What Are Enzymes?
- Enzymes are biological polymers that catalyse biochemical reactions, per the definition.
- The bulk of enzymes are proteins having catalytic properties that are essential for many processes.
- Enzymes carry out metabolic activities and other chemical reactions within the cell, which are essential for life.
- The early phase of the metabolic process is dependent on enzymes, which interact with a molecule known as the substrate.
- Enzymes convert substrates into products, which are unique molecules of their own.
- Due to their significance in supporting life processes, the regulation of enzymes has been a crucial aspect of clinical diagnosis.
- Except for the family of RNA catalysts termed ribozymes, all enzymes’ macromolecular components are composed of protein.
- The term ribozyme is derived from the enzyme of ribonucleic acid. Numerous ribozymes are ribonucleic acid molecules that catalyse processes in one of their own bonds or between other RNAs.
- Enzymes are present in all biological tissues and fluids. All reactions occurring in metabolic pathways are catalysed by intracellular enzymes.
- Enzymes at the plasma membrane control cell catalysis in response to cellular signals, whereas enzymes in the circulatory system control blood clotting. The majority of essential life processes depend on the functioning of enzymes.
Structure of Enzyme
- Enzymes are composed of a linear chain of amino acids, which results in a three-dimensional structure.
- The sequence of amino acids specifies the structure, which identifies the enzyme’s catalytic activity.
- Upon heating, the structure of the enzyme denatures, leading to a reduction of enzyme activity, which is normally correlated with temperature.
- Enzymes are often larger than their substrates, with sizes ranging from 62 amino acid residues to an average of 2500 residues for fatty acid synthase.
- Located adjacent to the binding sites, just a small portion of the structure is engaged in catalysis.
- Together, the catalytic site and the binding site comprise the enzyme’s active site. A tiny number of ribozymes serve as biological catalysts based on RNA. It has a complicated reaction with proteins.
Enzymes Classification
Historically, enzymes were given names based on their discoverer. With additional study, classification grew more exhaustive.
The International Union of Biochemists (IUB) classifies enzymes into six functional classes based on the sort of reaction they catalyse. Hydrolases, oxidoreductases, lyases, transferases, ligases, and isomerases are the six types of enzymes.
The classification of enzymes is described in depth below:
1. Oxidoreductases
- Examples include pyruvate dehydrogenase, which catalyses the oxidation of pyruvate to acetyl coenzyme A.
2. Transferases
- These catalyse the transfer of a chemical group from one compound to another.
- An enzyme that transfers an amino group from one molecule to another is an example.
3. Hydrolases
- They catalyse a bond’s hydrolysis. The enzyme pepsin, for instance, hydrolyzes peptide bonds in proteins.
4. Lyases
- Aldolase, an enzyme in glycolysis, catalyses the separation of fructose-1, 6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
5. Isomerases
- They accelerate the creation of a compound’s isomer.
- In glycogenolysis, phosphoglucomutase catalyses the conversion of glucose-1-phosphate to glucose-6-phosphate (transfer of phosphate group from one position to another in the same substance) (glycogen is converted to glucose for energy to be released quickly).
6. Ligases
- Ligases facilitate the bonding of two molecules.
- DNA ligase, for instance, catalyses the joining of two DNA pieces by the formation of a phosphodiester link.
Cofactors
Cofactors are non-protein compounds that bind to enzymes. Cofactors are required for the proper functioning of enzymes. Apoenzyme refers to an enzyme that is devoid of a cofactor. Together, an enzyme and its cofactor create the holoenzyme. There are three varieties of enzyme cofactors:
- Prosthetic groups: Prosthetic groups are cofactors that are permanently and strongly attached to an enzyme. FAD (flavin adenine dinucleotide) is an enzyme-associated prosthetic group.
- Coenzyme: Coenzymes only bind to enzymes during catalysis. The protein is disengaged from the enzyme at all other times. NAD+ is a widespread coenzyme.
- Metal ions: For the catalysis of certain enzymes, a metal ion is required to create coordinate bonds at the active site. Several enzymes require the metal ion Zn2+ as a cofactor.
