What is Frameshift Mutation?
- A frameshift mutation is a genetic alteration resulting from the insertion or deletion of nucleotides in a DNA sequence that is not a multiple of three. Unlike substitutions, which involve replacing one nucleotide with another, frameshift mutations disrupt the triplet grouping of codons essential for translating genetic information into proteins. This disruption alters the reading frame of the sequence, leading to significant changes in the amino acid sequence of the resultant polypeptide.
- Frameshift mutations are distinct from other mutation types, such as substitutions or indels in non-coding regions, because they directly impact the coding sequence. When nucleotides are inserted or deleted, the codons are shifted, causing a cascade of changes in the downstream amino acid sequence. The resulting protein is often truncated or elongated and typically loses its functional capabilities.
- The severity of the impact from a frameshift mutation depends on the position of the mutation within the coding sequence. Mutations occurring early in the sequence tend to produce more drastic changes in the protein structure compared to those occurring later. Additionally, frameshift mutations frequently introduce new stop codons, leading to prematurely terminated polypeptides or extended proteins, which generally disrupt normal protein function.
- Frameshift mutations can contribute to various genetic disorders and diseases. For instance, they are implicated in conditions such as Tay-Sachs disease and certain types of cancer. In some cases, frameshift mutations have been associated with resistance to infections, such as with the HIV retrovirus. However, their role in generating biological novelty, as seen with potential examples like nylonase, remains a subject of ongoing debate.
Definition of Frameshift Mutation
A frameshift mutation is a genetic alteration caused by the insertion or deletion of nucleotides in a DNA sequence, which shifts the reading frame of codons and results in the production of an altered and typically nonfunctional protein.
Causes of Frameshift Mutation
The main causes of frameshift mutations can be categorized as follows:
- Spontaneous Mutations:
- Errors During DNA Replication: Frameshift mutations often arise spontaneously due to errors in DNA replication. During DNA synthesis, if nucleotides are incorrectly added or removed, this can shift the reading frame of the genetic code.
- Mistakes in DNA Repair: During the repair of DNA damage, incorrect insertions or deletions can occur, leading to frameshift mutations.
- Induced Mutations:
- Chemical Agents: Certain chemicals, such as acridine dyes, can intercalate into the DNA strand, causing insertions or deletions that lead to frameshift mutations.
- Physical Agents: Exposure to physical mutagens like X-rays or UV radiation can cause DNA damage, potentially leading to frameshift mutations if the repair mechanisms are faulty or incomplete.
- Reactive Oxygen Species (ROS):
- Oxidative Damage: Reactive oxygen species, which are byproducts of cellular metabolism, can cause oxidative damage to DNA. This damage can result in insertions or deletions during repair processes, leading to frameshift mutations.
- Intercalating Agents:
- Molecular Interference: Certain intercalating agents insert themselves between DNA bases, causing distortions in the DNA helix. This can lead to insertions or deletions of nucleotides, shifting the reading frame and causing frameshift mutations.
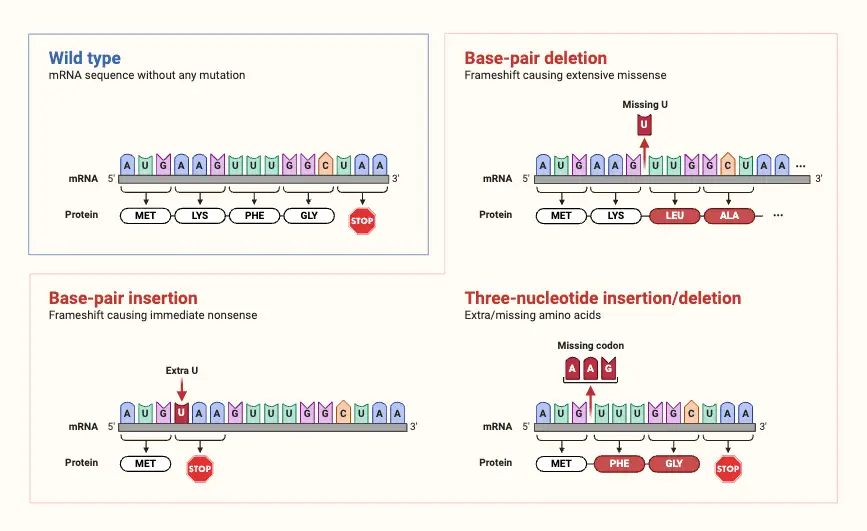
Types of Frameshift Mutations
Frameshift mutations are categorized based on the nature of the nucleotide change within the DNA sequence. These changes can be classified into two main types: deletion and insertion frameshift mutations. Each type impacts the reading frame of the genetic code differently.
