What is Ethanol?
- Ethanol (ethyl alcohol, EtOH) is a transparent, odourless, colourless liquid. The intoxicating component of beer, wine, and other alcoholic beverages is ethyl alcohol.
- It has a moderately sweet flavour in weak aqueous solution, but a scorching flavour in concentrated concentrations.
- It is also used as a biofuel in a number of places worldwide.
- Large industrial plants are the principal producers of ethanol, while some individuals have chosen to create their own.
- Agricultural crops have been used to produce ethanol for more than a century. Ethanol can be manufactured from a variety of starch, sugar, cellulose, etc.-containing basic materials.
- There are generally three kinds of raw materials that can be used to manufacture ethanol: 1) sugarcane, beets, sweet sorghum, and fruits 2) starchy substances, including corn, milo, wheat, rice, potatoes, cassava, sweet potatoes, etc. 3) cellulose-based materials such as wood, paper waste, crop wastes, etc.
- The majority of the third type of materials consists of biomass. Recently, biomass has been recognised as an essential biological resource for ethanol production.
What is Ethanol (Alcohol) Fermentation?
- Alcohol fermentation, scientifically termed as ethanol fermentation, is a biotechnological phenomenon where specific carbohydrates, notably glucose, fructose, and sucrose, undergo a transformation to produce ethyl alcohol (ethanol) and carbon dioxide. This intricate process is facilitated by microorganisms, predominantly yeasts, and in certain instances, bacteria.
- The core mechanism of alcoholic fermentation is rooted in the metabolic pathways of these microorganisms. Enzymatic reactions instigate the breakdown of sugars, leading to the formation of pyruvic acid. Subsequently, this acid is metabolized to yield ethanol and carbon dioxide. A pivotal step in this process is the regeneration of NAD+, a coenzyme essential for the conversion of pyruvate molecules into the end products. It’s noteworthy that while yeasts possess the versatility to operate both aerobically and anaerobically, ethanol fermentation is strictly an anaerobic process, occurring in the cytosol of the yeast cells.
- Among the myriad of microorganisms capable of facilitating this fermentation, Saccharomyces cerevisiae stands out as the most prevalent yeast species employed. Its efficacy in metabolizing sugars from diverse sources, such as grapes in winemaking or barley in beer production, renders it indispensable. However, the specificity of the beverage being produced often dictates the choice of yeast strain and the accompanying fermentation methodology to achieve the requisite characteristics.
- Beyond the realm of alcoholic beverages, the implications of ethanol fermentation extend to the domain of baking. The carbon dioxide released during this process imparts the characteristic fluffiness to bread, while the evaporating ethanol enhances its aroma and flavor.
- Furthermore, the broader spectrum of products resulting from alcoholic fermentation encompasses higher alcohols, esters, glycerol, and other compounds. These not only influence the flavor profiles of fermented foods and beverages but also contribute to their nutritional value and extended shelf life.
- In summation, alcohol fermentation is a multifaceted biochemical process, pivotal in the production of a range of consumables. Its intricate balance of chemical and physicochemical reactions, as elucidated by Lee (1983), underscores its significance in both the beverage industry and food science.
Ethanol Formula
The molecular formula for ethanol is C2H6O, which indicates that it contains two carbon atoms and one oxygen atom. The hydroxyl group (-OH) at the end of the two-carbon chain is denoted by the structural formula for ethanol, C2H5OH.
Properties of Ethanol
Ethanol, commonly known as alcohol, is a versatile compound with a range of properties that make it valuable in various industries, particularly in the production of alcoholic beverages and as a solvent in the pharmaceutical sector. Here are the primary characteristics of ethanol:
- Physical State: At standard atmospheric conditions, ethanol is a clear, colorless liquid.
- Melting and Boiling Points: Ethanol has a melting point of 156K (-117°C) and a boiling point of 351K (78°C).
- Solubility: Ethanol is miscible in water, meaning it can be mixed in any proportion with water without separating into two layers.
- Flammability: Ethanol is flammable and can be ignited easily when exposed to an open flame.