Uses of enzymes
Various applications of enzymes rely on the specificity and rapid catalysis attained by these molecules. This description falls into three categories: (A) clinical (B) industrial (C) laboratory
A. Laboratory uses
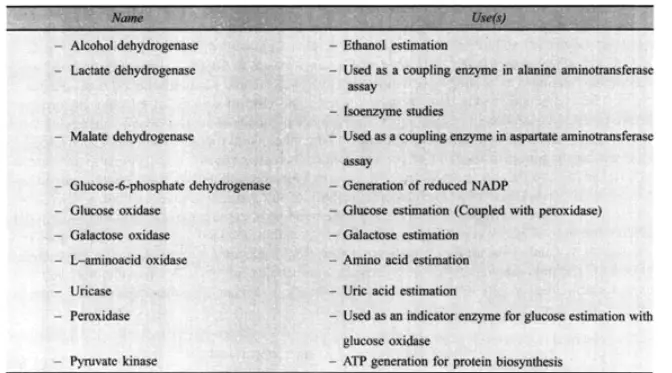
Several enzymes serve a crucial role in analytical procedures. They are frequently utilised in the estimation of a substance that serves as the enzyme’s substrate. Consequently, alcohol: NAD oxidoreductase can be used to assess the ethanol contained in a person’s urine and blood after consuming alcoholic beverages. Using NAD as a cofactor, this enzyme transforms ethanol into acetaldehyde. This is demonstrated by the subsequent reaction:
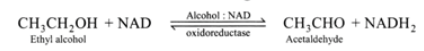
Using a spectrophotometer, the increase in ultraviolet absorption by NADH is evaluated at a wavelength of 3400 A. This wavelength of ultraviolet light is not absorbed by NAD. Several different biochemicals can be evaluated through the action of a single enzyme or a series of linked enzyme processes. For instance, the substrate of the first enzyme reaction can be assayed* or the product can be altered by the presence of a second enzyme. Consequently, glucose oxidase and peroxidase are employed to estimate the glucose concentration in blood. The H2O2 created in the oxidation of glucose by glucose oxidase is used to oxidise a colourless organic component added to form another compound whose concentration can be determined by measuring the intensity of its colour using a colorimeter at the proper wavelength. This H2O2 reaction is facilitated by peroxidase.
Hospital laboratories frequently employ enzymatic assay methods. Here, hundreds of precise estimates on, for example, blood or urine may be required daily to aid in patient diagnosis and treatment control. Therefore, a significant amount of automated procedures are applied in this work. One of these enzymatic test methods is used to determine the concentration of transaminases in the bloodstream. These enzymes are generated by damaged heart muscle in response to a coronary thrombosis, and their accumulation in the bloodstream is evaluated to determine the extent of such damage. alanine aminotransferase and aspartate aminotransferase are the two transaminase enzymes that are measured. Using another enzyme, lactate dehydrogenase, the amount of pyruvate produced by (a) is estimated. By means of this reversible enzyme, pyruvate is transformed to lactate, and the NADH2 loss that results is quantified.
Using malate dehydrogenase, the amount of oxaloacetate produced by (b) is quantified. Thus, oxaloacetate is transformed to malate by NADH2 loss.
It is interesting that lactate dehydrogenase is tested in blood serum by measuring the NADH2 produced in response to liver injury. This enzyme can exist in five distinct enzymatically active forms, which are referred to as its isoenzymes, which are present in distinct ways in various tissues of the body. Blood serum electrophoresis can distinguish between the isoenzymes. Thus, liver damage can be diagnosed because liver injury permits the release of hepatic lactate dehydrogenase into the bloodstream, which is predominantly one of five normal types. Normal blood serum contains a negligible amount of this type, making the rise easily detectable. Lactate dehydrogenase isoenzymes have a quaternary structure consisting of four subunits, each of which is a single protein chain. Each of these subunits can be one of two distinct types, such as A or B, yielding a total of five possible combinations: A4, A3B, A2B2, AB3, and B4.
Using enzymes in insolubilized form, formed by attaching them to a solid support such a cellulose derivative, facilitates attempts to automate enzyme tests of substrate. Columns of supporting material with enzyme coupled to it can be utilised continuously in automated systems, allowing typically highly expensive enzymes to be reused multiple times as opposed to being used only once and then destroyed by subsequent product processing. Enzymes coupled to solid supports can be more stable than the corresponding enzyme in solution if a favourable environment is created, and large-scale industrial applications are being developed for these products.
