Enzyme Immobilization
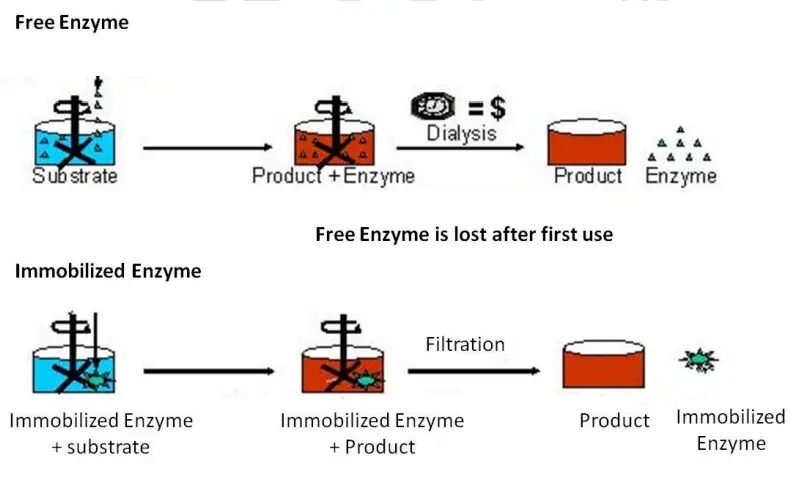
- Enzymes are catalysts that perform all vital biological reactions within an organism’s body. Their distinguishing characteristic is that they endure the reaction unchanged.
- Therefore, they can be utilised repeatedly. However, soluble enzymes are limited by their separation from the product and substrate.
- The majority of Enzymes in a living organism are either connected to the cell membrane or encapsulated within the cells.
- This result led to the hypothesis that pure separated enzymes may work better when immobilised on a solid substrate.
- The phrase immobilised enzyme refers to “catalytically active enzymes that are physically limited or localised in a specific region of space and can be used again and continuously.”
- The benefit of immobilisation is that it promotes work-up product isolation. Listed below are some potential advantages and disadvantages of immobility.
Soluble Enzyme + Substrate———– Product (single time usage of enzyme)
Immobilized Enzyme + Substrate———Product (Repeated usage of enzyme)
A number of essential considerations must be made when immobilising an enzyme.
- The enzyme’s biological activity should be maintained.
- The enzyme ought to be more stable than its soluble equivalent.
- The price of immobilisation shouldn’t be excessively high.
- It should be utilised frequently.
What is a Support Matrix?
It is a substance that facilitates the entrapment of an enzyme. For effective immobilization, a support matrix must possess the properties outlined in the following section.
Properties of Support Matrix
Ideal Properties of Support Matrix
- Mechanical durability.
- Biocompatible.
- Stable.
- Inertness.
- Regenerability.
- Ease of differentiation
- Expanding the specificity of enzymes.
- Product inhibition reduction.
- Decrease in microbial contamination.
- Cost-effective.
Classification of a Matrix
Support material are classified into two categories based on their morphology
- Non porus support: eg glass
- Porus support: eg silica
Porous support has a greater surface area than nonporous support.
Support material are classified into two categories based on their chemical nature
- Organic
- Inorganic
Two types of support matrices exist based on their chemical composition.
1. Organic matrix: There are natural polymers and synthetic polymers.
- Natural polymers: It has a favourable affinity for proteins. Natural polymers include polysaccharides (Cellulose, dextran, agar, agarose, chitin, alginate, etc.), proteins (Collagen, albumin), and carbon.
- Synthetic polymers: It is chemically and mechanically stable. Synthetic polymers include polystyrene, polyacrylate, polyacrylamide, and polyamides, among others.
2. Inorganic matrix: It separates minerals into Natural and Processed categories.
- Natural minerals: E.g.Bentonite, celite, centolite, silica, charcoal etc.
- Processed materials: E.g.Porous glass, metals and metal oxides.
Examples of Support Matrix
Some of the most often employed polysaccharides include
- Alginate: are polymers of n-acetyl glucuronic acid with a readily derivatizable hydroxyl group.
- Chitosan and chitin: Chitosan is chitin that has been deacetylated.
- Carrageenan: A linear sulfated polysaccharide, carrageenan is a carrageenan.