- Odor: It has a characteristic, somewhat pleasant odor, often associated with alcoholic beverages.
- Taste: Ethanol has a burning and slightly sweet taste.
- Density: It is less dense than water.
- Volatility: Ethanol is volatile, which means it evaporates quickly at room temperature.
- Toxicity: While ethanol is consumed in alcoholic beverages, it can be toxic in large amounts. Consumption of pure or high-concentration ethanol can be harmful and potentially fatal.
- Solvent Properties: Ethanol is an effective solvent and is used in the production of various pharmaceuticals and personal care products.
- Reactivity: Ethanol can react with various inorganic and organic compounds, making it useful in chemical synthesis.
- Biodegradability: Ethanol is biodegradable and poses minimal environmental hazards when released into the environment.
- Energy Content: Ethanol is used as a biofuel due to its high energy content when combusted.
In summary, ethanol’s unique set of properties makes it a widely used compound in various industries, from beverages to pharmaceuticals to fuels. However, its potential hazards, especially when consumed in large quantities, necessitate careful handling and usage.
Manufacture of Ethanol
- Ethanol can be manufactured using either chemical synthesis or fermentation.
- Prior to circa 1930, fermentation was the primary method of alcohol production.
- In 1939, for instance, 75% of the ethanol produced in the United States was via fermentation, whereas in 1968, over 90% was created by synthesis using catalytic ethylene hydration.
- Due to the rising cost of crude oil, the source of ethylene used in alcohol synthesis, the global focus has shifted to fermentation-based alcohol production.
Microbial production of Ethanol
- The microbial production of ethanol has evolved significantly over time, leveraging the metabolic capabilities of various microorganisms to transform organic feedstocks into this valuable compound. Historically, while alcohol was initially derived from fermentation processes, there was a period where its production was predominantly chemical, specifically through the catalytic hydration of ethylene.
- In contemporary times, the shift has been towards microbial fermentation as a primary method for ethanol synthesis, especially for chemical and fuel applications. Various plant-derived substrates, including sugar beets, potatoes, cassava, corn, and sugar cane, serve as the foundational materials for this microbial conversion.
- A plethora of microorganisms, both yeasts and bacteria, have been harnessed for industrial ethanol production. Among yeasts, Saccharomyces cerevisiae stands out as a predominant species in commercial alcohol manufacturing. However, other yeasts like S. uvarum, Candida brassicae, C. utilis, Kluyveromyces fragilis, and K. lactis have also been employed based on specific feedstocks and desired outcomes. For instance, Candida utilis is particularly adept at fermenting pentoses and is thus utilized for fermenting waste sulphite liquor. Additionally, recent experimental endeavors with Schizosaccharomyces have shown promising outcomes, and when fermenting milk whey, the strain K. fragilis is preferred.
- Beyond yeasts, certain bacterial species have also demonstrated proficiency in ethanol synthesis. Notably, Zymomonas mobilis exhibits a commendable osmotic resilience to elevated sugar concentrations compared to yeasts. This bacterium also showcases a substantial tolerance to ethanol and a rapid specific growth rate, making it a potential candidate for industrial applications.
- However, it’s crucial to acknowledge the challenges in microbial ethanol production. Yeasts, for instance, experience inhibitory effects when exposed to high ethanol concentrations. This inhibition curtails their growth rate, subsequently affecting the overall ethanol biosynthesis rate.
- In summation, the microbial pathway for ethanol production, utilizing a diverse array of microorganisms and feedstocks, offers a sustainable and efficient approach. Yet, understanding the intricacies and challenges of these microbial processes is essential for optimizing yields and ensuring economic viability.
1. Preparation of Medium
The preparation of an appropriate medium is paramount for the efficient production of ethanol. The choice of substrate plays a pivotal role in determining the yield and efficiency of the ethanol production process. Broadly, there are three primary substrates employed:
- Starch-containing Substrates: Starch is a complex carbohydrate that requires preliminary processing before it can be utilized by yeast for ethanol production. Since yeast inherently lacks amylase enzymes, external hydrolysis of starch is essential. This hydrolysis breaks down starch into simpler reducing sugars, which can then be fermented. Approximately one kilogram of starch necessitates the addition of one litre of amylases and three and a half litres of glucoamylases for effective hydrolysis.