- Deletion:
- Definition: Deletion refers to the removal of one or more nucleotides from the DNA sequence.
- Mechanism:
- Anaphase Movement: During cell division, particularly in anaphase, deletions can occur if chromosomes are not properly segregated.
- Nuclease Digestion: Enzymatic digestion by nucleases can also lead to deletions by removing segments of DNA.
- Types:
- Terminal Deletion: This involves the loss of nucleotides at the end of a chromosome. Such deletions affect the terminal sections and can disrupt gene function if they occur within coding regions.
- Intercalary Deletion: This involves the removal of an intermediate segment of the chromosome. Intercalary deletions can lead to more extensive alterations in the reading frame since they affect the internal regions of the coding sequence.
- Impact: Deletions are a common cause of frameshift mutations and typically result in a significant shift in the reading frame, altering the downstream amino acid sequence and often leading to nonfunctional proteins.
- Insertion:
- Definition: Insertion involves the addition of one or more nucleotides into the DNA sequence.
- Mechanism:
- Microsatellite Regions: Insertions frequently occur in microsatellite regions, which are repetitive DNA sequences prone to such mutations. This is often facilitated by the action of DNA polymerase during replication.
- Nucleotide Addition: The inserted nucleotides can vary in number and position, influencing the extent of the frameshift mutation.
- Impact: Insertions change the reading frame by introducing additional codons. This alteration can lead to the production of an altered protein with incorrect amino acid sequences or premature stop codons. The effect is often detrimental to protein function, resulting in truncated or nonfunctional proteins.
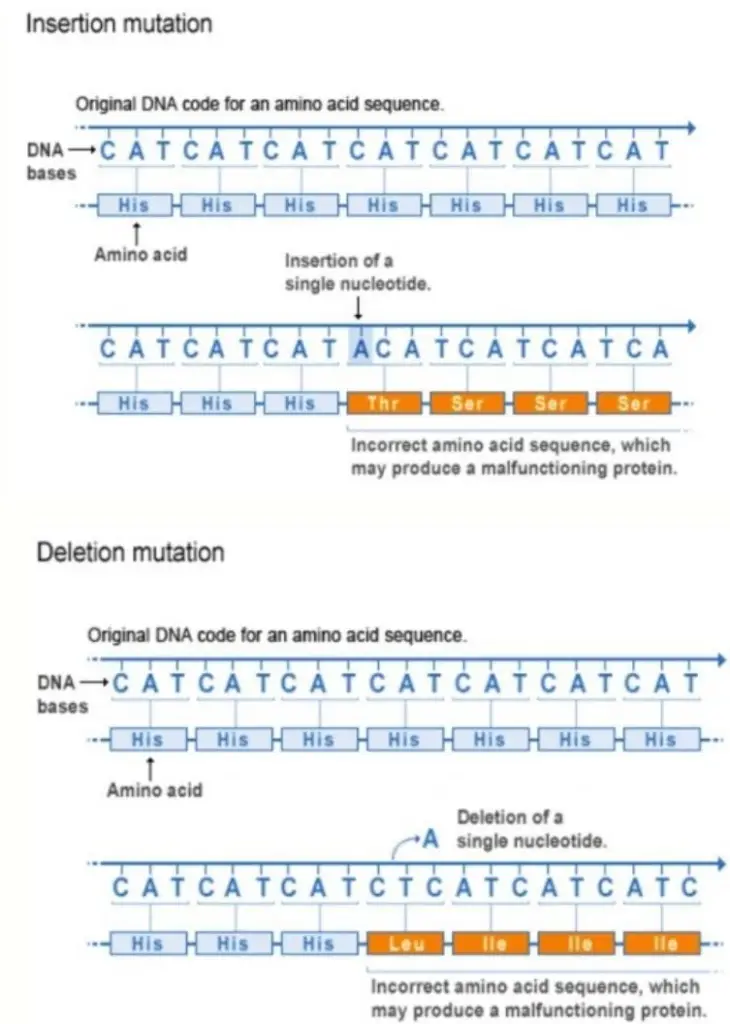
Effects of Frameshift Mutations
The primary effects of frameshift mutations include:
- Altered Protein Function:
- Non-functional Proteins: Frameshift mutations often result in a protein with an incorrect amino acid sequence, which can render the protein non-functional. This is due to the misalignment of codons caused by the shift in the reading frame.
