What is Ethanol Metabolism?
- Ethanol metabolism is a crucial biological process that allows the body to break down and eliminate alcohol. Therefore, understanding this process is essential for grasping the effects of alcohol on the human body.
- Ethanol, commonly known as alcohol, is a naturally occurring compound found in various plants and fruits. Besides its natural occurrence, it is also the primary intoxicating ingredient in alcoholic beverages. When consumed, ethanol undergoes a series of metabolic reactions to be broken down and eventually eliminated from the body.
- Upon ingestion, ethanol travels from the stomach to the small intestine. Then, it is rapidly absorbed into the bloodstream, distributing throughout the body. Interestingly, higher concentrations of ethanol are found in the blood and brain than in muscle or fat tissues. While a small percentage (2-8%) of the ingested alcohol is excreted through urine, sweat, or breath, the vast majority (92-98%) is metabolized in the liver.
- The liver, due to its rich enzyme content, plays a pivotal role in ethanol metabolism. Four primary enzymes are involved in this process: alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH), catalase, and the microsomal ethanol oxidizing system (MEOS). These enzymes facilitate the breakdown of the alcohol molecule, making its elimination from the body possible.
- The primary pathway for alcohol metabolism involves the enzyme ADH. Found mainly in the liver, but also in the brain and stomach, ADH catalyzes the conversion of ethanol to acetaldehyde. Acetaldehyde is a highly toxic substance and a known carcinogen. During this conversion, ethanol binds to ADH and loses electrons in the form of hydrogen atoms to a coenzyme called nicotinamide adenine dinucleotide (NAD), producing NADH.
- This process has significant implications for the body’s metabolic pathways. The conversion of ethanol to acetaldehyde reduces the cellular NAD to NADH ratio. A balanced NAD to NADH ratio is vital for various metabolic reactions. An altered ratio can inhibit essential reactions such as glycolysis, the tricarboxylic acid cycle (TCA cycle), fatty acid oxidation, pyruvate dehydrogenase, and gluconeogenesis. Elevated NADH levels can lead to metabolic disorders, including the formation of high lactic acid levels, which reduces the kidney’s ability to excrete uric acid. This accumulation can result in gout, a painful joint condition. Furthermore, increased NADH promotes fatty acid formation and reduces fat breakdown in the liver, leading to fatty liver disease, an early stage of alcohol-induced liver disease.
- In conclusion, ethanol metabolism is a complex process involving various enzymes and metabolic pathways. The liver’s role in this process is paramount, and any disruption in the metabolic reactions can have profound effects on the body. Understanding this process is essential for recognizing the impact of alcohol consumption on human health.
Definition of Ethanol Metabolism
Ethanol metabolism is the biological process by which the body breaks down and eliminates ethanol, primarily in the liver, through a series of enzymatic reactions that convert it into less toxic compounds, ultimately producing energy and releasing water and carbon dioxide.
Location of Ethanol Metabolism
- The location of ethanol metabolism is primarily within the human body’s cells, specifically those of the liver and kidney. Delving into the specifics, the liver stands out as the central hub for this metabolic process. In fact, more than 80% of the absorbed ethanol undergoes metabolism within the liver’s cells. This is due to the liver’s rich enzymatic content, which facilitates the breakdown of ethanol into less toxic compounds.
- Besides the liver, the kidney also plays a role, albeit to a lesser extent, in ethanol metabolism. The kidney’s involvement underscores the body’s comprehensive approach to managing and eliminating substances like ethanol.
- Therefore, while ethanol gets distributed throughout the body upon consumption, its metabolism predominantly occurs in the liver. The liver’s central role in this process is attributed to its high concentration of specific enzymes that are adept at breaking down ethanol. These enzymes, such as alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), are crucial in converting ethanol into acetaldehyde and subsequently into other compounds that can be easily eliminated from the body.
- In conclusion, the liver stands as the primary site for ethanol metabolism, with over 80% of the process occurring there. The kidney also contributes, emphasizing the body’s systematic approach to metabolizing and eliminating ingested substances. This intricate process ensures that ethanol is efficiently processed and removed, safeguarding the body’s overall health.
Distribution of Alcohol in the Body
The distribution of alcohol within the human body is a complex process influenced by various physiological factors. Understanding this distribution is crucial for assessing the effects of alcohol on different tissues and for legal and medical evaluations, such as breath analyzer tests. Let’s delve into the details of how alcohol distributes in the body:
- Equilibrium Concentration and Water Content: The equilibrium concentration of alcohol in any given tissue is largely determined by the tissue’s water content. Ethanol, being soluble in water, distributes from the blood into all tissues and fluids in proportion to their water content. Therefore, tissues with higher water content will have a higher concentration of ethanol. This distribution reaches equilibrium swiftly with the concentration of ethanol present in the plasma.