B. Enzymes in Industries
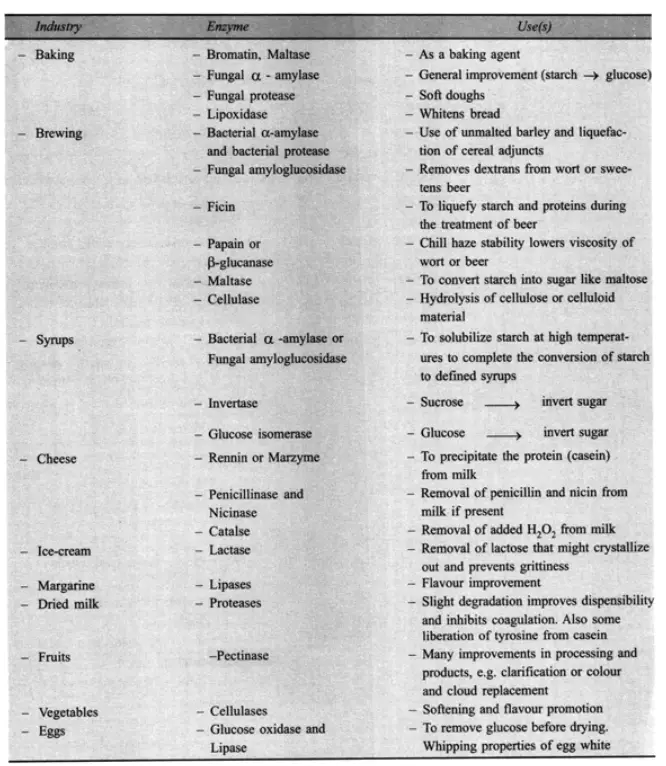

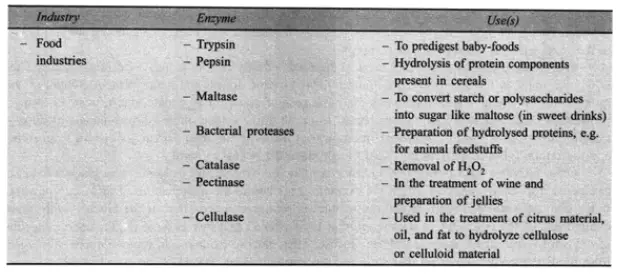
Recently, food makers have begun using enzymes that are commercially available. Enzymes are not particularly harmful. They have a high degree of action specificity and perform admirably under conditions of moderate temperature and pH. They respond swiftly, and their action might be quickly stopped if wanted. By modifying the constituent molecules of certain meals using enzymes, a number of advantageous changes can be brought about in their flavour, texture, colour, clarity, and solubility.
Numerous utilised enzymes belong to the group of hydrolases, which are enzymes that add water to specific chemical bonds, e.g. in macromolecules, resulting in fragmentation into smaller molecules. Amylase is a member of this category of enzymes. It is possible to isolate amylase-active enzymes from bacteria and fungi. They breakdown glucose’s ploymer, starch, mostly into maltose, the equivalent dimer, and glucose itself. In the baking industry, amylases are employed to treat flour prior to bread production. The finished loaf of bread possesses numerous good qualities. These include an enhanced flavour and a greater volume. This is caused by the excess CO emitted by the baker’s yeast during fermentation of the additional sugars produced from the starch due to amylase action. Amylases are also utilised in the manufacturing of sweet syrups from raw starches using a variety of raw starches. The procedure is incredibly cost-effective. Similarly, pectic enzymes are utilised for the breakdown of pectic compounds and contribute to the enhancement of the juice yield produced from various fruits. When added to juice, they reduce its viscosity and aid in the clarification of juices, especially wines.
Proteases, the protein hydrolyzing enzymes, are utilised in the dairy sector for cheese preparation, in the bakery for bread production, in the brewing business for chill-proofing, and most often as a meat tenderizer. The domestic use of crushed raw papaya fruit containing papain is an illustration of the tenderising effect of enzymes on meat. The most recent advancement in tenderising meat is the injection of proteolytic enzyme solutions into the circulatory system of animals before to slaughter, resulting in the uniform distribution of enzymes throughout the body tissues. Additionally, papain is used in the brewing business. Initially, barley is transformed into malt using the classic malting process. Prepare a mash that is conducive to yeast growth and fermentation of the current sugars, such as maltose, to ethanol plus carbon dioxide. Beer is, of course, the product. The public prefers this beverage to be transparent, as opposed to cloudy. Unfortunately, as beer is cooled, a cloudiness known as chill-haze can form. The addition of papain to beer is capable of preventing the production of haze. This proteolytic enzyme degrades a protein component of beer, hence preventing the formation of the proteintannin complex that causes haze.