- New Proteins: In some cases, the mutation can produce a novel protein with altered properties. However, this new protein is often dysfunctional or has an altered activity compared to the original protein.
- Abnormal Translation Termination:
- Premature Stop Codons: Frameshift mutations can introduce premature stop codons into the coding sequence. This leads to the truncation of the protein, producing an incomplete and often non-functional protein product.
- Impact on Cellular Functions: The production of truncated proteins can disrupt normal physiological processes, as these proteins may lack essential functional domains required for their activity.
- Dysregulation of Cellular Processes:
- Compensatory Mechanisms: Cells may attempt to compensate for the non-functional protein by upregulating the expression of the mutated gene. This can lead to an overproduction of the mutated protein or an increase in misfolded proteins.
- Cellular Stress: The accumulation of misfolded proteins can overwhelm cellular quality control mechanisms, potentially leading to cellular stress or apoptosis.
- Disease Associations:
- Genetic Disorders: Frameshift mutations are implicated in several genetic diseases. For instance, Crohn’s disease and cystic fibrosis can result from frameshift mutations that disrupt essential proteins involved in cellular functions.
- Cancer: Certain types of cancer are associated with frameshift mutations that lead to the production of abnormal proteins involved in tumorigenesis.
- Potential Benefits:
- Protective Effects: In some instances, frameshift mutations may confer a selective advantage. For example, a frameshift mutation in the CCR5 gene has been associated with resistance to HIV infection, demonstrating that such mutations can sometimes have beneficial effects.
Applications of Frameshift Mutation
The following outlines the key applications of frameshift mutations:
- Disease Diagnosis
- Genetic Testing: Frameshift mutations are linked to numerous genetic disorders. Their identification through genetic testing can facilitate the diagnosis of conditions such as Tay-Sachs disease, cystic fibrosis, and various cancers. Detecting specific frameshift mutations allows for accurate diagnosis and can help in determining the appropriate treatment plan.
- Screening Programs: Inherited genetic diseases often have frameshift mutations as a primary cause. Screening programs can use knowledge of these mutations to identify at-risk individuals or carriers of specific genetic conditions, leading to early intervention and management.
- Molecular Therapy
- Gene Therapy: Frameshift mutations can be deliberately induced in a controlled manner to correct or replace defective genes. For instance, researchers can design gene therapy approaches to counteract frameshift mutations that cause genetic diseases by inserting or correcting specific sequences within the genome.
- Targeted Therapies: In some therapeutic strategies, frameshift mutations are utilized to create non-functional proteins that disrupt disease pathways, particularly in cancer treatment. This approach targets cancer cells more precisely while minimizing harm to normal cells.
- Cancer Research
- Targeting Cancer Cells: Frameshift mutations can be exploited to target cancer-specific proteins or pathways. By understanding how frameshift mutations affect protein function, researchers can develop drugs that specifically target these mutated proteins or their associated pathways, thereby improving cancer treatment efficacy.
- Drug Development: The study of frameshift mutations in cancer cells can aid in the identification of novel drug targets and the development of new chemotherapeutic agents.
- Agricultural Biotechnology
- Mutation Breeding: Controlled induction of frameshift mutations can be used to create genetic variation in crops. This process, known as mutation breeding, helps in developing new plant varieties with desirable traits such as increased yield, disease resistance, or improved nutritional content.
- Crop Improvement: By inducing frameshift mutations, researchers can enhance specific attributes of crops, leading to the production of varieties that are better suited to environmental conditions or have improved agricultural performance.
- Protein Studies
- Structural and Functional Analysis: Frameshift mutations can be used to study the effects of protein structural changes on function. Researchers can induce these mutations to investigate how alterations in protein sequences impact stability, interactions, and overall function, providing insights into protein mechanics and biology.
- Protein Engineering: Understanding frameshift-induced alterations helps in designing proteins with novel functions or properties. This knowledge can be applied in protein engineering to create custom proteins for industrial or therapeutic purposes.
- Ecological and Evolutionary Studies
- Adaptive Evolution: In some cases, frameshift mutations can confer advantageous traits to organisms, enhancing their fitness in specific environments. Studying these mutations can provide insights into evolutionary processes and how genetic variations contribute to adaptation and survival.
Diseases caused by frameshift mutations
Several notable diseases are caused by frameshift mutations:
- Tay-Sachs Disease
- Mutation Details: A frameshift mutation in the HEXA gene causes Tay-Sachs disease.
- Effects: The absence of the Hex-A enzyme leads to the accumulation of GM2 gangliosides in neurons. This lipid buildup causes progressive neurological damage and is fatal.