- Ethanol and Biological Membranes: Despite being water-soluble, ethanol can pass through biological membranes. This is because ethanol is practically insoluble in fats and oils, allowing it to traverse various biological barriers. This characteristic ensures that ethanol can reach various parts of the body, influencing its effects on different tissues.
- Plasma Protein Binding: It’s noteworthy that there is no plasma protein binding of alcohol. This means that ethanol freely circulates in the bloodstream without being bound to proteins, facilitating its distribution throughout the body.
- Variability in Distribution: The distribution of alcohol can vary significantly among individuals. Factors such as body composition, specifically the proportions of fat and water, play a pivotal role. Given the low lipid: water partition coefficient of ethanol, individuals with varying body compositions can exhibit different blood alcohol concentrations even if they consume the same dose of alcohol per unit of body weight. For instance, women, who generally have a higher percentage of body fat compared to men, tend to have a smaller volume of distribution for alcohol. This results in women exhibiting higher peak blood alcohol levels than men when given the same dose of alcohol per kilogram of body weight. However, when the dose is adjusted per liter of body water, these differences diminish. Additionally, first-pass metabolism of alcohol in the stomach, which might be more pronounced in males, can also contribute to the observed differences in blood alcohol levels between genders.
- Breath Analyzer Test: The breath analyzer test, commonly used to estimate blood alcohol concentrations, relies on the diffusion of ethanol from the pulmonary arterial blood into the alveolar air. The ethanol vapor in one’s breath is in equilibrium with the ethanol dissolved in the blood’s water. This relationship is represented by a blood: breath partition coefficient of approximately 2100:1.
Human Metabolic Physiology
Human metabolic physiology is a complex system that governs how substances, including ethanol, are processed within the body. This intricate system ensures that the body efficiently utilizes, stores, and eliminates various compounds to maintain homeostasis and overall health.
- Ethanol Metabolism Pathway: The metabolism of ethanol in humans follows a sequential pathway. Initially, the enzyme alcohol dehydrogenase (ADH) catalyzes the conversion of ethanol into acetaldehyde. However, acetaldehyde is a toxic compound. Therefore, it is further metabolized into acetic acid by another enzyme, acetaldehyde dehydrogenase (ALDH). The subsequent step involves the enzyme acetic acid coenzyme A ligase (ACSL), which transforms acetic acid into acetyl-CoA. This compound, acetyl-CoA, then enters the citric acid cycle, also known as the Krebs cycle, where it undergoes further breakdown in the mitochondria. This metabolic process results in the production of water, carbon dioxide, and energy in the form of adenosine triphosphate (ATP).
- Individual Variability in Metabolism: Ethanol metabolism can vary among individuals due to factors such as genetic differences, gender, body composition, and the presence of liver diseases. Chronic and excessive alcohol consumption can lead to liver damage, affecting the liver’s ability to metabolize ethanol efficiently. Besides the liver, other organs, including the brain and the gastrointestinal tract, also play roles in ethanol metabolism.
- Evolutionary Perspective: The ability to metabolize ethanol is not exclusive to humans. Over millions of years, various organisms have evolved the capability to process ethanol, a product of fermentation. This metabolic pathway has been in existence for billions of years, allowing organisms to adapt to environments where ethanol is present.
- Physiological Structures Involved: The liver is the primary site for ethanol metabolism. Within the liver, hepatocytes, the primary liver cells, are equipped with specific enzymes that facilitate the oxidation of ethanol and its conversion into less harmful metabolites.
- Thermodynamics of Ethanol Metabolism: The metabolism of ethanol is accompanied by energy changes, which are governed by thermodynamic principles. Understanding these thermodynamic processes is crucial as they provide insights into the energy alterations that occur during the breakdown of ethanol and its subsequent conversion into other metabolites.