- Sugarcane and Sugar Beet Derivatives: This category includes sugarcane juice, molasses, and sugar beet juice. These substrates are rich in simple sugars, making them readily fermentable. Additionally, bagasse, a fibrous byproduct from sugarcane processing, can also be subjected to fermentation to produce ethanol.
- Wood and Wood Processing Wastes: These substrates encompass waste products from wood processing and other lignocellulosic materials. While not conventionally used, with appropriate hydrolysis of cellulose, they can serve as potential sources for ethanol production. Notably, sulphite waste-liquor, a residue from paper manufacturing, contains both hexose and pentose sugars. While hexose sugars are readily fermentable by most microorganisms, pentose sugars require specific microbial strains for effective conversion.
It’s worth noting that while whey, a byproduct from cheese production, can technically be used for ethanol production, its economic feasibility remains questionable.
Furthermore, as the quest for sustainable energy sources intensifies, several non-conventional substrates, including aquatic plant biomass, are being explored for their potential in ethanol production.
In conclusion, the preparation of the medium, underpinned by the choice of substrate, is a critical step in the microbial production of ethanol. Ensuring the optimal breakdown and availability of fermentable sugars is essential for maximizing yields and achieving efficient production processes.
2. Fermentation
- Fermentation is a pivotal biological process employed in the synthesis of ethanol. This process, which involves the metabolic conversion of organic substrates by microorganisms, can be executed in both continuous and batch modes.
- In continuous fermentation, ethanol production is sustained over extended periods. Large-scale fermenters are instrumental in facilitating this continuous mode, ensuring a steady output of ethanol. Notably, the methodologies and technologies employed in continuous fermentation vary globally, with countries like India, Brazil, Germany, and Denmark each adopting distinct approaches tailored to their specific needs and resources.
- The general conditions for fermentation, irrespective of the geographical location, tend to be consistent, typically maintained at a pH of 5 and a temperature of 35°C. However, the choice of microbial cultures and the specifics of culture conditions can differ. While the entire fermentation process can span several days, the onset of ethanol production is usually rapid, commencing within the initial 12 hours.
- Upon the completion of fermentation, the next steps involve the separation and recovery of the products. The microbial cells are isolated to yield yeast cell biomass, which finds utility as a source of single cell protein (SCP) in animal feed. Concurrently, the culture medium or the supernatant undergoes treatment to extract the ethanol.
- Batch fermentation, an alternative to continuous fermentation, is also employed in ethanol production. In this mode, a defined quantity of substrate undergoes fermentation in a single cycle. Interestingly, from a production standpoint, there isn’t a significant disparity in the outcomes of batch and continuous fermentations.
- A notable microorganism in ethanol fermentation is Saccharomyces cerevisiae. This yeast initiates ethanol production at concentrations of 10% (v/v) using 10-20g of dry cell weight per liter, all within a span of 12 hours. An innovative approach to expedite the fermentation process involves the continuous recycling of cells, which enhances the overall efficiency of ethanol synthesis.
- In summation, fermentation is a versatile and dynamic process, central to the production of ethanol. Its adaptability, combined with advancements in biotechnological tools, ensures its continued relevance in sustainable ethanol production.
3. Recovery
- The recovery of ethanol post-fermentation is a crucial step that ensures the purity and concentration of the final product. Distillation, a widely employed separation technique, plays a central role in this recovery process.
- Through multiple distillation stages, it is feasible to recover up to 95% of ethanol. However, achieving complete recovery, i.e., 100%, presents a challenge due to the formation of an azeotropic mixture. An azeotrope is a mixture of two or more components that has a constant boiling point composition, making its separation via simple distillation difficult. In the context of ethanol recovery, this azeotropic mixture consists of 5% water.
- To circumvent this challenge and enhance the recovery process, an additional component, benzene, is introduced. The presence of benzene facilitates the formation of two distinct azeotropic mixtures: a benzene-water-ethanol azeotrope and an ethanol-benzene azeotrope. By leveraging these azeotropic combinations, it becomes possible to separate and remove the 5% water content, resulting in the acquisition of absolute alcohol.