- Cystic Fibrosis
- Mutation Details: Two distinct frameshift mutations in the CFTR gene, one involving the insertion of two nucleotides and another a single nucleotide deletion, are implicated.
- Effects: These mutations disrupt the CFTR protein, impairing chloride and sodium ion transport across cell membranes. This dysfunction results in persistent lung infections, pancreatic damage, and other organ malfunctions.
- Leigh Disease
- Mutation Details: A frameshift mutation in the NDUFS4 gene, which encodes a component of the NADH dehydrogenase complex, is responsible.
- Effects: Leigh disease is a severe mitochondrial disorder characterized by progressive neurodegeneration, leading to symptoms such as feeding difficulties, hypotonia, seizures, and respiratory issues.
- Type A Niemann-Pick Disease
- Mutation Details: Frameshift mutations in the acid sphingomyelinase gene (fsP330) contribute to this disorder.
- Effects: This genetic condition results in defective sphingomyelin metabolism, causing severe neurological and visceral symptoms.
- Crohn’s Disease
- Mutation Details: Frameshift mutations in the NOD2 gene, including a cytosine insertion (3020insC), are associated.
- Effects: These mutations lead to a truncated NOD2 protein, impairing immune responses and contributing to inflammation and gastrointestinal symptoms typical of Crohn’s disease.
- Certain Cancers
- Mutation Details: Frameshift mutations can occur in various oncogenes and tumor suppressor genes.
- Effects: These mutations may contribute to cancer development by altering proteins involved in cell growth and division. Examples include lung cancer, colorectal cancer, and hereditary breast and ovarian cancers.
- Hypertrophic Cardiomyopathy
- Mutation Details: Frameshift mutations in the Troponin C gene, such as c.363dupG (p.Gln122AlafsX30), are implicated.
- Effects: This genetic mutation causes abnormal cardiac muscle growth, leading to hypertrophic cardiomyopathy, a condition associated with sudden cardiac death in young adults.
- Smith-Magenis Syndrome
- Mutation Details: This disorder results from interstitial deletions affecting the RAI1 gene.
- Effects: The syndrome is characterized by mental retardation, craniofacial and skeletal anomalies, developmental delays, and sleep disturbances.
- Hereditary Polyneuropathy
- Mutation Details: Dominant-negative frameshift mutations in the LRSAM1 gene are associated.
- Effects: These mutations lead to a progressive degeneration of peripheral nerves, resulting in sensory and motor deficits.
Examples of Frameshift Mutation
Here are illustrative examples of how frameshift mutations can impact the protein translation process:
- Example of a Single Nucleotide Insertion:
- Original Sequence:
AUG-AAT-AAC-GCU(This sequence codes for the amino acids: start-leucine-asparagine-alanine.) - Mutation: Insertion of an additional adenine (A) nucleotide after the start codon.
- Mutated Sequence:
AUG-AAA-TAA-CGC(This sequence codes for: start-lysine-isoleucine-alanine.) - Effect: The insertion of a single nucleotide shifts the reading frame, resulting in a completely altered sequence of amino acids. This change can disrupt the normal function of the protein or create a nonfunctional variant.
- Original Sequence:
- Example of a Deletion:
- Original Coding Sequence:
ATGGTCGATCTGACTCCTGAGGAGAAGTCT - Amino Acid Translation: Methionine-Valine-Arginine-Leucine-Threonine-Proline-Glutamic Acid-Glutamic Acid-Lysine-Serine
- Frameshift Mutation: Removing the underlined segment
ATresults inATGGTCGCTGACTCCfollowed by a stop codon. - Amino Acid Translation: Methionine-Valine-Proline-Aspartic Acid-Stop
- Effect: The deletion introduces a stop codon early in the sequence, truncating the protein and rendering it nonfunctional.
- Original Coding Sequence:
- Introduction of an Early Stop Codon:
- Original Coding Sequence:
ATGGTCGCATCTGACTCCTGAGGAGAAGTCT - Amino Acid Translation: Methionine-Valine-Arginine-Isoleucine-Threonine-Proline-Glutamic Acid-Glutamic Acid-Lysine-Serine
- Frameshift Mutation: Removing the underlined segment
ATchanges the sequence toATGGTCGCTGACTCCand introduces a premature stop codon. - Amino Acid Translation: Methionine-Valine-Proline-Aspartic Acid-Stop
- Effect: The premature stop codon leads to an incomplete protein, which is typically nonfunctional.