Ethanol Metabolism Steps
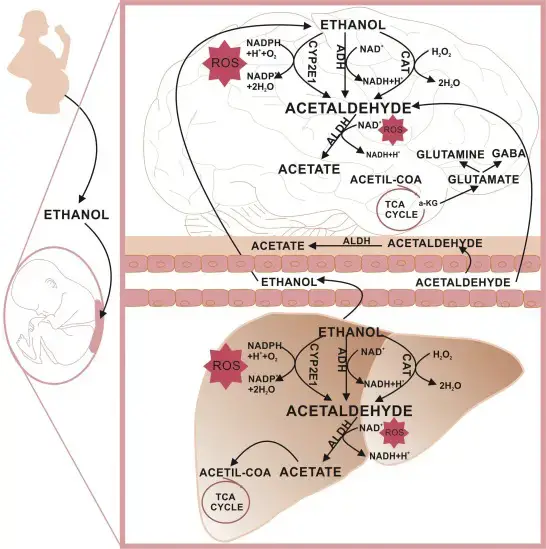
Ethanol metabolism is a sequential and intricate process that transforms ethanol, a simple alcohol, into energy and waste products that the body can easily eliminate. This process is vital for the body to handle the intake of alcoholic beverages and to prevent the accumulation of toxic by-products. Let’s delve into the detailed steps of ethanol metabolism:
- Step One: Conversion of Ethanol to Acetaldehyde The initial step in ethanol metabolism is the transformation of ethanol into acetaldehyde. This is facilitated by the enzyme alcohol dehydrogenase (ADH). During this conversion, energy is released in the form of heat. The energy change associated with this reaction, known as the enthalpy change (∆H), can be quantified to understand the thermodynamics of the process.
- Step Two: Metabolism of Acetaldehyde to Acetic Acid Following the formation of acetaldehyde, the molecule undergoes another transformation. The enzyme acetaldehyde dehydrogenase (ALDH) catalyzes the conversion of acetaldehyde to acetic acid. This reaction is exothermic, meaning it releases energy. The energy change, or the change in enthalpy (∆H), can be calculated to provide insights into the energy dynamics of this step.
- Step Three: Conversion of Acetic Acid to Acetyl-CoA The next phase in the pathway involves the transformation of acetic acid into acetyl-CoA. This step is orchestrated by the enzyme acetic acid coenzyme A ligase (ACSL). During this conversion, a high-energy thioester bond forms between acetic acid and coenzyme A (CoA). The subsequent hydrolysis of this bond releases energy, which can be measured by considering the change in enthalpy (∆H) of the reaction.
- Steps 4 through 11: Breakdown of Acetyl-CoA The acetyl-CoA produced then enters the citric acid cycle, where it undergoes multiple reactions leading to its complete breakdown. These reactions produce water and carbon dioxide as end products. The entire process of breaking down acetyl-CoA through the citric acid cycle and oxidative phosphorylation is highly exergonic, signifying the release of substantial amounts of energy.
Ethanol Metabolism Reaction
- First Reaction: Oxidation of Ethanol to Acetaldehyde The initial step in the metabolism of ethanol is its oxidation to form acetaldehyde. This reaction is facilitated by the enzyme alcohol dehydrogenase (ADH). ADH plays a pivotal role in ensuring that ethanol is converted into acetaldehyde, a compound that can be further metabolized by the body.
- Second Reaction: Conversion of Acetaldehyde to Acetic Acid Once acetaldehyde is formed, it doesn’t remain in that state for long. The enzyme aldehyde dehydrogenase (ALDH) takes charge at this juncture, catalyzing the conversion of acetaldehyde into acetic acid. This step is crucial as acetaldehyde is a toxic compound, and its rapid conversion into acetic acid prevents its accumulation in the body.
- Third Reaction: Transformation of Acetic Acid to Acetyl-CoA The journey of ethanol metabolism doesn’t end with the formation of acetic acid. The acetic acid is then converted into acetyl-CoA, a molecule central to many metabolic pathways. This transformation is orchestrated by the enzyme acetic acid coenzyme A ligase (ACSL). Acetyl-CoA is a pivotal molecule that feeds into the citric acid cycle, a major energy-producing pathway in cells.
- Subsequent Reactions: Metabolism of Acetyl-CoA Once acetyl-CoA is formed, it enters the mitochondria, the powerhouse of the cell. Here, from steps four through eleven, acetyl-CoA undergoes a series of reactions in the citric acid cycle. These reactions ultimately lead to the production of water and carbon dioxide, which are harmless end products that the body can easily eliminate.