- Cellulose: Cellulose is a linear polymer composed of D-glucose units (two are depicted) connected by β(1→4)-glycosidic linkages.
- Starch: Starch is a linear glucose polymer.
- Pectin: Pectin is a galacturonic acid-based heteropolysaccharide found in plant cell walls.
Some commonly used Proteins include:
- Collagen.
- Gelatin
As a result of their physical and chemical characteristics, synthetic supports are the most abundant support materials for protein immobilization.
- DEAE cellulose
- polyvinyl chloride (PVC)
- UV-activated polyethylene glycol (PEG)
- glutaraldehyde-activated nylon
- cyclodextrin glucosyltransferase
Inorganic materials as supports;
- Zeolites: Zeolites are crystalline microporous materials with well-defined features.
- Ceramics: Ceramics are inorganic and metal-free
- Celite: Celite is a very porous diatomaceous substance.
- Silica: oxide of silicon
- Glass: Glass is an extremely viscous fluid.
- Activated carbon: Activated carbon is derived from carbonaceous sources, including coal, coconuts, nutshells, peat, wood, and lignite.
- Charcoal: Charcoal, a carbon allotrope, has exceptional adsorption capability.
Physical Characteristics of the Matrices
- Mean particle size: Mean particle size is the parameter that determines a carrier’s porosity. The porous matrix is preferred over the non-porous matrix because it improves the surface area, hence increasing the enzyme’s loading capacity. This matrix feature defines the total surface area and influences a catalyst’s binding capacity. A porous material should have optimal pore distribution in order to maximise particle flow.
- Hydrophilic character: It determines the activity level of an immobilised enzyme.
- Mechanical strength: Mechanical strength can be defined as the binding of an enzyme that is inversely proportional to its ease of entrapment.
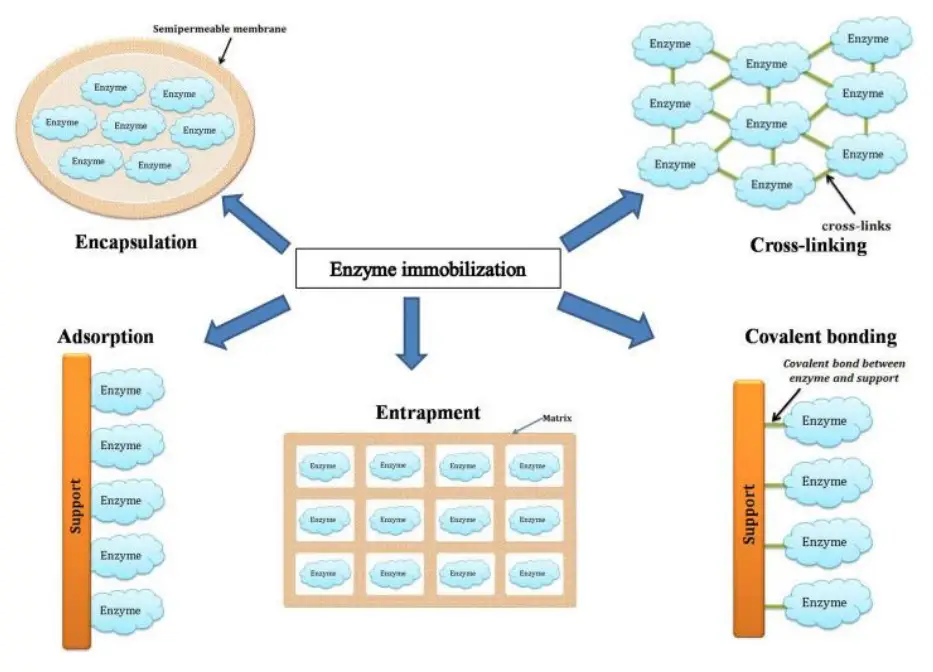
Enzyme immobilization Techniques / Methods of Immobilization
Based on the binding property, it is classified as either physical or chemical.
