| Genus and species | Entamoeba histolytica |
| Domain | Eukaryota |
| Phylum | Amoebozoa |
| Family | Entamoebidae |
| Etiologic agent of: | Amoebiasis; amoebic dysentery; extraintestinal amoebiasis, usually amoebic liver abscess; “anchovy sauce”); amoeba cutis; amoebic lung abscess (“liver-colored sputum”) |
| Infective stage | Tetranucleated cyst (having 2-4 nuclei) |
| Definitive host | Human |
| Portal of entry | Mouth |
| Mode of transmission | Ingestion of mature cyst through contaminated food or water |
| Habitat | Colon and cecum |
| Pathogenic stage | Trophozoite |
| Locomotive apparatus | Pseudopodia (“false foot””) |
| Motility | Active, progressive and directional |
| Nucleus | ‘Ring and dot’ appearance: peripheral chromatin and central karyosome |
| Mode of reproduction | Binary fission |
| Pathogenesis | Lytic necrosis (it looks like “flask-shaped” holes in Gastrointestinal tract sections (GIT) |
| Type of encystment | Protective and Reproductive |
| Trophozoite stage | |
| Pathognomonic/diagnostic feature | Ingested RBC; distinctive nucleus |
| Cyst Stage | |
| Chromatoidal body | ‘Cigar’ shaped bodies (made up of crystalline ribosomes) |
| Number of nuclei | 1 in early stages, 4 when mature |
| Pathognomonic/diagnostic feature | ‘Ring and dot’ nucleus and chromatoid bodies |
What is Entamoeba Histolytica?
- Entamoeba histolytica is an anaerobic parasitic amoebozoan belonging to the genus Entamoeba. This single-celled organism primarily targets humans and other primates, causing a disease known as amoebiasis. The global impact of E. histolytica is significant; it is estimated to infect approximately 35 to 50 million individuals each year. Alarmingly, this parasite is responsible for over 55,000 deaths annually, underscoring the public health challenge it presents.
- Historically, it was believed that around 10% of the global population was infected with E. histolytica. However, this estimate was revised following the realization that a substantial proportion of these infections, approximately 90%, were attributed to a closely related species, Entamoeba dispar, which does not cause disease in humans. While mammals such as dogs and cats can experience transient infections, they are not considered major contributors to the transmission of E. histolytica among humans.
- The term “histolytica” is derived from “histolysis,” which refers to the breakdown or disintegration of organic tissues. This nomenclature highlights the pathogenic nature of the organism, as it can invade and damage human tissues, particularly in the intestines.
- Understanding the biology and transmission mechanisms of E. histolytica is crucial for devising effective prevention and treatment strategies. The parasite is primarily spread through the fecal-oral route, often via contaminated food and water. Therefore, improving sanitation and hygiene practices is vital in reducing the incidence of amoebiasis.

History and Distribution of Entamoeba Histolytica
The history and distribution of Entamoeba histolytica illustrate the significant impact this parasite has had on human health throughout the years. First identified in the late 19th century, E. histolytica remains a global health concern, particularly in regions with inadequate sanitation.
- Discovery:
- E. histolytica was first discovered by Lösch in 1875 when he identified the parasite in the dysenteric feces of a patient in St. Petersburg, Russia.
- In 1890, the renowned physician William Osler documented a case of dysentery in a young man, who later succumbed to a liver abscess attributed to the parasite.
- Pathogenesis:
- In 1891, researchers Councilman and Lafleur elucidated the mechanisms behind intestinal and hepatic amoebiasis. They also coined the terms “amoebic dysentery” and “amoebic liver abscess,” which are critical in understanding the clinical manifestations of the infection.
- Global Prevalence:
- E. histolytica exhibits a worldwide distribution but is particularly prevalent in tropical regions. The parasite has been identified in various climates, from Alaska (61°N) to the Straits of Magellan (52°S).
- Approximately 10% of the global population is thought to be infected, with up to 50% of individuals in developing countries affected.
- Infection in Developed Countries:
- Notably, E. histolytica is not confined to developing nations; about 1% of the American population is reported to harbor the parasite.
- Asymptomatic and Symptomatic Infections:
- While 80% to 99% of infected individuals remain asymptomatic, invasive amoebiasis can result in severe health issues, affecting around 50 million people and causing 50,000 deaths annually.
- The majority of these fatalities occur in tropical regions of Asia, Africa, and Latin America, making E. histolytica the third leading parasitic cause of mortality, following malaria and schistosomiasis.
