What is Folin-Wu Method?
- The Folin-Wu Method represents a historical approach in the field of biochemistry for measuring blood sugar levels. Developed in the early 20th century, this method played a pivotal role in understanding and diagnosing conditions related to blood sugar, particularly diabetes. Despite its historical significance, the Folin-Wu Method has largely been replaced by more advanced techniques due to certain limitations in its specificity and accuracy.
- At its core, the Folin-Wu Method involves a chemical process where blood plasma reacts with a specific reagent, leading to a color change. This colorimetric change is indicative of the sugar levels in the blood. One of the key aspects of this method was its simplicity and the minimal requirement for specialized equipment, making it accessible in areas with limited resources.
- However, the method’s lack of specificity is a notable drawback. It does not exclusively react with glucose but also with other reducing sugars like fructose, lactose, and certain compounds such as glutathione. This non-specificity means that the blood sugar levels estimated by the Folin-Wu method can be inaccurately high, as it accounts for sugars other than glucose.
- Although it is now considered an outdated technique in many parts of the world, the Folin-Wu Method may still find application in regions where access to modern enzyme-based tests is limited. Its use in such scenarios underscores a significant point in global healthcare – the reliance on older methodologies where resources for newer technologies are scarce.
- In summary, the Folin-Wu Method is a key historical technique in biochemistry for blood sugar estimation. While it has been largely supplanted by more specific and accurate methods, its role in the early understanding of blood sugar levels and its continued use in resource-limited settings highlights its importance in the evolution of biochemical testing.
Principle of Folin-Wu Method
- The Folin-Wu Method is a biochemical technique primarily used for estimating glucose levels in blood. This method is based on a series of chemical reactions that result in a measurable color change, indicative of the glucose concentration.
- Initially, the process begins with the removal of proteins from the blood sample. This is achieved through the addition of 10% sodium tungstate and 2/3N sulfuric acid. The mixture undergoes a filtration process, resulting in a protein-free filtrate which contains glucose.
- Once the proteins are removed, the glucose present in the filtrate is subjected to a boiling process in an alkaline medium. During this step, glucose is converted into its enediol form. This enediol form plays a crucial role in the subsequent reaction where it reduces cupric ions (Cu²⁺) to cuprous oxide (Cu₂O), a precipitate.
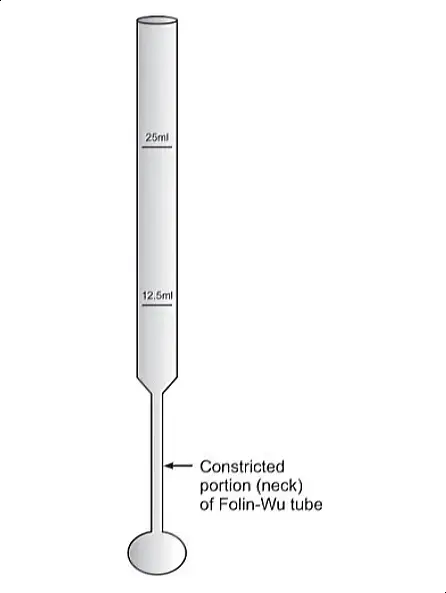
- To ensure accuracy in measurement, the method employs Folin-Wu tubes. These tubes are specifically designed to minimize the reoxidation of the formed cuprous oxide by atmospheric oxygen – a factor that could otherwise affect the outcome of the test.
- The cuprous oxide then reacts with phosphomolybdic acid, leading to the formation of phosphomolybdenum blue. This substance is notable for its distinctive blue color. The intensity of this blue color is directly proportional to the amount of glucose present in the sample.
- To quantify the glucose concentration, the final blue solution is measured spectrophotometrically at a wavelength of 680 nm. The intensity of the color, as measured at this specific wavelength, provides an estimate of the glucose level in the blood sample.
- In summary, the principle of the Folin-Wu Method involves a series of chemical reactions that convert glucose in a blood sample into a colored compound, the intensity of which can be measured to determine the glucose concentration. This method, though less common today, played a significant role in the historical development of biochemical testing for blood sugar levels.
Requirements
- Folin-Wu Tubes: These are specialized glass tubes designed to prevent the reoxidation of certain chemicals during the testing process. Their unique construction is essential for maintaining the integrity of the test results.
- Colorimeter: A device used for measuring the intensity of color in a solution. In the context of the Folin-Wu method, the colorimeter assesses the intensity of the blue color formed, which correlates with the glucose concentration.
- Reagents:
- 2/3 N H2SO4 (Sulfuric Acid): Prepared by adding 2 ml of sulfuric acid to approximately 50 ml of distilled water (D/W), and then diluting the mixture to a final volume of 100 ml.
- 10% Sodium Tungstate: Created by dissolving 10 grams of sodium tungstate in 100 ml of distilled water.
- Alkaline Copper Tartarate: This reagent has two components:
- Solution A: 40 grams of sodium carbonate and 7.5 grams of tartaric acid are dissolved in about 400 ml of distilled water.