Factors Affecting Alcohol Absorption
The absorption of alcohol in the human body is a multifaceted process influenced by various physiological and external factors. Understanding these factors is crucial in predicting the rate and extent of alcohol absorption, which in turn affects the blood alcohol concentration and the subsequent physiological and psychological effects of alcohol. Let’s delve into the factors that affect alcohol absorption:
- Site of Absorption: Alcohol absorption is more rapid in the duodenum and jejunum compared to the stomach. Therefore, the rate at which the stomach empties its contents into the duodenum becomes a significant determinant of alcohol absorption. Faster gastric emptying leads to quicker absorption of alcohol.
- Concentration Gradient and Passive Diffusion: Alcohol traverses biological membranes through passive diffusion, moving from an area of higher concentration to one of lower concentration. The higher the alcohol concentration, the steeper the concentration gradient, leading to more rapid absorption.
- Blood Flow: Efficient blood flow aids in swiftly removing alcohol from the site of absorption. This maintains the concentration gradient, promoting faster absorption of alcohol.
- Irritant Properties of Alcohol: High concentrations of alcohol can irritate the stomach lining, causing superficial erosions, hemorrhages, and paralysis of the stomach’s smooth muscle. These irritant properties can hinder alcohol absorption.
- Mode of Alcohol Ingestion: Peak blood alcohol levels are higher when alcohol is consumed as a single large dose compared to multiple smaller doses. This is likely because the alcohol concentration gradient is higher in the former scenario.
- Type of Alcoholic Beverage: Generally, the type of alcoholic beverage consumed doesn’t significantly influence the rate of alcohol absorption. Whether one drinks beer, wine, or spirits, the blood ethanol concentration remains relatively consistent, given the same alcohol content.
- Presence of Food: Consuming alcohol on an empty stomach leads to faster absorption compared to when alcohol is consumed with or after a meal. The presence of food in the stomach slows down gastric emptying, thus retarding alcohol absorption. Meals rich in fat, carbohydrates, or protein are equally effective in slowing down this process, emphasizing the commonly advised “don’t drink on an empty stomach” concept.
- Other Determinants: The final blood alcohol concentration is influenced by the total amount of alcohol consumed, the presence or absence of food in the stomach, factors affecting gastric emptying, and the rate at which alcohol is oxidized in the body.
Gene Expression and Ethanol Metabolism
Gene expression plays a pivotal role in determining how ethanol is metabolized within the human body. The intricate process of ethanol metabolism is influenced by the expression of specific genes, which in turn affects the activity of the associated enzymes. This relationship between gene expression and enzyme activity has profound implications for an individual’s alcohol tolerance and susceptibility to alcohol-related health issues. Let’s delve into the relationship between gene expression and the various stages of ethanol metabolism:
- Ethanol to Acetaldehyde Conversion The initial step in ethanol metabolism involves its conversion to acetaldehyde. This reaction is facilitated by the enzyme alcohol dehydrogenase (ADH). During this process, ethanol undergoes oxidation, transferring hydride ions (H-) to the coenzyme NAD+. The reaction can be represented as: Ethanol + NAD+ → Acetaldehyde + NADH + H+.
- Ethanol Metabolism in Human Adults In human adults, the conversion of ethanol to acetaldehyde is predominantly mediated by ADH. However, it’s noteworthy that there are different genetic variants of ADH present in the human population. These genetic variations lead to differences in the rate of ethanol metabolism, resulting in varying levels of alcohol tolerance among individuals.
- Ethanol Metabolism in Human Fetuses The metabolism of ethanol in fetuses differs significantly from that in adults. In fetuses, the enzyme responsible for converting ethanol to acetaldehyde is fetal alcohol dehydrogenase (FADH). However, the activity of FADH in fetuses is relatively low compared to that in adults. This reduced activity results in elevated acetaldehyde levels in the fetus, which can have detrimental effects.
- Acetaldehyde to Acetic Acid Conversion Acetaldehyde, a toxic compound, is further metabolized to acetic acid. This crucial step is catalyzed by the enzyme acetaldehyde dehydrogenase (ALDH). The reaction can be represented as: Acetaldehyde + NAD+ + H2O → Acetic Acid + NADH + H+.
- Acetic Acid to Acetyl-CoA Conversion The acetic acid produced is then converted into acetyl-CoA, a central molecule in various metabolic pathways. This conversion is facilitated by the enzyme acetic acid coenzyme A ligase (ACSL). The reaction is as follows: Acetic Acid + CoA + ATP → Acetyl-CoA + AMP + PPi.
- Breakdown of Acetyl-CoA Acetyl-CoA then enters the citric acid cycle within the mitochondria. Here, it undergoes a series of reactions, leading to its complete oxidation. This process produces water, carbon dioxide, and energy in the form of adenosine triphosphate (ATP).