One of the proteolytic enzymes employed in the food business (dairy) is also utilised in domestic settings. This is rennin, which has been extracted from calf stomach. This enzyme destroys casein, the primary protein component of milk. Due to a minor change in structure, the assault causes casein molecules to congregate, and casein curd is precipitated from milk. Rennin is the active ingredient in home remedies for creating junkets. In the industrial production of cheese from milk, rennin (also known as rennet) is used to make the required curd. In the food business, glucose oxidase and catalase are also utilised. Glucose oxidase can be used to eliminate glucose from some foods, such as eggs, prior to the production of dried egg powder. If the glucose is not eliminated, it reacts with other components of the egg, such as amino acids, to generate a brown colour. It is intriguing that this enzyme requires one oxygen molecule to oxidise one glucose molecule. Therefore, if sufficient glucose is present, glucose oxidase will eliminate traces of oxygen from foods. Catalase, an enzyme utilised in concert with glucose oxidase, degrades the H,O generated by this reaction into water and oxygen. Eventually, all of the oxygen in the system can be consumed with no H,O buildup. If oxygen is present during storage, many products lose their marketability.
In order to remove oxygen, dry foods and mayonnaise can be treated with glucose oxidase and catalase. Another enzyme is of great importance because it solves a significant challenge in the confectionery industry: how to encase a liquid sugar core in chocolate to produce chocolates with a soft centre. Obviously, it is simpler to cover a hard sugar centre, and this is done; nevertheless, an enzyme is added. This enzyme is referred to as invertase, and it hydrolyzes sucrose molecules into glucose and fructose slowly. These two highly soluble substances can dissolve in the limited amount of water present to form the centre liquid. The name of the enzyme, invertase, is derived from the mixture of glucose and fructose that it creates. Because the conversion of sucrose to glucose and fructose results in “inversion,” the mixture is referred to as invert sugar. This is the reversal that occurs in the plane of polarised light passing through the system as it rotates. It is related to the optical activity difference between sucrose and its derivatives. On an industrial scale, invertase is extracted from yeast by using a short treatment with papain to liberate invertase from debris created by the rupture of yeast cells with hot toluene. In the dairy business, another major class of lipolytic enzymes is lipases. Controlled lipolysis is required for the development of cheese’s distinctive flavour and aroma. Several more enzymes are gaining popularity in the food sector. Exploring novel, inexpensive sources for the large-scale commercial manufacturing of these enzymes will be worthwhile.
Enzymes are utilised by other industries besides the food industry. Enzymes are utilised in the pharmaceutical, leather, paper, textile, rubber, canning, and photography industries, among others. To yet, the majority of applications have been relatively easy. To utilise an enzyme for more sophisticated functions, a great deal of essential information about it is required; further research can offer this information. Clearly, an understanding of the enzyme’s specificity, stability, pH and temperature optimums, and potential inhibition is necessary for its successful application.
c. Clinical uses of enzymes
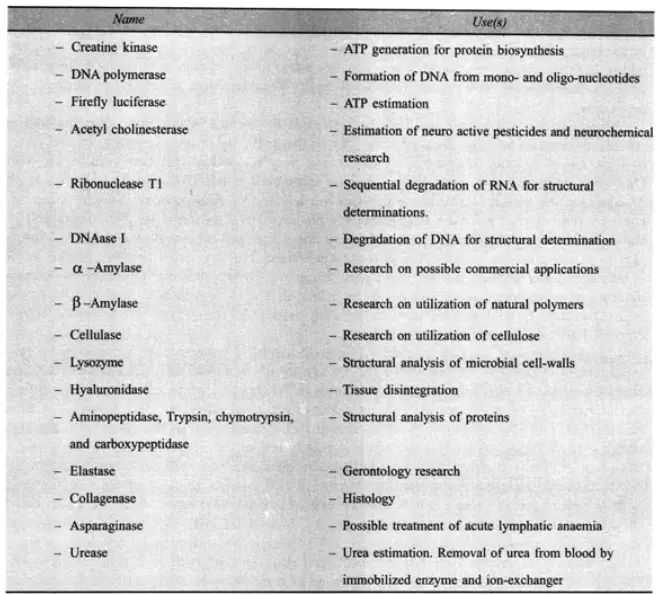
Some enzymes are utilised in the therapy of human ailments. The treatment of the small proportion of the population that is allergic to penicillin is an often cited example. If an allergy is identified, the bacterial enzyme penicillinase could be administered to eliminate the penicillin. It is interesting that the synthesis of this enzyme by certain penicillin-resistant bacterial strains enables them to degrade the penicillin used to treat them.
