Fehling’s test is a classical chemical test which is used to detect the presence of reducing sugars and aldehyde functional groups in a given sample. It is mainly used to differentiate reducing sugars from non-reducing sugars and ketones. It is based on the reducing property of aldehyde group which reduces copper ions under alkaline condition.
It is the test which uses a reagent known as Fehling’s solution. This solution is prepared freshly by mixing equal volume of Fehling’s A and Fehling’s B. Fehling’s A is a blue coloured aqueous solution of copper sulphate (CuSO₄), while Fehling’s B is a colourless alkaline solution containing potassium sodium tartrate (Rochelle salt) and sodium hydroxide. These two solutions together forms a deep blue complex.
When a solution containing reducing sugar or aldehyde (such as glucose) is heated with freshly prepared Fehling’s solution, the cupric ions (Cu²⁺) are reduced to cuprous ions (Cu⁺). As a result, a brick-red or reddish brown precipitate of copper(I) oxide (Cu₂O) is formed. If the test solution does not contain a reducing sugar, no reaction occurs and the blue colour of the solution remains unchanged.
This test was earlier used widely in medical field for the detection of glucose in urine, especially in diagnosis of diabetes mellitus. Since the reagent is unstable, it is always prepared freshly before performing the test.
Objectives of Fehling’s Test
The objectives of Fehling’s test are listed below–
- To detect the presence of aldehyde group (–CHO) in an organic compound.
- To distinguish between aldehydes and ketones, as aldehydes give positive test while ketones generally do not react.
- To identify the presence of reducing sugars such as glucose, lactose and maltose.
- To differentiate reducing sugars from non-reducing sugars like sucrose.
- To detect glucose in urine which helps in diagnosis of diabetes mellitus.
- To estimate the amount of reducing sugars in food and industrial samples by determining dextrose equivalent (DE).
Principle of Fehling’s Test
The principle of Fehling’s test is based on an oxidation–reduction reaction which is used to detect aldehyde group and reducing sugars in a given solution. It is based on the ability of reducing sugars to reduce copper ions in alkaline medium. In this test copper(II) ions are maintained in solution by tartrate ions in alkaline condition, otherwise the copper ions would precipitate as copper hydroxide due to presence of sodium hydroxide.
When a solution containing aldehyde group or reducing sugar is heated with Fehling’s solution, the aldehyde group is oxidized to carboxylic acid while the cupric ions (Cu²⁺) are reduced to cuprous ions (Cu⁺). As a result of this reduction reaction, insoluble copper(I) oxide (Cu₂O) is formed which appears as brick-red precipitate. The formation of this precipitate confirms the presence of reducing sugar in the test sample.
Fehling’s test reaction
The reaction in Fehling’s test is an oxidation–reduction reaction in which aldehyde group or reducing sugar reduces cupric ions in alkaline medium. During this reaction the aldehyde group is oxidized to carboxylic acid, while the blue coloured copper(II) ions are reduced to cuprous ions forming a red precipitate.
The reaction is as follows–
R–CHO + 2Cu²⁺ + 5OH⁻ → R–COO⁻ + Cu₂O↓ + 3H₂O
Here, the formation of brick-red precipitate of copper(I) oxide (Cu₂O) indicates a positive Fehling’s test.
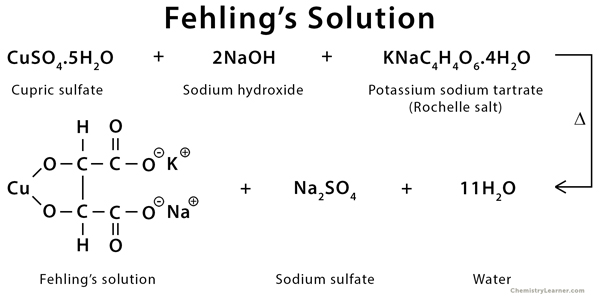
What is Fehling’s Solution?
Fehling’s solution is a chemical reagent which is used in Fehling’s test for the detection of aldehyde group and reducing sugars. It is not a single solution but is prepared freshly by mixing two separate solutions in equal proportion before use. The solution provides an alkaline medium and copper ions required for the redox reaction.