- Original Coding Sequence:
Differences Between Point Mutation and Frameshift Mutation
Point mutations and frameshift mutations are two distinct types of genetic mutations, each with unique mechanisms and consequences.
Point Mutation
- Definition
- Point mutations involve the substitution of a single nucleotide base in the DNA sequence. This substitution can alter one nucleotide, but it does not change the overall length of the DNA sequence or shift the reading frame.
- Types
- Transition: A transition mutation occurs when a purine base (adenine or guanine) is replaced by another purine base or a pyrimidine base (cytosine or thymine) is replaced by another pyrimidine base.
- Transversion: A transversion mutation involves the substitution of a purine base with a pyrimidine base, or vice versa.
- Effects
- Nucleotide Sequence: Although the sequence of the nucleotide changes, the reading frame of the mRNA is not affected.
- Protein Function: Depending on the location and nature of the substitution, point mutations can lead to silent, missense, or nonsense mutations. For example, sickle cell anemia is caused by a point mutation that alters the hemoglobin protein.
- Examples
- Sickle Cell Anemia: This condition is caused by a point mutation in the β-globin gene, where adenine is replaced by thymine, leading to a single amino acid change in the hemoglobin protein.
Frameshift Mutation
- Definition
- Frameshift mutations involve the insertion or deletion of one or more nucleotides in the DNA sequence. These changes disrupt the reading frame of the mRNA, leading to a shift in the entire downstream nucleotide sequence.
- Types
- Insertion: The addition of extra nucleotides into the DNA sequence.
- Deletion: The removal of nucleotides from the DNA sequence.
- Effects
- Nucleotide Sequence: Frameshift mutations alter the nucleotide sequence significantly by shifting the reading frame, which results in a completely different sequence of amino acids downstream of the mutation site.
- Protein Function: This alteration often leads to premature termination of the protein translation or the production of a malfunctioning protein. For instance, Tay-Sachs disease results from a frameshift mutation that disrupts the Hex-A enzyme function, leading to severe neurological damage.
- Examples
- Tay-Sachs Disease: Caused by a frameshift mutation in the HEXA gene, leading to a dysfunctional Hex-A enzyme and resultant lipid accumulation in the brain.
Comparison Table
| Aspect | Point Mutation | Frameshift Mutation |
|---|---|---|
| Type of Change | Replacement of one base pair with another | Insertion or deletion of base pairs |
| Effect on Nucleotide Sequence | Changes occur at a single nucleotide | Multiple nucleotide alterations |
| Effect on Reading Frame | Reading frame remains unaffected | Reading frame is altered |
| Types | Transition, transversion | Insertion, deletion |
| Examples | Sickle cell anemia | Tay-Sachs disease |
Facts
- Did you know that frameshift mutations can drastically alter protein function by shifting the reading frame of the genetic code, leading to completely different amino acid sequences?
- Have you heard that inserting or deleting just one nucleotide in a gene can lead to frameshift mutations, potentially resulting in a nonfunctional protein or a completely new protein?
- Are you aware that frameshift mutations often introduce premature stop codons, which can truncate proteins and disrupt essential biological functions?
- Can you believe that frameshift mutations are implicated in a variety of genetic diseases, such as Tay-Sachs disease, cystic fibrosis, and some cancers, due to their impact on protein synthesis?
- Did you know that frameshift mutations can be used deliberately in research to study the effects of protein structure changes on function, providing insights into protein mechanics?
- Have you heard that frameshift mutations can be induced in plants through mutation breeding to develop new crop varieties with improved traits like disease resistance or higher yield?
- Are you aware that some frameshift mutations can lead to beneficial adaptations in certain organisms, potentially enhancing their survival and fitness in specific environments?
- Can you believe that frameshift mutations can be targeted in cancer therapies to disrupt the function of cancer-specific proteins, offering a potential strategy for more effective treatments?
- Did you know that understanding frameshift mutations helps scientists design gene therapies to correct genetic disorders by compensating for or repairing the mutations?
- Have you heard that frameshift mutations can sometimes cause the formation of misfolded proteins that may trigger cellular stress responses, contributing to various diseases and conditions?
- https://biologydictionary.net/frameshift-mutation/
- https://www.biologyonline.com/dictionary/frameshift-mutation
- https://dnaofbioscience.blogspot.com/2016/05/what-is-frame-shift-mutation.html
- https://www.nature.com/scitable/definition/frameshift-mutation-frame-shift-mutation-frameshift-203/
- https://en.wikipedia.org/wiki/Frameshift_mutation