- Epidemiological Distribution in India:
- India presents a varied epidemiological landscape regarding E. histolytica prevalence:
- High Prevalence States (>30%): Chandigarh, Tamil Nadu, and Maharashtra.
- Moderate Prevalence States (10–30%): Punjab, Rajasthan, Uttar Pradesh, Delhi, Bihar, Assam, West Bengal, Andhra Pradesh, Karnataka, and Kerala.
- Low Prevalence States (<10%): Haryana, Gujarat, Himachal Pradesh, Madhya Pradesh, Odisha, Sikkim, and Puducherry.
- India presents a varied epidemiological landscape regarding E. histolytica prevalence:
Morphology of Entamoeba Histolytica
The morphology of Entamoeba histolytica is critical for understanding its life cycle and pathogenesis. This protozoan parasite exists in three distinct stages: trophozoite, precyst, and cyst. Each stage exhibits unique morphological features, which are essential for identification and understanding the organism’s behavior within its host.
- Trophozoite Stage:
- This is the active, feeding form of E. histolytica, which is responsible for causing disease.
- Shape: The trophozoite does not have a fixed shape, constantly changing due to its motility.
- Size: Typically ranges from 18 to 40 µm, with an average size between 20 and 30 µm.
- Cytoplasm: Divided into two components:
- Ectoplasm: The clear, transparent outer layer.
- Endoplasm: The granular inner layer, which contains ingested red blood cells (RBCs), tissue debris, and food materials.
- Nucleus: Features a single, spherical nucleus measuring 4 to 6 µm in diameter. The nucleus has a central karyosome surrounded by fine peripheral chromatin, which aids in identification.
- Motility: Trophozoites exhibit active motility using pseudopodia, which are temporary projections of the cytoplasm. This motility is critical for their colonization and invasion of host tissues.
- Oxygen Requirement: E. histolytica is an anaerobic organism, primarily residing in the large intestine of its host.
- Precyst Stage:
- This stage represents the transition between the trophozoite and cyst forms.
- Size: Generally smaller, measuring 10 to 20 µm in diameter.
- Shape: Typically round or slightly ovoid, with blunt pseudopodia extending from the periphery, although these are less pronounced than in trophozoites.
- Cytoplasmic Contents: Lacks ingested RBCs or food particles, indicating its preparatory nature for encystation.
- Cyst Stage:
- This is the infective form of E. histolytica that can survive outside the host and is responsible for transmission.
- Shape: Cysts are generally round or oval in shape.
- Size: Typically measure between 12 to 15 µm in diameter.
- Cyst Wall: Enclosed by a highly retractile membrane known as the cyst wall, which offers resistance to digestion by gastric juices, facilitating its survival in the gastrointestinal tract.
- Nucleus: A mature cyst typically contains four nuclei (quadri-nucleated), which is crucial for its identification.
- Cytoplasm: Exhibits chromatoid bodies and glycogen masses, but it lacks RBCs and food materials, signifying its dormant state.
- Excretion: Mature cysts are excreted in the stool of infected individuals and can remain viable in the environment for several days, posing a risk for transmission to new hosts.
Life Cycle of Entamoeba Histolytica
Entamoeba histolytica is a pathogenic protozoan responsible for amoebic dysentery and other gastrointestinal diseases. Understanding its life cycle is crucial for developing effective prevention and treatment strategies. The life cycle consists of distinct stages that facilitate its transmission and pathogenicity, primarily involving cysts and trophozoites.
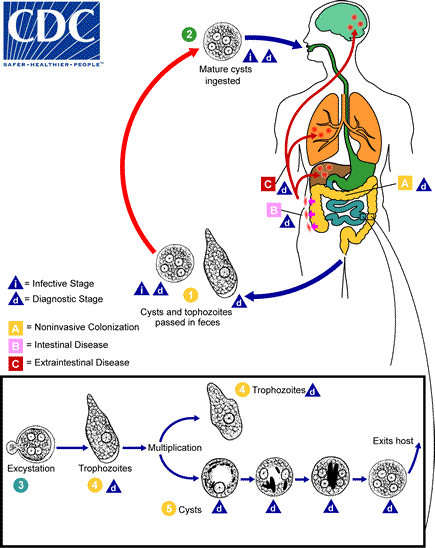
- Cyst and Trophozoite Stages:
- The life cycle begins with the presence of both cysts and trophozoites in feces.