Normal Penicillin inhibits bacterial growth (and division) by interfering with the bacterium’s capacity to complete cell wall synthesis. Penicillinase targets the penicillin molecule, removing its antibacterial properties by breaking one of its two atom rings. The enzyme lysozyme is an antibiotic that destroys the cell-wall carbohydrates of certain bacteria. It is a naturally occurring antibiotic present in human tears that is used to treat eye infections. From egg white, large quantities of lysozyme can be extracted. Trypsin is one of the most extensively used proteolytic enzymes utilised in medical treatment. By dissolving bodily fluids, including blood clots, this is used to clean wounds. Let us conclude by discussing an enzyme that has been utilised to treat individuals with certain forms of leukaemia, a form of cancer. The following mechanism explains, in basic terms, the therapeutic value of this enzyme. It was determined that the white leukaemia cells in the bloodstream required a continual supply of asparagine from the blood in order to survive. Asparagine is the amide version of the amino acid aspartic acid and has the following chemical formula:
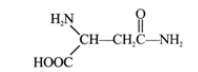
This amino acid is typically synthesised within the cell from aspartic acid, and it is integrated into proteins through the typical methods. This enzyme can be extracted from E. coli bacteria. When asparaginase is injected into the bloodstream, asparagine is hydrolyzed into aspartic acid. However, due to a metabolic disorder, leukemic white cells are unable to utilise this, unlike normal cells. It has been discovered that L-asparaginase-containing microcapsules inhibit the development of asparagine-dependent cancers in mice. When the mice were injected with the soluble enzyme, the tumours were not inhibited due to the immune system’s rejection of the foreign protein. Similar procedures were used to provide encapsulated catalase to acatalusaemic mice. Chang suggests the use of encapsulated enzymes for removing uremic metabolites in cases of clinical abnormalities associated with enzyme deficiencies due to inherited metabolic disorders. This technology is still in its infancy, but its future holds immense promise. Enzyme therapy is fraught with complications, even in the ostensibly straightforward instances discussed above. Nonetheless, it is anticipated that enzymes will be employed to cure numerous ailments in the future. In certain instances, they may be used in their unrefined forms. On occasion, though, they are employed in highly purified states (e.g. urease for urea estimtaion). Enzymes produced by microorganisms and the corresponding microorganism (s).

The future is extremely bright for additional applications. For specific applications in all three sectors, entirely new enzymes will be designed.
General Aspects Of Enzymes’ Production
Enzymes are generated commercially by two methods:
- Semisolid culture
- Submerged culture
There is intense competition among enzyme producers. As a result, manufacturers are hesitant to reveal their processes. It is not possible to conduct a survey of the employed production methods. Nonetheless, it is evident that the submerged culture method has gained traction over the past three to four decades. These two techniques are discussed briefly here.
1. Semisolid culture
On top of a suitable semi-solid substrate, the enzyme-producing culture is developed. Typically, nutritional salts are added to wet wheat or rice bran as the substrate. The medium for production is made by combining bran with a solution containing any nutritional salts necessary. Acid is used to achieve the ideal pH level for optimal mould development. The medium is then autoclaved with steam while being stirred. This sterile media is distributed on metal trays to a depth of ten centimetres, with a total quantity of thousands of kg. A transfer of this nature is conducted under aseptic circumstances. Alternately, cultivation could be conducted in spinning drums. Either the autoclave or trays are infected with fungal spores after cooling. A collection of trays are contained within a big vessel. The movement of adequately humidified air across the surface of the culture ensures aeration. Temperature must be maintained within strict limitations. Additionally, heat is produced during fermentation. The trays should therefore be supplied with a cooling system. It should be remembered that direct air cooling is impractical due to the drying of the culture. Water is then utilised to extract the enzyme. Enzymes generated by semisolid cultures or surface cultures are:

Precautions to be taken
- In a semisolid culture, it is vital to maintain aseptic conditions during fermentation, as contaminations are a key concern.