- In essence, the recovery process, underpinned by distillation and the strategic use of azeotropic mixtures, ensures the efficient and effective extraction of ethanol, optimizing its purity and concentration for subsequent applications.
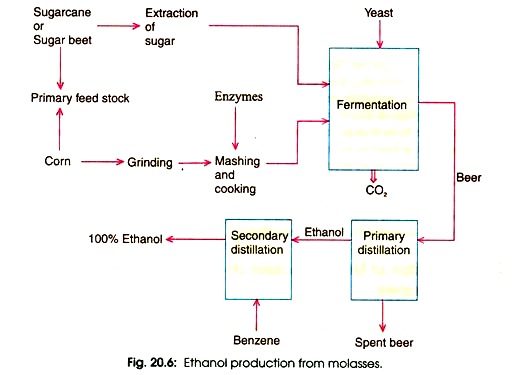
General procedure for production of ethanol from Sugarcane
The production of ethanol from sugarcane is a systematic process that involves several stages, each crucial for ensuring the quality and purity of the final product. This document elucidates the general methodology for producing ethanol from sugarcane, adhering to the principles of scientific literature:
- Acquisition of Sugarcane: The initial step in ethanol production is the procurement of sugarcane, the primary feedstock. This crop is rich in sugars, which are essential for the fermentation process.
- Sugar Extraction: Post-harvest, the sugarcane undergoes processing to extract its inherent sugars. The residual byproduct of this extraction is molasses, a dark, viscous liquid. Molasses is significant as it contains approximately 60% fermentable sugar, making it a valuable resource for ethanol production.
- Molasses Dilution: To facilitate effective fermentation, molasses is diluted using water in a 1:5 ratio by volume.
- Nutrient Supplementation: To ensure optimal yeast growth and fermentation, the diluted molasses may require supplementation. If the nitrogen content in molasses is insufficient, ammonium sulphate is added to meet the yeast’s nitrogen requirements.
- Acidification: The molasses solution is acidified using a minimal amount of sulfuric acid. This acidification not only promotes yeast growth but also inhibits the proliferation of undesirable bacteria, ensuring a controlled fermentation environment.
- Fermentation Process: The acidified molasses solution is transferred to large fermentation tanks and maintained at a temperature of 35°C. Over a span of two to three days, enzymes present in yeast, specifically sucrose and zymase, catalyze the conversion of sugar into ethyl alcohol through the following reactions: C12H22O11+H2O→2C6H12O6
C6H12O6→C2H5OH+2CO2 - Fractional Distillation: Post-fermentation, the resultant liquid, termed “wash,” contains approximately 15 to 18% ethanol. To increase its purity, the wash undergoes fractional distillation, yielding rectified spirit or commercial alcohol with an ethanol concentration of around 92%.
Fractional distillation is a pivotal technique in the purification and separation of liquid mixtures based on differences in their boiling points. This method is particularly significant in the production of ethanol, a compound with myriad applications. The following elucidation provides a comprehensive understanding of the fractional distillation process, specifically in the context of ethanol production:- Feedstock Preparation: The initial phase in the production of ethanol involves the preparation of the feedstock for subsequent fermentation. Various sources, including cereal grains like corn, rye, barley, and wheat, as well as plants like sugarcane, serve as primary feedstocks. In certain instances, starch-rich vegetables, such as potatoes, are also employed. The preparation methodologies vary, but the ultimate objective is to obtain a liquid solution rich in fermentable carbohydrates. This solution undergoes clarification and is subjected to high temperatures (20 to 30 minutes) to eliminate potential bacterial contaminants.
- Fermentation Process: Upon preparation, the feedstock solution undergoes fermentation. Yeast cells, equipped with the enzyme Zymase, are introduced to catalyze the conversion of simple carbohydrates into ethanol and carbon dioxide. The fermentation process, though seemingly straightforward, is intricate. The primary products are ethanol, carbon dioxide, and heat. However, the impure yeast culture can also produce secondary compounds, including glycerin and methanol.