- Cysts are predominantly found in formed stools, whereas trophozoites are typically present in diarrheal stools.
- Infection Route:
- Infection occurs through the ingestion of mature cysts. This can happen via consumption of fecally contaminated food and water or through direct contact with contaminated hands.
- Additionally, exposure to fecal matter during sexual contact can lead to infection through the transmission of cysts and trophozoites.
- Excystation:
- Once ingested, cysts travel to the small intestine, where they undergo a process known as excystation.
- During excystation, trophozoites are released into the intestinal lumen.
- Migration and Infection Types:
- Following excystation, trophozoites migrate to the large intestine.
- Here, two primary outcomes can occur:
- Non-invasive Infection:
- In some individuals, trophozoites remain confined to the intestinal lumen, resulting in asymptomatic carriage.
- These asymptomatic carriers continue to excrete cysts in their feces, contributing to environmental contamination.
- Intestinal Disease:
- In other cases, trophozoites can invade the intestinal mucosa, leading to inflammation and ulcers, a condition known as intestinal amoebiasis.
- Extraintestinal Disease:
- In severe cases, trophozoites may enter the bloodstream and disseminate to extraintestinal sites such as the liver, brain, and lungs, causing extraintestinal amoebiasis.
- Non-invasive Infection:
- Reproduction:
- Trophozoites multiply through binary fission, a form of asexual reproduction that increases their numbers within the host.
- As trophozoites proliferate, they can encyst to form new cysts, completing the life cycle.
- Environmental Stability:
- Cysts are notably resilient; they can survive in the external environment for days to weeks, maintaining their infectious potential due to the protective nature of their cyst wall.
- In contrast, trophozoites are highly sensitive to environmental conditions and are rapidly destroyed outside the human body. If ingested, they cannot withstand the harsh gastric environment.
Transmission of Entamoeba Histolytica
The active (trophozoite) stage can only be found in the host , and also in fresh feces. Cysts remain outside the host, in water, in soils and on food items, especially in moist conditions in the last. The infection may occur when someone puts something in their mouth that has been in contact with the feces of someone affected by E. histolytica, swallows something, like food or water, which is contaminated by E. histolytica, or takes in E. histolytica cysts (eggs) collected from surfaces that are contaminated or on fingers.
The cysts are easily destroyed by heat or frigid temperatures. They last for just some months away from the host. If the cysts are swallowed they can cause infection by excysting (releasing the stage of trophozoites) within the digestive tract. There are many symptoms that can be present, including bleeding diarrhea, fulminating dysentery as well as weight loss as well as abdominal pain, fatigue and amoeboma. Amoeba’s can bore in the intestinal walls, which can cause lesions as well as intestinal discomfort and can also enter into the bloodstream. It can then be able to reach the vital organs of your body. This is primarily the liver, but also the brain, lungs and the spleen. The most common result of the invading of tissues is an abscess, which could be fatal if left untreated. Red blood cells that are digested are often found in the amoeba cells cytoplasm.
- Geographical distribution: All over the world and more frequent in subtropics and tropics particularly in areas of inadequate sanitation (developing and under-developed nations).
- Habitat: Trophozoites from E. histolytica reside in the submucosal and mucosal layer of the intestine of a man. The life-cycle of Entamoeba histolytica is divided into two stages that includes a motile trophozoite as well as a non-mot cyst. Trophozoites are present inside intestinal lesions lesions that extend into the intestine as well as diarrheal stools. Likewise, cysts are predominant in stools with no diarrhea.
- Infective form: A mature quadrinucleate cyst. it has a spherical form with a refractile wall.
Laboratory Diagnosis of Entamoeba Histolytica
The laboratory diagnosis of Entamoeba histolytica is crucial for identifying intestinal amoebiasis and its extraintestinal manifestations, such as amoebic liver abscess. The diagnostic process encompasses various methods, each with its own strengths and limitations, facilitating accurate detection of the pathogen.
- Stool Examination:
- Specimen Collection: Fresh stool samples should be collected in a wide-mouth container and analyzed promptly. It is essential to inspect the stool both macroscopically and microscopically for accurate diagnosis.
- Macroscopic Appearance: The stool of patients with amoebiasis typically appears foul-smelling, copious, and semi-liquid, often mixed with blood and mucus. Its color may range from brownish-black to bright red, and it does not adhere to the container.
- Microscopic Appearance:
- Saline Preparation: The cellular exudate in the stool is scanty, primarily consisting of nuclear masses (pyknotic bodies) from pus cells, along with some epithelial cells and macrophages.