- Large quantities of spores should not be allowed to escape into the environment.
Advantages
- This procedure requires a relatively little investment.
- It allows substrates with a high dry matter content to be utilised. Therefore, it produces a high enzyme concentration in the fermented crude material.
- It must be used to cultivate some moulds that are extremely difficult to cultivate in fermentors due to wall growth.
- This technique permits the fungi to grow in their “natural” state. This results in the normal differentiation of mycelium, conidiophores, conidia, etc., as well as the production of a greater variety of enzymes than submerged fermentation. In some instances, this phenomenon is desired (e.g. in the production of pectic enzymes).
Disadvantages
- This method demands additional room.
- It demands extra labour.
- It increases the chance of infection.
- This fermentation system is not readily amenable to modern control approaches.
- Automation is difficult to implement in such systems.
2. Submerged culture
Widespread usage of submerged culture techniques is now prevalent in the manufacture of enzymes. The fermentation machinery is identical to that used in the production of antibiotics. It is a cylindrical stainless steel container. The tank is outfitted with an agitator, an aeration device, a cooling system, and additional auxiliary equipment (e.g. means of foam control, monitoring of pH. temperature, oxygen tension, etc.). The amount of production medium added to the fermentation tank is between 1,000 and 30,000 litres.
The formulation of the production medium and, to a lesser extent, the regulation of fermentation conditions play crucial roles in the success of enzyme fermentations. In other words, these processes are complicated due to the chemical composition of manufacturing media. The production medium should essentially have an energy source, carbon and nitrogen supplies, as well as any unique growth needs (e.g. essential amino acids or vitamins). However, growth alone is not sufficient to achieve a large enzyme production. The presence of particular chemicals in the production medium can either stimulate or impede enzyme development. The presence of lactose (an inducer) in the media, for instance, activates B-galactosidase. Additional inducer instances are provided in Table.
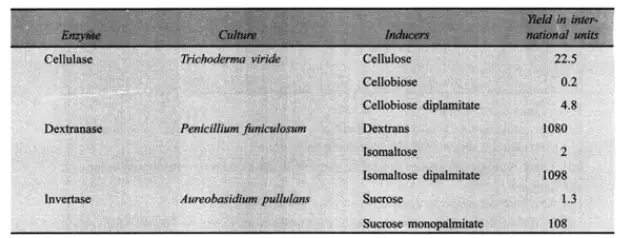
Since inducers are quite costly, it is better to employ constitutive mutants that do not need the inducer. Several enzymes have been designed with such mutations. On the other hand, certain chemicals present in the medium function as enzyme repressors. For instance, glucose inhibits the synthesis of some enzymes (e.g. a-amylases). Under these conditions, glucose levels should be kept low. This can be accomplished by providing glucose in increments or by utilising a sugar that is slowly metabolised (e.g. lactose or partly hydrolyzed starch). The addition of certain surfactants in the production medium boosts enzyme yields. Tween 80 and other non-ionic detergents are commonly used. Table 19.5 displays the impact of 0.1% Tween 80 on the enzyme yield.

Triton (Rohm and Haas) occasionally produces superior results. However, the mechanism of action of these surfactants is unknown. The majority of enzyme fermentations occur at neutral pH. Consequently, it is essential to maintain the pH within the required range during fermentation. This is possible by incorporating a buffer system; (e.g. phosphates or calcium carbonate to the medium). The alternative to this is the addition of certain chemicals that, upon metabolism, result in the desired pH change. This occurs owing to the development of acid or base, depending on the circumstance. Consequently, salts of organic acids and nitrates tend to increase the pH, whereas ammonium salts tend to decrease the pH.