Fehling’s solution consists of two components namely Fehling’s A and Fehling’s B. Fehling’s A is an aqueous solution of copper sulphate (CuSO₄) which is blue in colour, while Fehling’s B is a colourless alkaline solution containing potassium sodium tartrate (Rochelle salt) and sodium hydroxide. The tartrate ions help in keeping the copper ions in solution under alkaline condition and prevent their precipitation as copper hydroxide.
Requirements for Fehling’s Test
The requirements for Fehling’s test are given below–
Reagents
- Fehling’s solution A (aqueous copper sulphate solution).
- Fehling’s solution B (alkaline solution of potassium sodium tartrate and sodium hydroxide).
- Test solution containing reducing sugar or aldehyde.
- Distilled water (for control if required).
Laboratory Apparatus
- Clean and dry test tubes.
- Test tube holder.
- Pipette or dropper.
- Water bath or heating source.
- Test tube stand.
Fehling’s Test Procedure
The procedure of Fehling’s test is carried out in the following steps–
- Take a clean and dry test tube.
- Add 1 ml of Fehling’s solution A and 1 ml of Fehling’s solution B in the test tube to prepare Fehling’s solution freshly.
- Mix the solution properly so that a deep blue coloured solution is formed.
- Add 1–2 ml of the test solution into the test tube containing Fehling’s solution.
- Place the test tube in a boiling water bath and heat for few minutes.
- Observe the colour change or formation of precipitate in the test tube.
- Appearance of brick-red precipitate indicates positive Fehling’s test, while no change in blue colour indicates negative result.
Result of Fehling’s Test
The result and interpretation of Fehling’s test are described below–
Positive Result
- Formation of brick-red or reddish brown precipitate after heating.
- The blue colour of Fehling’s solution gradually disappears and insoluble copper(I) oxide (Cu₂O) is formed.
Interpretation
- Indicates the presence of reducing sugars such as glucose, maltose and lactose.
- Confirms the presence of aliphatic aldehyde group (–CHO) which is oxidized to carboxylic acid.
- Alpha-hydroxy ketones like fructose also give positive result due to conversion into aldehyde in alkaline medium.
- In medical test, presence of glucose in urine indicates diabetes mellitus.
Negative Result
- No change in colour of the solution after heating.
- The solution remains deep blue and no precipitate is formed.
Interpretation
- Indicates absence of reducing sugars or aldehyde group.
- Confirms the presence of non-reducing sugars like sucrose.
- Aromatic aldehydes and simple ketones do not give positive Fehling’s test.

Uses of Fehling’s Test
The uses of Fehling’s test are listed below–
- To differentiate between aldehydes and ketones.
- To detect the presence of reducing sugars such as glucose, fructose, lactose and maltose.
- To distinguish reducing sugars from non-reducing sugars like sucrose.
- To detect glucose in urine for diagnosis of diabetes mellitus.
- To estimate dextrose equivalent (DE) in food and starch industries.
- To demonstrate oxidation–reduction reaction in laboratory experiments.
- To detect other reducing compounds like formic acid and alpha-hydroxy ketones.
Advantages of Fehling’s Test
The advantages of Fehling’s test are listed below–
- It gives clear and visible result by formation of brick-red precipitate.
- It helps in distinguishing aldehydes from ketones.
- It is useful in identification of reducing sugars.
- It differentiates reducing sugars from non-reducing sugars.
- It can detect small amount of reducing sugar in a sample.
- It was used earlier in medical field for detection of glucose in urine.
- It is simple, economical and easy to perform in laboratory.
- It is useful for teaching oxidation–reduction reaction in practical classes.
Limitations of Fehling’s Test
The limitations of Fehling’s test are listed below–
- Fehling’s solution is unstable and must be prepared freshly before use.
- It does not give positive result with aromatic aldehydes.
- It cannot clearly differentiate between aldoses and ketoses, as some ketoses give positive test.
- Other reducing substances like formic acid and ascorbic acid may give false positive result.
- It gives only qualitative result and cannot estimate exact amount of sugar.
- The reagent is strongly alkaline and needs careful handling in laboratory.
FAQ
1. What is Fehling’s Test?
Fehling’s test is a chemical test which is used to detect the presence of aldehyde group and reducing sugars in a given solution. It is mainly used to differentiate reducing sugars from non-reducing sugars and ketones based on reduction of copper ions in alkaline medium.