- Presence of Red Blood Cells (RBCs): RBCs may appear in clumps and vary in color from yellow to brown-red. The detection of motile trophozoites, particularly those with ingested RBCs, confirms the presence of E. histolytica.
- Cysts and Staining: Cysts have a smooth, thin cell wall and contain refractile chromatoid bars. Staining techniques, such as iodine staining, are often employed to visualize cysts and trophozoites, allowing for identification of nuclear characteristics. Trichrome stain further enhances the visibility of intracellular features.
- Cyst Excretion: Since cyst excretion is often intermittent, at least three consecutive stool samples should be examined to increase the likelihood of detecting the pathogen.
- Mucosal Scrapings:
- Scraping obtained via sigmoidoscopy can provide additional information. This method allows for the direct wet mount examination and can be supplemented with iron hematoxylin and immunofluorescent staining to enhance visualization.
- Stool Culture:
- Stool cultures are valuable, particularly for diagnosing chronic and asymptomatic cases. Media used include Boeck and Drbohlav media, NIH polygenic media, Craig’s medium, Nelson’s medium, and Robinson’s medium.
- Serodiagnosis:
- Serological tests are essential for detecting invasive amoebiasis. Various tests include:
- Indirect Hemagglutination (IHA) Test: A titer of 1:256 or greater indicates amoebic hepatitis.
- Latex Agglutination Test: Useful for antibody detection.
- Enzyme-Linked Immunosorbent Assay (ELISA): Commercial kits increasingly utilize ELISAs to identify E. histolytica antigens, offering greater sensitivity than microscopy and specific detection capabilities.
- It is important to note that serological tests may remain positive for years following successful treatment, complicating the interpretation in some cases.
- Serological tests are essential for detecting invasive amoebiasis. Various tests include:
- Molecular Diagnosis:
- Advanced techniques, including DNA probes and radioimmunoassays, are emerging for rapid and specific detection of E. histolytica in stool samples. These methods enhance diagnostic accuracy and speed.
- Diagnosis of Extraintestinal Amoebiasis:
- Microscopy: Examination of pus from liver abscesses can demonstrate trophozoites of E. histolytica, although they may not be present in all cases. Aspirates taken from the abscess periphery are more likely to contain the organism.
- Liver Biopsy: Trophozoites can also be identified in liver biopsy specimens, particularly in cases of hepatic amoebiasis or amoebic hepatitis.
- Serological Tests: Serological assays are valuable for diagnosing hepatic amoebiasis. Various tests, including IHA, latex agglutination, and ELISA, can assist in the diagnosis, although they may yield false-positive results.
- Imaging Techniques: Radiological examinations, such as X-rays, ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI), can detect space-occupying lesions in the liver, indicative of amoebic liver abscess. The diagnosis of an amoebic liver abscess is confirmed with imaging and a positive serological test.
Prevention/Prophylaxis of Entamoeba histolytica
Prophylaxis against Entamoeba histolytica infections is crucial in preventing the spread of amoebiasis, a disease characterized by intestinal and extraintestinal manifestations. Effective preventive measures primarily focus on minimizing exposure to fecal-oral transmission routes, as well as educating communities about safe practices.
- General Prophylactic Measures:
- Protecting food and water sources from contamination is essential. This involves:
- Ensuring proper sanitation and hygiene in food preparation areas.
- Using safe drinking water, preferably from treated or bottled sources.
- Washing fruits and vegetables thoroughly before consumption to eliminate potential contaminants.
- Protecting food and water sources from contamination is essential. This involves:
- Control of Human Carriers:
- Identifying and treating asymptomatic carriers of E. histolytica is vital in preventing outbreaks. This can be achieved through:
- Regular screening for E. histolytica among high-risk populations, including food handlers and individuals in close contact with affected persons.
- Excluding infected individuals from food handling and preparation roles until they have been effectively treated and cleared of the parasite.
- Identifying and treating asymptomatic carriers of E. histolytica is vital in preventing outbreaks. This can be achieved through:
- Health Education:
- Educating the public on proper hygiene practices plays a pivotal role in controlling the spread of infection. Effective strategies include:
- Promoting handwashing with soap and water, particularly after using the restroom and before food preparation or consumption.
- Providing information about the symptoms of amoebiasis, encouraging individuals to seek medical attention if symptoms develop.
- Facilitating awareness campaigns in communities to highlight the importance of safe food and water practices.