Economy is crucial for a medium formulation. The cost of raw materials accounts for sixty to eighty percent of the variable expenses in a typical fermentation process. Common agricultural raw resources that are readily available and of constant quality are recommended. To clarify the nutritional requirements of the culture for the production of a desired enzyme, specified or synthetic media can be utilised for this purpose. After determining what is essential, it is possible to hunt for cheaper materials that yield the same outcome. Moreover, empirical determination of the optimal concentration of each medium component is laborious. When creating the production medium, the enzyme recovery procedures must also be taken into account. The composition should be such that suspended particles and viscosity are as low as possible at the end of fermentation. Furthermore, elements that could impede the separation of solid and liquid phases must be absent.
The majority of production medium are still batch-steam-sterilized in the fermentor. At around 120°C for 1-2 hours, total sterility of the medium is guaranteed. Alternately, the medium for production can be sterilised independently via continuous sterilisation. The latter technique has a number of advantages, including reduced colour development as a result of Mallard interactions between proteinaceous components and carbohydrates and improved fuel economy when heat recovery is implemented. Additionally, the condensate does not dilute the manufacturing medium. After fermentation is complete, the fermented liquor is rapidly cooled to approximately 5°C to prevent degradation. Either filtration or centrifugation of pH-adjusted, chilled broth is employed to separate microorganisms. The filtrate’s colloidal particles are removed using coagulating or flocculating agents (e.g. calcium phosphate). As a body feed, diatomaceous earth (2-4%) may be added to the fermented broth prior to filtration. Suspended solids are extracted using either vacuum drum filtration or a disc-type centrifuge with a self-cleaning bowl. In order to increase the enzyme’s purity, it is precipitated using acetone, alcohols, or inorganic salts (e.g. ammonium or sodium sulfate). Fractional precipitation results in higher purity levels than single-step precipitation. Because of explosion risks, salts are favoured over solvents for large-scale activities.
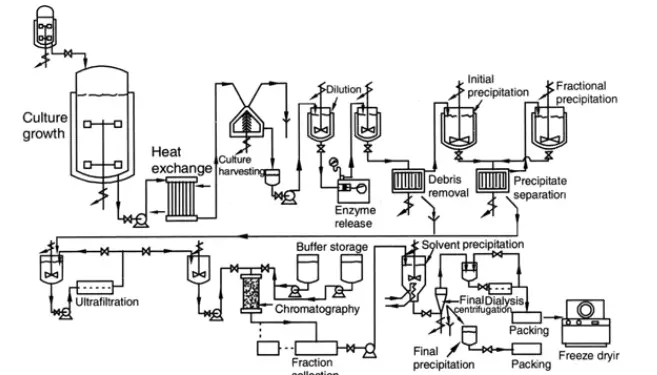
In order to extract endocellular enzymes, microbial cells must be disintegrated. A homogenizer or a bead mill can do this. Afterwards, exoenzymes are purified using the same techniques used for endogenous enzymes. The various purification procedures for endocellular enzymes are depicted schematically in Figure.
Advantages
- This procedure takes less room and labour.
- The approach reduces the likelihood of infection.
- Modern control methods are simple to implement.
- The automation of such processes is simpler.
- Additionally, the yields are often greater in terms of dry matter.
Disadvantage
- Initial investment costs are expensive.
Amylases
Amylases are the most essential enzymes in food technology (e.g. bread-making, beer-making, etc.). Therefore, a- and B-amylase concentrations are prepared and employed in a number of ways. The activity of these enzyme preparations must be thoroughly standardised in accordance with their intended function. Amylases are a significant group of enzymes. Their ability to hydrolyze 1, 4-glucosidic bonds in polysaccharides is defining (e.g. starch and glycogen). Alpha-amylases and Beta-amylases are the two primary groupings. Alpha-Amylases are endo-enzymes. They attack all the glucose-glucose bonds within the starch molecule. The bond between C-1 and the oxygen atom connected to the neighbouring glucose group is hydrolyzed. Eventually, the process leads to the complete breakdown of dextrins into glucose.
Thus, amylose, a linear starch, is destroyed more rapidly than amylopectin, a branching starch. The efficiency of alpha-amylases varies depending on their source. Beta-amylases hydrolyze starch and other amyloses by separating maltose molecules until the process is inhibited by 1, 3-linkages or branch points. The remaining molecule is known as limit dextrin.
Alpha-Amylases are manufactured using fungus (such as Aspergillus niger and A. oryzae) and bacteria (i.e. Bacillus amyloliquefaciens and B. licheniformis). Accordingly, Alpha-amylases are classified as either fungal Alpha-amylases or bacterial a-amylases, depending on the microorganisms used in their manufacture.
