The Esculin hydrolysis test is a biochemical test which is used for the identification and differentiation of certain bacteria, mainly Enterococcus species and Group D streptococci from other streptococci. It is also used for identifying some other organisms such as Listeria species and members of Bacteroides fragilis group. It is based on the ability of the organism to hydrolyze esculin by the presence of the enzyme esculinase (β-glucosidase). This process occurs when esculin is broken down into glucose and esculetin. The esculetin so formed reacts with ferric ions present in the medium and produces a dark brown or black coloured complex, which indicates a positive result.
The test is commonly performed using bile esculin agar medium, which contains bile salts and ferric citrate. The bile salts inhibit the growth of most Gram-positive bacteria except bile tolerant organisms, while ferric ions act as indicator. In this medium, organisms which are capable of hydrolyzing esculin will show blackening of the medium either around the colonies or in more than half of the agar slant. If the organism does not hydrolyze esculin, there is no black colour development and the medium remains unchanged, which is considered as a negative result.

Objectives of Esculin Hydrolysis Test
- To determine the ability of the organism to produce the enzyme esculinase (β-glucosidase) and hydrolyze esculin into glucose and esculetin.
- To isolate and identify Enterococcus species and Group D streptococci which can hydrolyze esculin in presence of bile.
- To differentiate enterococci and Group D streptococci from non-Group D streptococci which do not hydrolyze esculin.
- To aid in the differentiation of certain members of Enterobacteriaceae based on esculin hydrolysis reaction.
- To identify organisms such as Listeria species which are capable of hydrolyzing esculin.
- To select and identify members of Bacteroides fragilis group among anaerobic bacteria.
- To help in identification of Yersinia enterocolitica from food and animal sources.
- To identify Cryptococcus neoformans based on production of dark pigment from esculin.
Principle of Esculin Hydrolysis Test
The principle of Esculin hydrolysis test is based on the ability of certain bacteria to produce the enzyme esculinase (β-glucosidase). It is the process in which the glycoside esculin present in the medium is hydrolyzed into glucose and esculetin. This process occurs when organism possessing the enzyme is inoculated into the medium containing esculin as a substrate. The glucose is utilised by the bacteria for metabolism, while esculetin is released into the surrounding medium.
The liberated esculetin then reacts with ferric ions present in the medium, which is usually supplied in the form of ferric citrate or ferric ammonium citrate. This reaction results in the formation of a dark brown or black coloured phenolic iron complex. The blackening of the medium or the appearance of a black halo around the colonies is taken as positive result. In bile esculin agar, bile salts are also present which inhibits the growth of many Gram positive bacteria and thus select organisms which are bile tolerant as well as capable of hydrolyzing esculin.
Requirement
Media
- Bile esculin agar (BEA) slants or plates used as differential medium for esculin hydrolysis.
- Bile esculin azide agar (Enterococcosel agar) used as selective medium for isolation of enterococci and Group D streptococci.
- Bacteroides bile esculin (BBE) agar used for identification of Bacteroides fragilis group under anaerobic condition.
- Esculin broth used for tube method of esculin hydrolysis test.
- Esculin agar without bile used for differentiation of certain Gram negative bacteria and fungi.
Reagents
- Ferric ammonium citrate (1%) used as indicator for formation of black coloured complex.
- Esculin solution (0.02%) used for rapid esculin spot test.
- Distilled water used for moistening esculin discs or filter paper.
Supplies and Equipment
- Sterile inoculating loop or inoculating needle used for transfer of culture.
- Incubator maintained at 35–37°C for incubation of test cultures.
- Anaerobic jar or anaerobic system required for BBE agar plates.
- Ultraviolet lamp used for detection of loss of fluorescence in esculin medium.
- Esculin discs used for rapid detection methods.
- Glass slides and filter paper used for spot test method.
- Bunsen burner or incinerator used for sterilization of inoculating tools.
Procedure of Esculin Hydrolysis test
General Preparation
- Allow the bile esculin agar medium to attain room temperature before inoculation.
- Check the surface of the medium to ensure it is moist and free from excess water.
Tube (Slant) Method
- Using a sterile inoculating loop or needle, pick well isolated colonies from an 18–24 hours old pure culture.
