephrine), and phosphodiesterase-3 inhibitors (e.g., milrinone) increase intracellular calcium levels, thereby enhancing actin and myosin interaction.
- The administration of these inotropic agents effectively shifts a depressed Frank-Starling curve upward, improving stroke volume and cardiac output at a given preload.
- Management of Pulmonary Congestion:
- In cases of systolic heart failure accompanied by pulmonary congestion, clinicians often prescribe diuretics (e.g., furosemide or hydrochlorothiazide) or pure venous vasodilators (e.g., nitrates) to alleviate fluid overload.
- These treatments reduce preload without significantly altering stroke volume, as the Frank-Starling curve may remain almost horizontal at higher preload levels in patients with significant contractile dysfunction.
- However, excessive diuresis or venous vasodilation can lead to hypotension due to a drop in stroke volume.
- Arteriolar Vasodilation Therapy:
- Arteriolar vasodilators, such as hydralazine, provide another treatment strategy for systolic heart failure with pulmonary congestion. By reducing afterload, these agents increase stroke volume, enhancing left ventricular emptying and decreasing preload, which alleviates pulmonary symptoms.
- Combining vasodilators with positive inotropic agents may yield greater improvements in stroke volume than either treatment alone. However, it is important to note that the Frank-Starling curve may not return to normal performance levels, even with combination therapy.
- Delicce AV, Makaryus AN. Physiology, Frank Starling Law. [Updated 2023 Jan 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470295/
- https://en.wikipedia.org/wiki/Frank%E2%80%93Starling_law