- Distillation Phase: Post-fermentation, the resultant mixture, often termed “wash,” contains ethanol concentrations ranging from a mere few percent to approximately 14%. Given the close boiling points of ethanol (78.4°C) and water (100°C), their complete separation via simple distillation is challenging. Instead, an azeotropic mixture, comprising 96% ethanol and 4% water, is formed. This mixture, known as Rectified Spirit (RS), contains 94% v/v ethanol.
- Dehydration: To achieve higher ethanol purity, the rectified spirit undergoes a dehydration process. Since 96% ethanol forms an azeotrope with water, specialized techniques are required for further concentration. Azeotropic distillation and Molecular Sieve Technology are two prevalent methods employed for the dehydration of rectified spirit, yielding absolute alcohol.
In summary, the production of ethanol from sugarcane is a meticulous process that requires careful attention to each stage. By adhering to the outlined procedure, one can ensure the production of high-quality ethanol suitable for various applications.
Process of alcohol fermentation from glucose
Alcohol fermentation is a biochemical process that converts glucose into ethanol and carbon dioxide. This transformation is facilitated by a series of enzymatic reactions, primarily occurring in anaerobic conditions. The following elucidation provides a detailed overview of this intricate process.
1. Chemical Representation of Alcohol Fermentation:
The overarching chemical reaction for alcohol fermentation can be delineated as:
C6H12O6→2C2H5OH+2CO2
This equation signifies the conversion of one glucose molecule into two ethanol molecules and two carbon dioxide molecules.
2. Stages of Alcohol Fermentation:
Alcohol fermentation is a biological process that converts glucose into ethanol and carbon dioxide. This transformation is facilitated by specific enzymes and is a fundamental pathway in many microorganisms, especially yeasts.
Chemical Representation of Alcohol Fermentation: C6H12O6→2C2H5OH+2CO2
This equation delineates the conversion of glucose into ethanol and carbon dioxide.
Stages of Alcohol Fermentation:
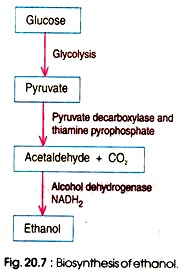
1. Glycolytic Pathway: Glycolysis is the inaugural phase of alcohol fermentation. It encompasses the catabolism of glucose into two pyruvate molecules. This metabolic pathway is ubiquitous in organisms and is pivotal for carbohydrate degradation.
The glycolytic reactions can be encapsulated as: C6H12O6+2ADP+2Pi+2NAD+→2CH3COCOO−+2ATP+2NADH+2H2O+2H+
The culmination of glycolysis yields two pyruvate molecules, two molecules of NADH, and a net gain of two ATP molecules. In anaerobic conditions, these pyruvate molecules undergo further transformations to produce ethanol.
2. Conversion of Pyruvate to Ethanol: The transition from pyruvate to ethanol is a two-step process:
- Decarboxylation of Pyruvate: The initial step involves the decarboxylation of pyruvate, leading to the release of carbon dioxide and the formation of acetaldehyde. The enzyme pyruvate decarboxylase catalyzes this reaction. CH3COCOO−+H+→CH3CHO+CO2
- Reduction of Acetaldehyde: Subsequently, acetaldehyde undergoes a reduction reaction. Here, NADH donates electrons to acetaldehyde, culminating in the formation of ethanol. Concurrently, the NAD molecule is regenerated. The enzyme alcohol dehydrogenase facilitates this reaction. CH3CHO+NADH+H+→C2H5OH+NAD+
In essence, alcohol fermentation from glucose is a coordinated series of biochemical reactions that yield ethanol, a molecule with significant industrial and recreational applications. This process underscores the metabolic versatility of microorganisms and their ability to produce valuable compounds from simple substrates.