- Inoculate the surface of the bile esculin agar slant by streaking in zig-zag or fish tail pattern.
- Heavy inoculum should be avoided as it may overcome the inhibitory action of bile salts.
- Stabbing of the butt is not required for routine identification of streptococci and enterococci.
- Keep the cap of the tube slightly loosened to allow proper aeration.
- Incubate the inoculated tubes aerobically at 35–37°C.
- Observe the medium after 18–24 hours for blackening.
- If no change is seen, incubation may be continued up to 48–72 hours before reporting as negative.
Plate Method
- Using a sterile loop, streak the bile esculin agar plate by quadrant streaking technique to obtain isolated colonies.
- Incubate the plates at 35–37°C for 18–24 hours.
- For Bacteroides bile esculin agar, incubation is done under anaerobic condition.
- Examine the plate for development of brown to black colour around the colonies.
Rapid Disk Method
- Place an esculin disk on a clean glass slide and moisten it with one drop of distilled water.
- Using a sterile loop, rub two to three well isolated colonies directly onto the disk surface.
- Allow the disk to stand at room temperature for about 10 minutes.
- Observe for appearance of dark brown or black colour on the disk surface.
Interpretation of Esculin Hydrolysis Test
- Positive result (tube or plate) – Blackening of the medium due to formation of dark brown or black coloured complex after hydrolysis of esculin.
- Positive result (slant) – Black colour development in more than half of the agar slant within 24–48 hours.
- Positive result (fluorescence test) – Loss of natural fluorescence of esculin when observed under ultraviolet light.
- Positive result (rapid disk or spot test) – Appearance of dark brown or black colour on the disk or filter paper within 10 minutes.
- Negative result (visual observation) – No black colour formation and the medium remains yellowish or amber in colour.
- Negative result (slant) – Absence of blackening or black colour in less than half of the slant.
- Negative result (fluorescence test) – Presence of bright fluorescence under ultraviolet light indicating esculin is not hydrolyzed.
- Bacteroides fragilis group – On Bacteroides bile esculin agar, colonies are surrounded by dark brown to black zone.
- Listeria monocytogenes – Formation of small black colonies on bile esculin agar medium.

Esculin Hydrolysis Test – List of Organisms
Esculin Positive (+)
- Gram positive bacteria
- Enterococcus species (Enterococcus faecalis, Enterococcus faecium)
- Group D streptococci (Streptococcus bovis, Streptococcus equinus)
- Listeria monocytogenes
- Aerococcus species
- Leuconostoc species
- Pediococcus species
- Lactococcus species
- Gram negative bacteria
- Bacteroides fragilis group
- Klebsiella species
- Enterobacter species
- Serratia species
- Yersinia enterocolitica
- Proteus vulgaris
- Proteus rettgeri
- Fungi
- Cryptococcus neoformans
Esculin Negative (–)
- Gram positive bacteria
- Streptococcus pyogenes (Group A)
- Streptococcus agalactiae (Group B)
- Viridans streptococci (non Group D)
- Clostridium perfringens
- Gram negative bacteria
- Escherichia coli
- Proteus mirabilis
- Proteus morganii (Morganella morganii)
- Salmonella species
- Shigella species
- Providencia species
- Fusobacterium nucleatum
Quality Control Organisms
For Bile Esculin Agar and Esculin Hydrolysis test
- Positive control
- Enterococcus faecalis (ATCC 29212)
- Enterococcus faecalis (NCTC 12697)
- Enterococcus faecium (ATCC 882)
- Enterococcus hirae (ATCC 8043)
- Negative control
- Streptococcus pyogenes (ATCC 19615)
- Streptococcus agalactiae (NCTC 8181)
- Escherichia coli (ATCC 25922)
- Proteus mirabilis (ATCC 25933)
For Bile Esculin Azide Agar (Enterococcosel agar)
- Positive control
- Enterococcus faecalis (ATCC 29212)
- Negative control
- Escherichia coli (ATCC 25922)
- Streptococcus pyogenes (ATCC 19615)
- Staphylococcus aureus (ATCC 25923)
For Bacteroides Bile Esculin (BBE) agar
- Positive control
- Bacteroides fragilis (ATCC 25285)
- Bacteroides thetaiotaomicron (ATCC 29741)
- Negative control
- Clostridium perfringens (ATCC 13124)
- Proteus mirabilis (ATCC 12453)
- Fusobacterium nucleatum (ATCC 25586)
Uses of Esculin Hydrolysis test
- To identify Enterococcus species and Group D streptococci based on esculin hydrolysis in presence of bile.