2. What is Fehling’s solution?
Fehling’s solution is a chemical reagent used in Fehling’s test. It is not a single solution but is prepared freshly by mixing two separate solutions, Fehling’s A and Fehling’s B, in equal volume before use.
3. What is the principle of Fehling’s test?
The principle of Fehling’s test is based on oxidation–reduction reaction. In alkaline medium, aldehyde group or reducing sugar reduces blue coloured copper(II) ions to cuprous ions forming a brick-red precipitate of copper(I) oxide.
4. What are the uses of Fehling’s Test?
Fehling’s test is used to detect reducing sugars, to differentiate aldehydes from ketones, to distinguish reducing and non-reducing sugars, and for detection of glucose in urine for diagnosis of diabetes mellitus.
5. How is Fehling’s solution prepared?
Fehling’s solution is prepared freshly by mixing equal volume of Fehling’s solution A and Fehling’s solution B just before performing the test.
6. What is Fehling’s A?
Fehling’s A is an aqueous solution of copper sulphate (CuSO₄). It is blue in colour and provides copper(II) ions required for the reaction.
7. What is Fehling’s B?
Fehling’s B is an alkaline solution containing potassium sodium tartrate (Rochelle salt) and sodium hydroxide. It provides alkaline medium and keeps copper ions in solution.
8. What is the difference between Fehling’s Test and Benedict’s Test?
Fehling’s test requires freshly prepared reagent and gives mainly qualitative result, while Benedict’s test uses a single stable reagent and can give semi-quantitative result by different colour changes. Benedict’s test is more commonly used for urine sugar testing.
9. What is the procedure for Fehling’s test?
In this test, equal volume of Fehling’s A and Fehling’s B are mixed, then test solution is added and the mixture is heated in water bath. Formation of brick-red precipitate indicates positive result.
10. How does Fehling’s Test work?
Fehling’s test works by oxidation of aldehyde group and reduction of copper(II) ions to copper(I) oxide in alkaline medium, which forms a visible precipitate.
11. What do the results of Fehling’s Test mean?
Brick-red precipitate indicates presence of reducing sugar or aldehyde group, while no colour change indicates absence of reducing sugar.
12. What is the main objective of Fehling’s test?
The main objective of Fehling’s test is to detect reducing sugars and aldehyde group in a given sample.
13. What are the limitations of the Fehling Test?
The test reagent is unstable, aromatic aldehydes do not give positive result, some ketoses give false positive result, and the test is only qualitative in nature.
14. Who developed Fehling’s Test?
Fehling’s test was developed by German chemist Hermann von Fehling.
15. Why are Fehling solutions A and B kept separate?
Fehling’s solutions A and B are kept separate because the mixed solution is unstable. They are mixed freshly before use to prevent precipitation of copper hydroxide and loss of activity.
- Aakash Institute. (n.d.). Fehling solution in biology: Definition, types and importance.
- Allen. (n.d.). Fehling’s solution: Preparation, tests and application.
- AquaPhoenix Scientific. (2015, December 1). Safety data sheet: Fehlings solution B. Fisher Science Education.
- Ashenhurst, J. (2025, March 21). Reducing sugars. Master Organic Chemistry.
- BYJU’S. (n.d.). Fehling test.
- BYJU’S. (n.d.). Fehlings solution.
- BYJU’S. (n.d.). Tests of carbohydrates.
- Carl Roth. (2024, October 10). Safety data sheet: Fehling’s solution II.
- Chemistry Stack Exchange. (2017). Why is sodium potassium tartarate used in Fehling’s solution?
- Labster. (n.d.). Fehling’s test: Procedure.
- Lancashire, R. J. (n.d.). Fehling’s test for reducing sugars. University of the West Indies, Mona.
- Quick Biochemistry Basics. (n.d.). Difference between fehling and benedict test [Video]. YouTube.
- Technical Analysis and Mechanistic Review of Fehling’s Test for Carbonyl Group Detection. (n.d.).
- Testbook. (n.d.). Fehling test – Reaction, reagent, mechanism, and difference between Fehling’s and Benedict’s test.
- Vedantu. (n.d.). Fehling test: Principle, procedure, and results explained.
- Wikipedia. (n.d.). Fehling’s solution.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.