Diseases Related to Ethanol Metabolism
The by-products and intermediates formed during this process can have detrimental effects on various tissues and organs. Let’s delve into the diseases and health issues related to ethanol metabolism:
- Liver Cirrhosis due to Acetaldehyde: Acetaldehyde, a primary by-product of ethanol metabolism, is an unstable molecule. Its instability makes it prone to forming free radicals. These free radicals are toxic to the liver and can lead to liver damage over time. Prolonged exposure to acetaldehyde and its associated free radicals can result in cirrhosis, a condition characterized by the scarring of liver tissue.
- Fetal Alcohol Syndrome and Acetaldehyde: Acetaldehyde’s harmful effects are not limited to the liver. It can also damage embryological neural crest tissue, which plays a crucial role in the development of the nervous system. This damage is believed to be a contributing factor to the neurological manifestations observed in fetal alcohol syndrome, a condition that affects children born to mothers who consumed alcohol during pregnancy.
- Hypoglycemia in Alcoholics: Individuals with alcoholism are at an increased risk of developing hypoglycemia, especially after consuming ethanol. The reason for this lies in the altered metabolic pathways due to the increased ratio of NADH/NAD+ resulting from ethanol metabolism. This altered ratio leads to the reduction of pyruvate to lactate and oxaloacetate to malate. Since both pyruvate and oxaloacetate are crucial intermediates in the gluconeogenesis pathway, their reduction impairs the body’s ability to produce glucose, leading to hypoglycemia.
- Impaired Gluconeogenesis: The metabolism of ethanol and the subsequent increase in the NADH/NAD+ ratio have a direct impact on gluconeogenesis. As mentioned earlier, the conversion of pyruvate to lactate and oxaloacetate to malate hinders the gluconeogenesis process. This impairment can lead to a decrease in blood glucose levels, posing health risks, especially in individuals who rely on gluconeogenesis as a primary source of glucose, such as those fasting or on low-carbohydrate diets.
Importance of Ethanol Metabolism
- Detoxification: Ethanol, commonly found in alcoholic beverages, is a psychoactive substance. The body recognizes ethanol as a toxin. Through metabolism, ethanol is converted into less harmful substances, ensuring that it doesn’t accumulate and cause toxicity.
- Energy Production: The end products of ethanol metabolism, especially acetyl-CoA, enter the citric acid cycle (or Krebs cycle). This cycle is a series of chemical reactions used by all aerobic organisms to release stored energy. Thus, ethanol can serve as an energy source.
- Prevention of Acetaldehyde Accumulation: One of the primary products of ethanol metabolism is acetaldehyde, which is more toxic than ethanol itself. Efficient metabolism ensures that acetaldehyde is quickly converted to acetic acid, preventing its accumulation and potential harm.
- Genetic and Evolutionary Significance: The ability to metabolize ethanol has evolutionary implications. Over time, as human ancestors consumed fermented fruits and other natural sources of ethanol, those with efficient ethanol metabolism had survival advantages, leading to the preservation of genes associated with this trait.
- Influence on Behavior and Central Nervous System: Ethanol affects the central nervous system, leading to altered behavior, impaired judgment, and reduced motor skills. Efficient metabolism ensures that these effects are temporary and do not persist for extended periods.
- Health Implications: Chronic consumption of alcohol without efficient metabolism can lead to various health issues, including liver diseases (like fatty liver, hepatitis, and cirrhosis), gastrointestinal problems, cardiovascular diseases, and increased risk of certain cancers.
- Metabolic Interactions: Ethanol metabolism influences other metabolic pathways. For instance, the increased NADH/NAD+ ratio during ethanol metabolism affects gluconeogenesis, fatty acid metabolism, and the electron transport chain, among others.
- Medicinal and Industrial Relevance: Understanding ethanol metabolism is crucial in the medical field, especially when prescribing medications that might interact with alcohol. In industrial settings, knowledge of ethanol metabolism is vital in biotechnological applications, such as biofuel production.
References
- Wilson, David F.; Matschinsky, Franz M. (2020). Ethanol metabolism: The good, the bad, and the ugly. Medical Hypotheses, 140(), 109638–. doi:10.1016/j.mehy.2020.109638
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012 Nov;16(4):667-85. doi: 10.1016/j.cld.2012.08.002. PMID: 23101976; PMCID: PMC3484320.
- C. Onyekwelu, K. (2019). Ethanol. IntechOpen. doi: 10.5772/intechopen.79861