- To differentiate enterococci and Group D streptococci from non Group D streptococci.
- To help in differentiation of certain members of Enterobacteriaceae on the basis of esculin hydrolysis reaction.
- To select and identify Bacteroides fragilis group when performed on Bacteroides bile esculin agar.
- To aid in identification of Listeria species especially Listeria monocytogenes.
- To isolate and identify Yersinia enterocolitica from food and animal feeding stuffs.
- To identify Cryptococcus neoformans based on brown black pigment production from esculin.
- To assist in identification of some oxidase positive aerobic Gram negative rods.
Advantages of Esculin Hydrolysis test
- It is useful for differentiation of Enterococcus species and Group D streptococci from non Group D streptococci.
- It helps in identification of Klebsiella, Enterobacter and Serratia group among Enterobacteriaceae.
- It allows selection and presumptive identification of Bacteroides fragilis group on Bacteroides bile esculin agar.
- It gives rapid results when performed as spot or disk test.
- It is a simple and cost effective biochemical test.
- It can be applied to wide range of organisms including Gram positive cocci and Gram negative rods.
- It is useful in identification of Cryptococcus neoformans based on pigment production.
Limitations of Esculin Hydrolysis test
- It gives only presumptive identification and further biochemical or serological tests are required for confirmation.
- Some organisms other than enterococci and Group D streptococci can also hydrolyze esculin and give false positive result.
- Certain viridans streptococci are capable of growing in bile and hydrolyzing esculin when bile concentration is low.
- Heavy inoculum may overcome the inhibitory effect of bile salts and produce false positive reaction.
- Hydrogen sulphide producing organisms may react with iron in the medium and cause blackening which can be misinterpreted.
- Escherichia coli may show delayed positive reaction on prolonged incubation due to inducible enzyme.
- Some strains of Bacteroides may fail to hydrolyze esculin or may be inhibited by high bile concentration.
- Gram negative bacteria are not inhibited on bile esculin agar lacking sodium azide and may interfere with interpretation.
- Improper storage or prolonged exposure of media to light and heat may affect test results.
Precautions
- Heavy inoculum should not be used as it may overcome the inhibitory effect of bile salts and give false positive result.
- Prolonged incubation should be avoided as some organisms may show delayed esculin hydrolysis.
- Black colour due to hydrogen sulphide production should be carefully interpreted as it may interfere with results.
- Only fresh and properly stored media should be used for the test.
- Tube caps should be kept loosely closed during incubation to allow proper aeration.
- The test should always be performed from pure culture and further confirmatory tests are required.
- Stabbing of the butt is not required during inoculation of bile esculin agar slants.
- Esculin disks should not be over moistened while performing rapid disk test.
- Standard laboratory safety precautions should be followed while handling cultures and media.
Describe the biochemical reaction that produces a black precipitate
The biochemical reaction responsible for black precipitate formation in Esculin hydrolysis test occurs in two sequential steps. In the first step, the organism possessing the enzyme esculinase (β-glucosidase) hydrolyzes the glycoside esculin present in the medium. This hydrolysis breaks the β-glucosidic linkage of esculin and results in the formation of glucose and esculetin. The glucose is utilised by the bacteria for energy, while esculetin is released into the surrounding medium.
In the next step, the liberated esculetin which is a phenolic compound reacts with ferric ions present in the medium, usually supplied as ferric citrate or ferric ammonium citrate. This chemical reaction leads to the formation of an insoluble dark brown or black coloured phenolic iron complex. The deposition of this complex around the colonies or throughout the medium produces the characteristic black precipitate, which is taken as positive indication of esculin hydrolysis.
The chemical process can be summarized by the following equations:
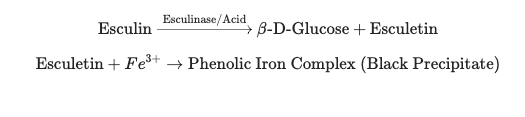
Bacterial genera differentiated by Esculin Hydrolysis test
- Gram positive cocci
- Enterococcus species and Group D Streptococcus differentiated from non Group D viridans streptococci.