- Educating the public on proper hygiene practices plays a pivotal role in controlling the spread of infection. Effective strategies include:
- Environmental Sanitation:
- Improving sanitation infrastructure is essential for reducing the incidence of E. histolytica infections. This may involve:
- Ensuring adequate sewage disposal systems to prevent fecal contamination of water supplies.
- Implementing waste management strategies to reduce exposure to contaminated materials.
- Improving sanitation infrastructure is essential for reducing the incidence of E. histolytica infections. This may involve:
- Monitoring and Surveillance:
- Establishing systems for monitoring and surveillance of E. histolytica infections can aid in early detection and response to outbreaks. Effective measures include:
- Regular reporting and analysis of infection rates in specific communities or regions.
- Collaborating with health departments to track cases and implement prompt public health interventions.
- Establishing systems for monitoring and surveillance of E. histolytica infections can aid in early detection and response to outbreaks. Effective measures include:
- Behavioral Interventions:
- Encouraging the adoption of healthy personal habits can significantly decrease the risk of infection. Key interventions involve:
- Advising individuals to avoid consuming food from unsafe sources, such as street vendors or unregulated markets.
- Promoting the boiling or filtering of water in areas where water quality is questionable.
- Encouraging the adoption of healthy personal habits can significantly decrease the risk of infection. Key interventions involve:
Treatment
The treatment of Entamoeba histolytica infections, commonly known as amoebiasis, is multifaceted and involves various classes of antiamoebic drugs. The choice of treatment depends on the type of infection—whether it is intestinal, extraintestinal, or asymptomatic.
- Classification of Anti-Amoebic Drugs:
- Luminal Amoebicides: These agents act specifically within the intestinal lumen and are ineffective against systemic infections. Key luminal amoebicides include:
- Diloxanide furoate
- Iodoquinol
- Paromomycin
- Tetracycline
- Tissue Amoebicides: These drugs target systemic infections but are less effective in treating intestinal forms of amoebiasis. Notable tissue amoebicides are:
- Emetine
- Chloroquine
- For amoebic liver abscess, chloroquine is typically administered at a dosage of 1 gram for the first two days, followed by 5 grams daily for three weeks.
- Combined Action Amoebicides: Some medications possess the ability to act on both luminal and tissue infections. The primary drugs in this category include:
- Metronidazole
- Tinidazole
- Ornidazole
- These compounds are considered the first line of treatment for amoebic colitis and amoebic liver abscess due to their dual action.
- Luminal Amoebicides: These agents act specifically within the intestinal lumen and are ineffective against systemic infections. Key luminal amoebicides include:
- Recommended Treatment Protocol:
- Despite their effectiveness, metronidazole and tinidazole do not achieve high concentrations in the intestinal lumen. Therefore, to ensure complete eradication of the infection, it is recommended that patients with amoebic colitis or liver abscess also receive a luminal agent, such as paromomycin or iodoquinol. Paromomycin is often the preferred choice.
- The following table summarizes the recommended dosages and durations of antiamoebic drugs:
| Drug | Dosage | Duration (in days) |
|---|---|---|
| Tinidazole | 2 g/day orally | 3 |
| Metronidazole | 750 mg three times a day (or IV) | 5-10 |
| Paromomycin | 30 mg/kg, four times a day (divided doses) | 5-10 |
| Iodoquinol | 650 mg orally, three times a day | 20 |
- Asymptomatic Infection Treatment:
- Asymptomatic individuals with documented E. histolytica infection should be treated to mitigate the risks of developing amoebic colitis or amoebic liver abscess later and to reduce the potential for transmission to others. Treatment should involve luminal agents like paromomycin or iodoquinol, utilizing the dosages specified above.
- Supportive Care:
- In conjunction with pharmacological treatment, oral rehydration and electrolyte replacement should be administered as necessary, particularly in cases involving diarrhea or significant fluid loss.
- Considerations for Special Populations:
- Treatment regimens may need to be adjusted based on patient factors, including age, nutritional status, and presence of other medical conditions. Additionally, close monitoring is essential to assess the effectiveness of the chosen therapeutic approach and to identify any potential adverse effects.
Pathogenesis of Entamoeba histolytica
The pathogenesis of Entamoeba histolytica, a protozoan parasite responsible for amoebiasis, encompasses complex mechanisms that result in both intestinal and extraintestinal disease manifestations. Understanding these mechanisms is crucial for comprehending the varying clinical presentations associated with this organism.