- Physical Methods
- Adsorption
- Entrapment
- ENcapsulation
- Chemical Methods
- Covalent Binding
- Cross Linking
A. Physical Methods of Enzyme immobilization
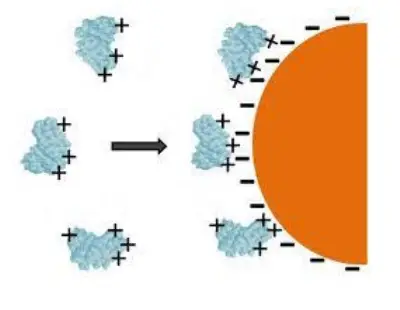
1. Adsorption
- This is the earliest and simplest technique. Enzyme clings to the surface of the water-insoluble carrier matrix in this form.
- Similar to electrostatic or hydrophobic affinity binding to a particular ligand, the binding is nonspecific.
- Typically, the bond between enzymes and the carrier matrix is strong, but it can be reduced by numerous causes, including:
- Addition of substrate
- pH or ionic strength
- In enzyme adsorption, the bonding is accomplished via weak forces, such as the hydrogen bond and the Vander Waal force.
- The matrix employed The particle size of the matrix must be modest (500Å-1mm D).
- Examples: In this type, various carrier materials are used, including:
- Mineral support (E.g. Aluminium oxide, clay)
- Organic support (E.g. Starch)
- Modified sepharose and ion exchange resins
Methods of Immobilization by Adsorption
- Static method: The static approach is an effective yet time-consuming procedure. It involves agitation-free immobilisation of enzyme and carrier molecule.
- Dynamic process: This dynamic procedure requires the continual mixing of an enzyme with the carrier.
- Reactor loading: Reactor loading entails introducing both the enzyme and the carrier into the reactor while stirring the entire material. It is commonly employed in the industrial manufacturing of immobilised enzyme.
- Electro-deposition: Electro-deposition involves keeping a carrier close to the electrode in an enzyme bath and then passing an electric current through it. This causes an enzyme to travel toward the carrier. The enzyme is finally deposited on the surface.
Advantages of Adsorption
- It has no pore diffusion limitation.
- It is a straightforward and economical method.
- In this method, no reagents are necessary.
- There is only a slight decline in enzyme activity.
- It has less of an effect on an enzyme.
- It has minimal activation requirements.
- The enzyme that has been adsorbed can be recycled, regenerated, and reused.
- It has a high enzyme loading efficiency.
Disadvantages of Adsorption
- It offers a small surface area for enzyme binding.
- Typically, desorption of an enzyme from its carrier occurs.
- The yield is also modest.
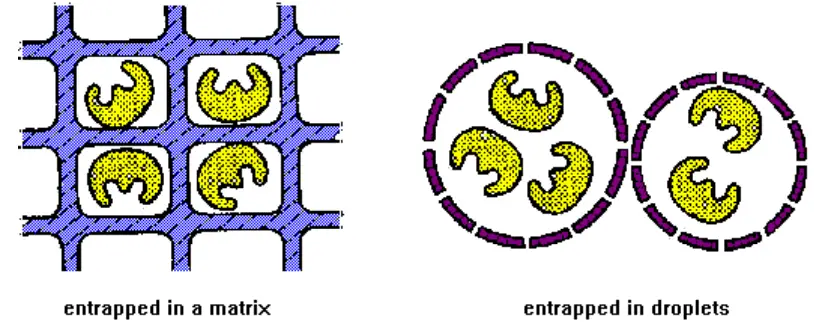
2. Entrapment
- This method entraps an enzyme within a porous polymer or gel matrix. Also known as lattice entrapment. Covalent or noncovalent bonds can exist between an enzyme and its matrix.
- The matrix employed It is water-soluble and its nature varies depending on the enzymes present.
- Examples: polyacrylamide gels, cellulose triacetate, agar, gelatine, alginate, etc. are utilised as carrier materials.
Methods of enzyme entrapment
It involves the incorporation of an enzyme into the matrices listed below:
- Gels: This process involves entrapping an enzyme within the gel matrix.
- Fibres: Entrapment of an enzyme within the matrix of a fibre.
- Microcapsules: Involves trapping inside a microcapsule.
Advantages of Entrapment Method
- It has a high enzyme loading capacity.
- It is a quick method.
- Here, enzyme distortion is minimal.
- It is simple to implement.
Disadvantages of Entrapment Method
- Substrate and product diffusion creates difficulties.