- Aerococcus species differentiated from other Gram positive cocci.
- Leuconostoc species differentiated in selective diagnostic conditions.
- Pediococcus species differentiated from similar cocci.
- Gram negative rods (Enterobacteriaceae)
- Klebsiella, Enterobacter and Serratia group differentiated from Escherichia, Salmonella and Shigella.
- Proteus vulgaris and Proteus rettgeri differentiated from Proteus mirabilis and Morganella morganii.
- Anaerobic bacteria
- Bacteroides fragilis group differentiated from other anaerobes such as Fusobacterium and Clostridium.
- Other bacteria
- Listeria species differentiated from other Gram positive rods based on esculin hydrolysis.
- Aeromonas species differentiated from other oxidase positive Gram negative rods.
FAQ
What chemical compounds are produced when esculin is hydrolyzed?
When esculin is hydrolyzed, two chemical compounds are produced as end products of the reaction. The glycoside esculin is broken down by the action of the enzyme esculinase (β-glucosidase) or by acid hydrolysis. This cleavage results in the formation of glucose and esculetin. The glucose formed is utilised by the organism for metabolic activities, while esculetin which is a phenolic compound remains free in the medium and later reacts with ferric ions to produce the characteristic black coloured complex.
Why is sodium azide added to certain bile esculin formulations?
Sodium azide is added to certain bile esculin formulations mainly to increase the selectivity of the medium. It acts as an inhibitory agent against Gram negative bacteria by interfering with the cytochrome oxidase system of these organisms. Due to this action, growth of Gram negative bacilli such as Escherichia coli and other accompanying flora is suppressed, while bile tolerant organisms are allowed to grow.
The presence of sodium azide therefore helps in selective isolation of Enterococcus species and Group D streptococci from specimens containing mixed microbial population. Because of this strong inhibitory effect, the concentration of bile in the medium can be reduced, which helps in better growth and faster recovery of the target organisms without loss of selectivity.
How does esculetin react with ferric ions in the medium?
Based on the provided sources, the reaction between esculetin and ferric ions in the medium occurs through the following biochemical steps:
Production of Esculetin: First, organisms capable of expressing the enzyme esculinase hydrolyze the glycoside esculin into glucose and the aglycone esculetin (6,7-dihydroxycoumarin).
Reaction with Iron: The liberated esculetin reacts with ferric ions (Fe3+), which are supplied in the medium by ingredients such as ferric citrate or ferric ammonium citrate.
Formation of Complex: This reaction produces an insoluble phenolic iron complex.
Visual Result: This complex appears as a dark brown or black precipitate, which causes the blackening of the medium surrounding the colonies.
How does sodium azide affect the growth of gram-negative bacteria?
Based on the provided sources, sodium azide affects Gram-negative bacteria in the following ways:
Inhibition of Growth: Sodium azide acts as a potent inhibitor that prevents the growth of most Gram-negative bacteria.
Mechanism of Action: It exerts its inhibitory effect by interfering with the cytochrome oxidase system of the bacteria.
Selective Function: In formulations like Bile Esculin Azide Agar (Enterococcosel Agar), sodium azide is specifically included to suppress the Gram-negative microbial flora often found in clinical specimens (such as feces), allowing for the selective isolation of Gram-positive organisms like enterococci. This distinguishes it from standard Bile Esculin Agar, where Gram-negative rods (Enterobacteriaceae) are not inhibited and may grow.
What causes the brown-black pigmentation in Cryptococcus neoformans cultures?
Based on the provided sources, the brown-black pigmentation in Cryptococcus neoformans cultures on esculin media is caused by the following biochemical process:
- Hydrolysis: The organism hydrolyzes esculin to release glucose and esculetin (6,7-dihydroxycoumarin).
- Enzymatic Action: Unlike bacteria which rely on iron to form a complex, Cryptococcus neoformans utilizes a fungal enzyme known as phenoloxidase.
- Melanin Formation: This enzyme acts on the esculetin (a diphenol), converting it into a melanin-like pigment.