- Entamoeba histolytica is primarily recognized for causing intestinal and extraintestinal amoebiasis. While infection can occur, the majority of individuals (approximately 90%) remain asymptomatic, with disease manifestations occurring in only about 10% of cases.
- The incubation period for E. histolytica infection is notably variable, typically ranging from 4 days to 4 months. This variability complicates the identification of initial infection and disease progression.
Intestinal Amoebiasis: Mechanisms of Pathogenesis
- Asymptomatic Presence:
- The trophozoites of E. histolytica reside in the intestinal lumen without causing illness unless they penetrate the intestinal mucosa.
- Strain Variation:
- Not all E. histolytica strains are pathogenic; the differentiation between invasive and non-invasive strains can be achieved using various techniques, including complement-mediated lysis tests, phagocytic assays, genetic markers, and monoclonal antibodies.
- Invasion Process:
- The pathogenic metacystic trophozoites penetrate the columnar epithelial cells within the crypts of Lieberkühn in the colon. This penetration is facilitated by:
- Motility: The trophozoites exhibit significant motility, aiding in their invasive capacity.
- Enzymatic Activity: The tissue-lytic enzyme histolysin contributes to mucosal damage, allowing the amoeba to invade deeper layers.
- Adherence Factors: Amoebic lectin serves as a crucial virulence factor, mediating adherence to host tissues.
- The pathogenic metacystic trophozoites penetrate the columnar epithelial cells within the crypts of Lieberkühn in the colon. This penetration is facilitated by:
- Lesion Formation:
- As trophozoites invade the intestinal wall, they induce the formation of discrete ulcers characterized by:
- Morphology: The ulcers typically present with a pinhead center and raised edges, forming a flask-shaped structure.
- Ulcer Development: Initial lesions can heal spontaneously; however, deeper penetration often leads to rapid replication, necrosis, and abscess formation. The lesions may break down, leading to the classic amoebic ulcer, which appears as multiple, superficial lesions primarily localized in the colon, especially in the cecum and sigmoid regions.
- As trophozoites invade the intestinal wall, they induce the formation of discrete ulcers characterized by:
- Clinical Manifestations:
- The spectrum of intestinal amoebiasis ranges from asymptomatic carriage to fulminant colitis, with amoebic dysentery being the most severe presentation.
- Symptoms typically include:
- Large, foul-smelling stools mixed with blood-streaked mucus.
- Characteristic presence of E. histolytica trophozoites containing ingested erythrocytes.
- In severe cases, patients may present with febrile illness and toxicity, particularly during fulminant colitis, characterized by confluent ulceration and necrosis of the colon.
Extraintestinal Amoebiasis: Complications and Spread
- Hepatic Involvement:
- The liver is the most frequently affected organ in cases of extraintestinal amoebiasis. Although trophozoites may reach the liver during an episode of dysentery, they only cause significant disease in a minority of cases (approximately 2–10% in endemic areas).
- Complications may include amoebic hepatitis, often without prior symptoms of intestinal disease. This occurs due to repeated invasion from the colon or the influence of toxic substances from the intestine.
- Liver Abscess Formation:
- Approximately 5–10% of individuals with intestinal amoebiasis develop liver abscesses, which typically contain necrotic liver tissue and exhibit a characteristic “anchovy sauce” pus.
- These abscesses can be solitary or multiple, usually located in the upper right lobe, and may lead to severe complications if untreated, including rupture into adjacent organs or the abdominal cavity.
- Pulmonary and Other Metastatic Involvement:
- Although rare, E. histolytica can spread hematogenously to the lungs, leading to pulmonary amoebiasis, often following the rupture of a liver abscess.
- Additional complications can include abscesses in other organs such as the brain, kidneys, and spleen, with potential for severe tissue destruction and fatal outcomes.
- Cutaneous and Genitourinary Involvement:
- Skin lesions may arise from direct extension of infection around the anus or from colostomy sites.
- Genitourinary amoebiasis can occur, especially in males, where lesions may resemble malignancy and affect various structures, including the penis and female genitalia.
Factors affecting virulence of Entamoeba histolytica
The virulence of Entamoeba histolytica is influenced by a combination of intrinsic factors related to the parasite itself and extrinsic factors associated with the host environment. Understanding these factors is essential for comprehending the organism’s pathogenic potential and the clinical manifestations of amoebiasis.