- Solution B: 4.5 grams of copper sulfate are dissolved in approximately 100 ml of distilled water. The two solutions (A and B) are then mixed and the volume is adjusted to 1000 ml with distilled water.
- Phosphomolybdic Acid: Prepared by dissolving 35 grams of molybdic acid and 5 grams of sodium tungstate in 200 ml of 10% sodium hydroxide (NaOH). This mixture is added to 200 ml of distilled water and boiled for 45 minutes to remove ammonia. After cooling, slowly add 125 ml of 89% phosphoric acid and then adjust the volume to 500 ml with distilled water.
- Distilled Water: Used as a solvent and for dilution purposes in various steps of the procedure.
- Glucose Standards:
- Stock Solution (1g/dl): Prepared by dissolving 1 gram of glucose in 100 ml of saturated benzoic acid solution (0.3% concentration).
- Working Standard (10mg/dl): This is made by diluting the stock solution in a 1:100 ratio with saturated benzoic acid.
Procedure of Folin-Wu Method
Step 1: Preparation of Protein-Free Filtrate
- Mixing the Blood Sample: Begin by adding 1 ml of blood to 7 ml of distilled water in a container. Mix these components thoroughly.
- Adding Reagents: To this mixture, add 1 ml of 10% sodium tungstate. Then, add 1 ml of 2/3N sulfuric acid (H2SO4). After adding these reagents, mix the contents well.
- Settling and Filtration: Allow the mixture to stand undisturbed for about 5 minutes. This standing time is crucial for the reaction to occur. Following this, either centrifuge the mixture or filter it using Whatman number 1 filter paper to obtain the protein-free filtrate.
Step 2: Testing Procedure
- Setting up Folin-Wu Tubes: Arrange three Folin-Wu tubes and add the following to each:
- Blank Tube: Add 1 ml of distilled water.
- Standard Tube: Add 1 ml of the working glucose standard.
- Test Tube: Add 1 ml of the protein-free filtrate prepared earlier. To each of these tubes, also add 1 ml of alkaline copper tartarate.
- Boiling: Place all three tubes in a boiling water bath for 10 minutes. This step is important for the necessary chemical reactions to occur.
- Cooling and Adding Reagent: After the boiling process, remove the tubes and allow them to cool. Once cooled, add 1 ml of phosphomolybdic acid reagent to each tube.
- Eliminating Air Bubbles and Dilution: Vigorously shake the tubes to eliminate any air bubbles. Then, add distilled water to each tube up to the 12.5 ml mark.
- Measurement: Finally, mix the contents of each tube well and measure the absorbance at 680 nm, or using a red filter. Use the blank tube to set the zero on the measuring device.
This process results in a color change proportional to the glucose concentration in the blood sample. By comparing the absorbance of the test sample against the standard, the glucose level in the blood can be estimated. The Folin-Wu method, while historical, showcases the foundational techniques in biochemical analysis of blood sugar levels.
Calculations of Folin-Wu Method
- Measurement of Optical Density (OD): The OD of both the test sample and the standard solution is measured using a colorimeter. The OD is a measure of the amount of light absorbed by the solution, which is in turn proportional to the concentration of glucose present.
- Formula for Calculation: The concentration of glucose in the blood specimen is calculated using the following formula:
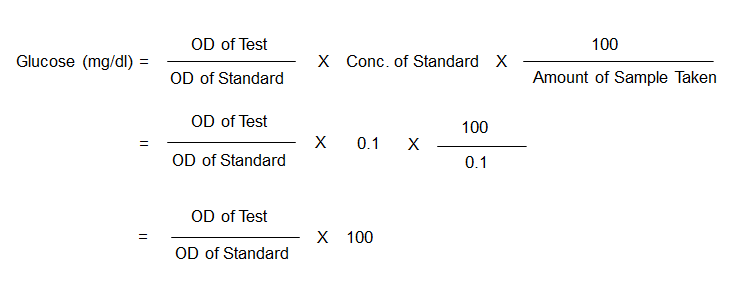
- Substitution of Values: In the given scenario, the blood sample was diluted 1:10 for protein precipitation, and then 1 ml of this diluted sample was used for the test, making the actual volume of blood used for the test 0.1 ml. Additionally, 1 ml of the working standard solution, which is at a concentration of 10 mg/dl, contains 0.1 mg of glucose.
- Final Calculation: The glucose concentration is then obtained by inserting the measured OD values for both the test and the standard into the simplified formula.
This calculation will yield the concentration of glucose in the blood sample in mg/dl, which can be used to assess the glucose levels for clinical or research purposes. It is important to ensure that the measurements of OD are accurate and that the standard solution is properly prepared to achieve reliable results.
References
- Kolhatkar, A., Ochei, J., & McGraw, T. (2008). Medical Laboratory Science: Theory and Practice.
- Naigaonkar, A.V., (2007). A Manual Of Medical Laboratory Technology.
- Geetha, D. K. (2011). Practical Biochemistry. Jaypee Brothers Medical Publishers (P) Ltd, UK.