- Pigmentation: The accumulation of this melanin-like pigment results in the characteristic brown-black coloration of the colonies.
- Acumedia Manufacturers, Inc. (2017). Bile esculin azide agar (7133) [Product information]. Neogen Corporation.
- BD. (2016). BD BBL™ Bacteroides bile esculin agar (BBE) // CDC anaerobe laked sheep blood agar with KV [Instructions for use]. Becton, Dickinson and Company.
- Biokar Diagnostics. (2017). BEA agar (Bile, esculin & azide) [Technical data sheet].
- BioMérieux. (n.d.). VITEK® 2.
- Cai, T., & Cai, B. (2023). Pharmacological activities of esculin and esculetin: A review. Medicine, 102(40), e35306.
- Chuard, C., & Reller, L. B. (1998). Bile-esculin test for presumptive identification of enterococci and streptococci: Effects of bile concentration, inoculation technique, and incubation time. Journal of Clinical Microbiology, 36(4), 1135–1136.
- CliniSciences. (n.d.). Bile esculin azide agar (Enterococcosel agar) [Data sheet].
- Comprehensive analysis of the esculin hydrolysis test: Biochemical foundations, taxonomic applications, and technical methodologies in clinical microbiology. (n.d.).
- Dalynn Biologicals. (2014). Bacteroides bile esculin (BBE) agar [Technical sheet].
- Dalynn Biologicals. (2014). Bile esculin agar [Technical sheet].
- Dalynn Biologicals. (2014). Bile esculin azide agar (Enterococcosel agar) [Technical sheet].
- Edberg, S. C., Gam, K., Bottenbley, C. J., & Singer, J. M. (1976). Rapid spot test for the determination of esculin hydrolysis. Journal of Clinical Microbiology, 4(2), 180–184.
- Edberg, S. C., Pittman, S., & Singer, J. M. (1977). Esculin hydrolysis by Enterobacteriaceae. Journal of Clinical Microbiology, 6(2), 111–116.
- Edberg, S. C., Chaskes, S. J., Alture-Werber, E., & Singer, J. M. (1980). Esculin-based medium for isolation and identification of Cryptococcus neoformans. Journal of Clinical Microbiology, 12(3), 332–335.
- Hardy Diagnostics. (2020). Bacteroides bile esculin (BBE) agar for anaerobic bacteria [Instructions for use].
- Hardy Diagnostics. (2020). Bile esculin agar (BEA) [Instructions for use].
- Hardy Diagnostics. (2020). Bile esculin with azide media [Instructions for use].
- HiMedia Laboratories. (2018). Bile esculin agar (M972) [Technical data].
- Ling, T. K. W., Liu, Z. K., & Cheng, A. F. B. (2003). Evaluation of the VITEK 2 system for rapid direct identification and susceptibility testing of Gram-negative bacilli from positive blood cultures. Journal of Clinical Microbiology, 41(10), 4705–4707.
- Livingston, S. J., Kominos, S. D., & Yee, R. B. (1978). New medium for selection and presumptive identification of the Bacteroides fragilis group. Journal of Clinical Microbiology, 7(5), 448–453.
- Merck Millipore. (n.d.). Bile aesuclin azide agar [Product information].
- Micromaster. (n.d.). Bile esculin agar (DM037) [Product specification sheet].
- Okada, J. S. (1979). Esculin hydrolysis in the enterobacteriaceae [Master’s thesis, University of the Pacific]. Scholarly Commons.Sigma-Aldrich. (n.d.). 06105 Bile esculin azide agar (Enterococcus selective agar) [Data sheet].
- Tankeshwar, A. (n.d.). Bile-esculin test for Enterococcus species. Microbe Online.
- UK Health Security Agency. (2025). UK standards for microbiology investigations: Aesculin hydrolysis test (TP 2, Issue 4.1).
- Uyirgene. (n.d.). Bile-esculin test.
- VUMIE. (2022, November 1). Esculin hydrolysis test. Virtual Microbiology Lab Simulator Software.
- Wikipedia. (n.d.). Aesculin. Retrieved from Wikipedia.
- Wikipedia. (n.d.). Bile esculin agar. Retrieved from Wikipedia.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.