- It results in the loss of low-molecular-weight enzymes.
- There may be the possibility of microbial contamination.
- In addition, it causes enzyme inactivation and sometimes enzyme activity loss.
- It has few industrial applications.
3. Encapsulation
- It involves membrane confinement. In an aqueous solution, an enzyme is contained within the semipermeable membrane of a capsule.
- This procedure permits the exchange of substrate and product, but not an enzyme. The efficiency of encapsulation depends on the stability of the enzyme.
- The capsule’s matrix consists of a semi-permeable membrane, which may be polymeric, lipoid, non-ionic, etc.
- Examples: It includes nitrocellulose, nylon semi-permeable matrix etc.
Methods of encapsulation
It can be attained through the following methods:
- Encapsulation in a reaction vessel: Encapsulation in a reaction vessel entails separating a chamber with a semipermeable membrane. In one compartment are enzymes, while in the other are substrate and product.
- Encapsulation by hollow fibre membrane: It involves trapping an enzyme within a semipermeable matrix (cellulose, triacetate etc.). Here, an enzyme is captured within the matrix.
- Microencapsulation: Utilizing 1-6-diaminohexane, enzyme molecules are enclosed within a microcapsule through chemical polymerization.
- Encapsulation by liposomes: Using phospholipid, an enzyme attaches to the concentric lipoidal membrane of the liposome.
Advantages of Encapsulation
- There is no leakage of enzymes.
- It has no impact on enzyme activity.
- It’s a straightforward procedure.
- It possesses a high enzyme loading efficiency.
Disadvantages of Encapsulation
- Utilizes a carrier with restricted pore size.
- It is not very economical.
B. Chemical Methods of Enzyme immobilization
1. Covalent Binding
- It is a common practise. An enzyme molecule forms a covalent link with the carrier during this procedure.
- Here, the binding strength is strong, or a complex structure is stable due to this bonding.
- Additionally, no enzymes are lost during the procedure. The functional group of an enzyme’s active component forms a covalent bond with the carrier molecule.
- The functional groups involved in the binding process include -NH2, -NH3, -COO, -OH, -SH, -O, and -S, among others. The charged condition of these functional groups determines the order of their responsiveness to the carrier: -S– > -SH > -O– > -NH2 > -COO– > -OH >> -NH3+
- The following are examples of polymeric carriers employed in covalent bonding:
- Carboxylic acid and related polyglutamic acid groups
- Amide group of a polypeptide Amino and similar polysaccharide groups
- Polysaccharides (celluloses, agarose, sepharose, etc.), polyvinyl alcohol, silica, and porous glasses are among the most frequently used polymers.

Methods used for Covalent Binding
The subsequent procedures are involved.
- Diazoation: It is based on the diazo bond between protein and aryldiazonium electrophilic groups of the matrix.
- Development of Peptide bond: formation of a bond between the amino/carboxyl groups of the support and the amino or carboxyl groups of the enzyme.
- Poly functional reagents: Use of a bi-functional or multi-functional reagent (glutaraldehyde) that establishes a link between the amino group of the support and the amino group of the enzyme.
- Amidination reaction: The matrix containing amido ester functional groups can be employed to immobilise proteins through the amidination process.
- Thiol–disulphide exchanged reaction: This technique is utilised for protein bonding through the thiol groups of both the carrier and the protein.
- Akylation and arylation: This approach is based on the alkylation of the amino, phenolic, and thiol groups of proteins with a matrix including reactive halides, vinyle, sulphonile, etc.
Advantages of Covalent Binding
- It provides an enzyme and a carrier with a strong binding force.
- There is no leaking of enzymes.
- It’s a straightforward and popular method.
- pH and ionic strength have no impact on the process.
Disadvantages of Covalent Binding
- Enzyme is altered chemically, resulting in functional conformational loss.
- When active site reactions occur, conformational changes inactivate the enzyme.
- This can be circumvented by immobilising the enzyme in the presence of substrate or a competitive inhibitor.
- Strong connection between enzyme and support.
- No leakage or desorption problem.
- Method that is comparatively straightforward.
- Different functional group assistance options are offered.