The above-mentioned two kinds of fungus produce fungal alpha-amylases. On wheat bran these fungus are cultivated (semisolid culture). It’s also feasible to manufacture fungal Alpha-amylases through submerged-culture using the following medium:

Due to the existence of mycelial body, there is a problem with aeration and agitation since the medium has a very high viscosity. When glucose is present, the manufacture of amylases is hindered. Bacterial -amylases are generated by the two bacterial species listed above. The only method for producing bacterial amylase is submerged culture using the following medium:

A temperature between 30 and 40 degrees Celsius is acceptable. 7.0 is the optimal pH for the fermentation medium. It is necessary to keep the pH close to neutral because amylase is denatured below 6. As a pH buffer, calcium carbonate is employed. When the bacterial count reaches 10°-1O’ cells per milliliter, the production of alpha-amylase begins and continues for another 100-150 hours. 20 percent sodium chloride is used to preserve liquid preparations of bacterial a-amylases. The most active preparations contain 2% amylase protein activity. In contrast, the most active solid preparations contain 5% amylase protein activity.
Proteases
Complex combinations of proteinases and peptidases are typically referred to as proteases. Since peptidases are endogenous enzymes, their quantities in the production medium are minimal. As with a-amylases, proteases are made by bacteria (Bacillus subtilis and B. licheniformis) and fungus (i.e. Aspergillus niger and A. oryzae). Due to their relative instability and susceptibility to deactivation upon dehydration, it is crucial to exercise caution during their manufacture. Alkaline serine proteases and acid proteases are the two types of proteases. The production techniques for each of these proteases are explained briefly here:
a. Alkaline serine proteases
Subtilisin Carlsberg is the most often used protease in detergents. Bacillus licheniformis is grown in submerged culture to produce it. The bacterium is cultured on a medium consisting of the following ingredients:

There are alternative media for this purpose. The fermentation temperature range of 30° to 40°C has been determined to be optimal. In order to get excellent results, the pH of the manufacturing medium is maintained at 7. After 10 to 20 hours, when maximum cell development has occurred, the enzyme is produced. This continues at a virtually constant rate until fermentation is complete. At the conclusion of the productive fermentation, the only protein remaining in the production medium is protease. The explanation for this is the protease-mediated hydrolysis of all proteins present in the medium. The yield may equal 10% of the medium’s initial protein content. The enzyme is primarily distributed as dust-free granules. These granules are composed of 1-5% enzyme protein. The enzyme is stable in liquid formulations. Approximately 2% of the enzyme is found in liquid formulations.
b. Acid proteases
The majority of these enzymes are generated by fungus. These enzymes are produced by the fungi Mucor pusillus, M. miehei, Aspergillus oryzae, Aspergillus phoenicis, and Aspergillus niger var. Macropus. Depending on the species of fungus utilised, acid proteases can be produced through either semisolid or submerged growth. Mucor pusillus, for example, is grown on a semisolid medium. The medium is composed of 60% wheat bran and 40% water. The optimal fermentation temperature is 30°C. The fermentation takes three days to complete. The wheat bran yield is 3,200 Soxhlet units per gramme. With the addition of ammonium salts, the enzyme production can be enhanced. Finally, water is used to extract the enzyme. Mucor miehei, on the other hand, is grown through submerged culture. The composition of the production medium employed for this purpose is as follows:

The optimal fermentation temperature is 30°C. The duration of fermentation is seven days. Approximately 3,500 Soxhlet units are produced every millilitre of fermenting broth. Enzyme preparations are sold at doses ranging from 10,000 to 150,000 Soxhlet units per millilitre. These formulations include 0.2% to 3.0% active enzyme.
Pectinases
Pectinases are generated by Aspergillus (such as A. niger) and Penicillium species. This fungus is developed on sugar beet molasses because it contains pectins. A. niger can be used to manufacture pectinases in semisolid culture. At the conclusion of the fermentation process, the mycelial material is dried and ground into a powder. Cold water is then used to extract a combination of pectin esterase, polygalacturonase, polymethylgalacturonase, and pectin transeliminase. After the enzyme combination has been extracted, it is treated to concentration and refrigeration without solidification.