- Intrinsically Virulent Factors of E. histolytica:
- Amoebic Cystine Proteinase: This enzyme plays a crucial role in virulence by inactivating complement factor C3, a component of the immune response that normally aids in the destruction of pathogens. By neutralizing this factor, E. histolytica can evade host defenses, allowing for increased survival and infection.
- Amoebic Lectin: This virulence factor mediates the adherence of trophozoites to epithelial cells in the intestinal lining. The ability to attach firmly enhances the parasite’s capacity to invade host tissues, facilitating colonization and pathogenicity.
- Ionophore Protein: This protein may contribute to the virulence of E. histolytica by affecting ion transport across cell membranes, potentially influencing trophozoite survival in the harsh intestinal environment.
- Extrinsic Host Factors:
- Nutritional Status: Malnutrition can compromise the immune response, making individuals more susceptible to infections by E. histolytica. Insufficient nutrient availability may hinder the body’s ability to mount an effective defense against the parasite.
- Stress and Alcoholism: Both physical and psychological stressors can impair immune function, while alcohol consumption is known to affect liver health and overall immune response, thereby facilitating the course of infection.
- Corticosteroid Therapy: The use of corticosteroids suppresses the immune system, reducing the body’s ability to fight off infections, including those caused by E. histolytica.
- Immunodeficiency: Individuals with weakened immune systems, such as those with HIV/AIDS or other immunocompromising conditions, are at a higher risk for severe amoebic infections due to the lack of robust immune defenses.
- Colonic Environment:
- Mucosal Glycoproteins: Glycoproteins present in colonic mucus serve as a barrier to trophozoite attachment. Alterations in the composition and quality of this mucus can influence the attachment of the amoeba to epithelial cells, thus affecting the virulence of the organism.
- Bacterial Flora: The composition of the colonic microbiome can also impact virulence. Certain bacterial populations may inhibit or promote the colonization of E. histolytica, influencing the severity of infection.
- Zymodeme Classification:
- E. histolytica strains can be classified into at least 22 zymodemes based on electrophoretic mobility of specific isoenzymes, including acetylglucosaminidase, aldolase, hexokinase, NAD-diaphorase, peptidase, and phosphoglucomutase. Of these, only nine are known to be invasive, indicating that most strains are non-invasive commensals.
- Geographic distribution of these zymodemes highlights the variability of pathogenic strains, with nonpathogenic zymodemes being more common, accounting for approximately 90% of the population even in endemic areas.
- Species Classification:
- It has been suggested that pathogenic (E. histolytica) and nonpathogenic strains (potentially reclassified as E. dispar) are morphologically identical but represent distinct entities. The pathogenic strains are characterized by their ability to invade tissues, whereas nonpathogenic strains lack erythrocytes in their trophozoites and predominantly contain bacteria.
Epidemiology of entamoeba histolytica
The epidemiology of Entamoeba histolytica is crucial for understanding its impact on global health and its transmission dynamics. As the third leading cause of death from parasitic infections, this organism presents significant public health challenges, particularly in developing regions.
- Global Prevalence:
- E. histolytica infections are prevalent worldwide, with an estimated 50 million symptomatic cases annually, leading to approximately 100,000 deaths.
- Despite the high number of infections, about 90% of those infected remain asymptomatic, complicating efforts to control the spread.
- Pathogenic vs. Nonpathogenic Forms:
- Infected individuals may harbor either E. histolytica or E. dispar.
- E. histolytica is the pathogenic form, responsible for conditions such as amoebic colitis and extraintestinal amoebiasis.
- Conversely, E. dispar is generally nonpathogenic and does not cause observable disease.
- Geographical Distribution:
- The prevalence of E. histolytica is significantly higher in areas with low socioeconomic status and inadequate public health measures.
- High infection rates are reported in countries such as India, various regions of Africa, Mexico, and Central and South America.
- For instance, a study in Bangladesh indicated that 2.2% of dysentery cases in preschool children were due to E. histolytica.
- In rural Mexico, seroprevalence rates for E. histolytica have reached as high as 42%.
- Risk Factors for Infection:
- Transmission primarily occurs through the fecal-oral route, emphasizing the importance of hygiene.
- Common risk factors include poor hand hygiene practices, defecating in open water sources (e.g., rivers), and close proximity to animals that may carry the parasite.
- In developed countries, such as the United States, amebiasis is rare, accounting for about five deaths per year. Cases often occur among individuals who have traveled to endemic areas or who are immigrants from these regions.
- Demographic Vulnerability:
- Amoebic colitis affects both males and females across all age groups.