- Wide applicability
2. Cross Linking
It is often referred to as “Copolymerization.” Using polyfunctional reagents, the immobilised enzymes form covalent bonds with the different groups of an enzyme. It does not require a support matrix. Cross-linking results in the development of three-dimensional cross-linked aggregates. The most frequently employed polyfunctional agents include glutaraldehyde and diazonium salts, among others.
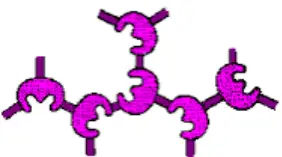
Advantages of Cross Linking Method
- There is minimal or no enzyme loss.
- It results in a highly stable enzyme.
- It is a simple and inexpensive method to implement.
- It has a broad range of applications in the commercial production of the enzyme.
Disadvantages of Cross Linking Method
- It inhibits enzyme activity.
- In general, the polyfunctional chemicals utilised in this technique denature enzymes.
- It is not very economical.
Advantages of Enzyme immobilization
- Stable and more functionally efficient
- Can be used several times.
- The products lack enzymes.
- Ideal for enzyme-based reaction systems.
- Easy regulation of enzyme action.
- Fit for industrial and medical applications.
- Minimize wastewater disposal problems.
- high ratio of substrate to enzyme
- Minimum response time
- Continuous enzyme utilisation
Disadvantages of Enzyme immobilization
- The potential of an enzyme losing its biological function through immobilisation or usage.
- Often, immobilisation is a costly endeavour needing sophisticated technology.
- Some enzyme become unstable following immobilisation.
- Occasionally, enzymes are rendered inactive by the system’s generated heat.
Applications of Enzyme Immobilization
- Industrial production: Used extensively in the commercial manufacture of industrial-grade antibiotics, drinks, amino acids, and secondary metabolites, etc.
- Biomedical applications: Immobilized enzymes are most frequently utilised in rapid diagnostic tests, such as ELISA, and in the treatment of numerous pathogenic disorders.
- Food industry: Enzymes such as Pectinases, Cellulases, and Amylases are immobilised on suitable carriers or matrices and successfully employed in the commercial manufacturing of fruit and vegetable jams, jellies, and syrups. Lactase is immobilised on cellulose fibres, allowing milk and whey to make lactose-free milk.
- Large Scale Using bioreactors for the production of bio-diesel from vegetable oils. They provide a continuous procedure that cuts costs in half.
- Waste water management: Treatment of sewage and industrial effluents via packed bed reactors
- Textile industry: The textile industry, such as scouring and bio-polishing.
- In the detergent industry: In the detergent industry, immobilisation of lipases to breakdown lipids found in stains or dirt is commonplace.
- Immobilized enzymes for bioremediation: Enzymes immobilised for bioremediation Bioremediation is a technology that utilises biological organisms and enzymes to remove pollutants from a contaminated place.
- Biodiesel production: Biodiesel manufacturing has acquired significance due to its ability to replace fossil fuels that are expected to run out within a century.
- Enzyme immobilised for phenolic derivative elimination.
References
- Datta S, Christena LR, Rajaram YR. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 2013 Feb;3(1):1-9. doi: 10.1007/s13205-012-0071-7. Epub 2012 Jun 6. PMID: 28324347; PMCID: PMC3563746.
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/enzyme-immobilization
- https://www.easybiologyclass.com/enzyme-cell-immobilization-techniques/
- https://www.slideshare.net/jahir143/enzyme-immobilization-20940809
- http://rnlkwc.ac.in/pdf/study-material/botany/Immobilization%20of%20Enzymes.pdf
- http://biochem.du.ac.in/web/uploads/71%20Enzyme%20immobilization.pdf
- https://www.biotecharticles.com/Applications-Article/Enzyme-Immobilization-Methods-and-Applications-3702.html
- https://agscientific.com/blog/importance-of-enzyme-immobilization.html
- https://www.creativebiomart.net/Enzyme-Immobilization.htm
- http://ecoursesonline.iasri.res.in/mod/page/view.php?id=4068
- https://biologyreader.com/immobilization-of-enzyme.html
- http://web.ist.utl.pt/ist11061/fidel/enzymatic/appendix/immob.html
- https://www.biologydiscussion.com/enzymes/immobilization/immobilization-of-enzymes-and-cells-methods-effects-and-applications/10208