Uses of ethanol (Alcohol) Fermentation
Alcohol fermentation, a biochemical process mediated by yeasts, is pivotal in various sectors due to its ability to transform sugars into ethanol and carbon dioxide. This process, deeply rooted in scientific principles, has diverse applications, as delineated below:
- Production of Alcoholic Beverages: Central to the beverage industry, alcohol fermentation facilitates the creation of a range of alcoholic drinks, encompassing beer, wine, and spirits. Utilizing sugars from an array of sources, yeasts undergo metabolic activities, yielding ethanol, which imparts the characteristic alcoholic content to these beverages.
- Baking Industry Applications: In the realm of baking, alcohol fermentation holds paramount importance. The process not only augments the organoleptic properties of bread, enhancing its flavor and aroma, but also plays a structural role. The evolution of carbon dioxide during fermentation acts as a leavening agent, inducing the dough to rise and bestowing bread with its characteristic soft and porous texture.
- Biofuel Generation: With the escalating need for sustainable energy sources, alcohol fermentation’s role in producing bioethanol comes to the fore. Bioethanol, derived from renewable resources, presents an eco-friendly alternative to traditional fossil fuels, marking a stride towards sustainable energy solutions.
- Industrial and Pharmaceutical Synthesis: Beyond edibles and energy, alcohol fermentation is instrumental in the industrial production of an array of chemicals and pharmaceutical entities. Notably, it aids in the synthesis of essential compounds such as organic acids and antibiotics, underscoring its significance in the chemical and medical sectors.
- Culinary and Perfumery Applications: The food and perfume industries harness alcohol fermentation to craft distinct flavors and fragrances. The process aids in extracting and developing aromatic compounds, enhancing the sensory attributes of various products.
- Cultural and Ritualistic Relevance: Transcending its tangible applications, alcohol fermentation holds profound cultural and traditional connotations. The beverages produced through this process are integral to numerous religious ceremonies across diverse societies, symbolizing communal unity and spiritual reverence.
In essence, alcohol fermentation, underpinned by scientific tenets, manifests its versatility across sectors, from food and beverages to energy and medicine. Its multifarious applications underscore its profound impact on human civilization and technological advancements.
Alcoholic Beverages
Alcoholic beverages, characterized by their ethanol content, are products of intricate biochemical processes involving fermentation and, in some cases, distillation. These beverages, derived from a plethora of raw materials, encompass a spectrum ranging from wines and beers to distilled spirits. This article elucidates the scientific underpinnings of the production of these beverages.
- Classification and Origin: Alcoholic beverages can be broadly categorized based on their raw materials and production methods. They originate from the fermentation of sugars present in fruits, grains, and other organic substrates. While wine, beer, and distilled liquors are globally recognized, numerous indigenous beverages, specific to certain regions, also contribute to the vast repertoire of alcoholic drinks.
- Wine Production: Wine, a revered alcoholic beverage, is predominantly derived from the fermentation of grapes, although other fruits can also serve as substrates. The yeast strain Saccharomyces cerevisiae plays a pivotal role in wine fermentation. This yeast metabolizes the inherent sugars of the fruit, culminating in the production of ethanol and carbon dioxide. The resultant wines typically possess an alcohol concentration ranging between 6% and 14%.
- Beer Brewing: Beer, with its ubiquitous presence, stands as the most consumed alcoholic beverage globally. The brewing process entails the fermentation of malted grains, with barley being the quintessential choice. The enzymatic action of yeast facilitates the conversion of grain starches into ethanol and carbon dioxide. Notably, beer brewing employs two primary yeast species: Saccharomyces cerevisiae, a top-fermenting yeast yielding ales, and Saccharomyces pastorianus, a bottom-fermenting yeast instrumental in lager production. Beers typically exhibit an alcohol content of approximately 4% to 6%.
- Distilled Spirits: Beyond wines and beers, the realm of alcoholic beverages extends to distilled spirits. These are products of distillation, a process that concentrates the alcohol content by separating it from the water and other components. Distilled spirits encompass a wide array of beverages, each with distinct characteristics, flavors, and production methodologies.
In summation, alcoholic beverages, with their rich history and cultural significance, are products of meticulous scientific processes. The interplay of raw materials, yeast strains, and fermentation conditions dictates the flavor profiles, aromas, and alcohol content of these beverages, making them integral components of global culinary landscapes.