- Increased risks are noted among gay and bisexual males due to potential fecal-oral contamination during sexual activity.
- Factors contributing to more severe infections and higher mortality rates include:
- Pregnancy
- Use of corticosteroids
- Presence of malignancies
- Malnutrition
- Alcoholism
- Amoebic Liver Abscess:
- This condition disproportionately affects middle-aged men, particularly those between 18 and 50 years of age.
- The risk of developing a liver abscess is at least three times higher in this demographic, underscoring the need for targeted awareness and prevention strategies.
FAQ
How to prevent Entamoeba histolytica?
Prophylaxis of Entamoeba histolytica involves taking measures to prevent the transmission of the parasite. This includes practicing good hygiene, ensuring safe water and food, sanitizing surfaces, practicing safe sex, seeking medical care, and taking precautions when traveling to areas with poor sanitation.
How common is Entamoeba histolytica?
Entamoeba histolytica infection is estimated to affect up to 50 million people worldwide, with the highest prevalence in developing countries with poor sanitation and hygiene practices. It is more common in areas with inadequate access to clean water and proper sanitation facilities. The infection can cause a range of symptoms, from mild diarrhea to severe dysentery, and can be life-threatening if left untreated.
Where is Entamoeba histolytica found?
Entamoeba histolytica is found in areas with poor sanitation, where contaminated food or water can spread the parasite. It is commonly found in developing countries with inadequate sanitation and hygiene practices.
What does Entamoeba histolytica eat?
Entamoeba histolytica is a parasitic protozoan that feeds on bacteria and other microorganisms found in the intestine of its host, which can include humans and other animals.
What is Entamoeba histolytica?
Entamoeba histolytica is a parasitic protozoan that can cause amoebiasis, a disease that affects the intestines and can spread to other parts of the body.
How is Entamoeba histolytica transmitted?
Entamoeba histolytica is transmitted through ingestion of cysts, which are the dormant and infective form of the parasite. Cysts can contaminate food, water, or surfaces that come into contact with fecal matter.
What are the symptoms of Entamoeba histolytica infection?
Symptoms of Entamoeba histolytica infection can range from mild diarrhea to severe dysentery, and may include abdominal pain, bloody stools, and fever.
How is Entamoeba histolytica infection diagnosed?
Entamoeba histolytica infection can be diagnosed through stool samples, blood tests, and imaging studies such as ultrasound and CT scans.
What is the treatment for Entamoeba histolytica infection?
The treatment for Entamoeba histolytica infection typically involves a course of antibiotics and supportive care to manage symptoms.
Can Entamoeba histolytica infection be prevented?
Entamoeba histolytica infection can be prevented by practicing good hygiene, ensuring safe water and food, sanitizing surfaces, practicing safe sex, seeking medical care, and taking precautions when traveling to areas with poor sanitation.
Who is at risk for Entamoeba histolytica infection?
People who live in or travel to areas with poor sanitation and hygiene practices are at higher risk for Entamoeba histolytica infection.
Can Entamoeba histolytica infection be fatal?
In severe cases, Entamoeba histolytica infection can be life-threatening if left untreated or if it spreads to other parts of the body.
Is there a vaccine for Entamoeba histolytica infection?
There is currently no vaccine for Entamoeba histolytica infection.
How common is Entamoeba histolytica infection?
Entamoeba histolytica infection is estimated to affect up to 50 million people worldwide, with the highest prevalence in developing countries with poor sanitation and hygiene practices.
- https://www.onlinebiologynotes.com/entamoeba-histolytica-morphology-life-cycle-pathogenesis-clinical-manifestation-lab-diagnosis-treatment/
- https://universe84a.com/collection/entamoeba-histolytica/
- https://www.vedantu.com/biology/entamoeba-histolytica-life-cycle
- https://microbeonline.com/entamoeba-histolytica-life-cycle-diseases-laboratory-diagnosis/
- https://labpedia.net/amoebiasis-entamoeba-histolytica-life-cycle-diagnosis-and-intestinal-amoebas/
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/entamoeba
- https://cpha.tu.edu.iq/images/E.histolytica.pdf.pdf
- https://www.biologydiscussion.com/parasites/the-structure-and-life-cycle-of-entamoeba-with-diagram/2735
- http://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000035ZO/P000888/M027352/ET/1519016883M18Morphology,Lifecycle,PathogenecityEntamoebaPart1Quad1.pdf
- http://lamarck.unl.edu/Parasitology-UNL/Lecture/notes/Protista.pdf
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.