What is Eosin Methylene Blue (EMB) Agar?
- Eosin Methylene Blue (EMB) agar is a differential microbiological medium that is widely used in medical laboratories to distinguish between different types of gram-negative bacteria. It was first developed by Holt-Harris and Teague in 1916 and has undergone further modifications by Levine.
- The primary purpose of EMB agar is to differentiate between lactose fermenting and nonfermenting microbes. It contains lactose and sucrose as fermentable carbohydrates, allowing for the differentiation of organisms based on their ability to ferment these sugars. Lactose fermenters, such as Escherichia coli, produce colonies that appear black or have dark centers with transparent and colorless outer margins on the agar. On the other hand, nonfermenters of lactose and sucrose result in colorless colonies.
- The differentiation is achieved through the use of two indicator dyes, eosin, and methylene blue. These dyes inhibit the growth of gram-positive bacteria to some extent and act as differential indicators in response to carbohydrate fermentation. The ratio of eosin and methylene blue is adjusted to approximately 6:1 in order to provide optimal differentiation with minimal toxicity.
- EMB agar has shown to be more sensitive and stable compared to other agars used for similar purposes. It enables faster differentiation between sugar fermenters and nonfermenters. Additionally, it has the ability to differentiate between fecal and nonfecal types of the coli aerogenes group, as well as distinguish salmonellae and other nonlactose fermenters from coliforms.
- The medium consists of peptic digest of animal tissue, which serves as a source of carbon, nitrogen, and other essential growth nutrients. Phosphate is added to buffer the medium. The inclusion of sucrose as an alternative carbohydrate source is particularly useful for detecting typically lactose-fermenting, gram-negative bacilli that may not ferment lactose or do so slowly.
- When gram-negative bacteria that ferment lactose are present on the EMB agar, they produce purplish black colonies as a result of taking up the methylene blue-eosin dye complex when the pH drops. The dye complex is absorbed into the colony. Nonfermenters, on the other hand, raise the pH of the surrounding medium through oxidative deamination of protein, which solubilizes the methylene blue-eosin complex. This results in colorless colonies.
- It’s important to note that some strains of Salmonella and Shigella species do not grow in the presence of eosin and methylene blue. Therefore, further tests are required to confirm the identification of such isolates.
- To use EMB agar, the test sample can be directly streaked on the medium plates. The plates should be incubated, protected from light. It is recommended to inoculate a non-selective medium alongside EMB agar for comparison and to obtain isolated colonies. Confirmatory tests should be performed to identify the isolated colonies accurately.
- In summary, Eosin Methylene Blue (EMB) agar is a differential microbiological medium that slightly inhibits the growth of gram-positive bacteria and provides a color indicator to distinguish between lactose fermenting and nonfermenting gram-negative organisms. It combines the formulations of Holt-Harris and Teague and Levine, containing peptic digest of animal tissue, phosphate, lactose, sucrose, eosin, and methylene blue. The medium allows for rapid identification of gram-negative pathogenic microbes in medical laboratories.
Purpose of Eosin Methylene Blue (EMB) Agar
The purpose of Eosin Methylene Blue (EMB) agar is multifaceted and serves several important functions in microbiology.
- Selectivity for gram-negative bacteria: EMB agar is a selective medium that inhibits the growth of gram-positive bacteria, allowing for the isolation and differentiation of gram-negative bacteria. This selectivity is achieved through the inclusion of eosin and methylene blue dyes, which have limited inhibitory effects on gram-positive bacteria.
- Isolation and differentiation of gram-negative bacilli: EMB agar is particularly useful in isolating and distinguishing various types of gram-negative bacilli, including enteric bacilli and coliforms. Coliforms refer to a group of gram-negative bacteria commonly found in the intestines of warm-blooded animals, while fecal coliforms specifically indicate the presence of bacteria originating from fecal matter. By using EMB agar, microbiologists can differentiate between these types of bacteria based on their ability to ferment lactose.
- Differentiation of lactose fermenters: One of the primary purposes of EMB agar is to differentiate between lactose fermenting and nonfermenting bacteria. Bacteria that can ferment lactose produce colonies with distinct colors on EMB agar, while those unable to ferment lactose appear as colorless colonies. This differentiation is crucial in identifying and characterizing different bacterial species.
- Water quality testing: EMB agar is extensively used in water quality tests to detect and distinguish coliforms and fecal coliforms in water samples. The presence of these bacteria indicates possible contamination with pathogenic microorganisms. By observing the colony colors on EMB agar, microbiologists can assess the safety and quality of water sources.
- Differentiation within the colon-typhoid-dysentery group: EMB agar is also valuable for differentiating bacterial species within the colon-typhoid-dysentery group. Escherichia coli colonies exhibit a distinctive metallic sheen with a dark center when grown on EMB agar. Aerobacter aerogenes colonies, on the other hand, have a brown center. Nonlactose-fermenting gram-negative bacteria typically appear pink on EMB agar. These distinguishing characteristics aid in the identification and classification of specific bacterial strains.
Principle of Eosin Methylene Blue (EMB) Agar
The principle of Eosin Methylene Blue (EMB) agar revolves around its selective and differential properties, as well as the reactions of bacteria to the components of the medium.
EMB agar contains a combination of two dyes, eosin and methylene blue, in a ratio of 6:1. Gram-negative bacteria that ferment lactose produce acid as a byproduct, which lowers the pH of the medium. The acidic environment promotes the absorption of the dyes by the colonies, resulting in dark purple coloration. Certain lactose-fermenting bacteria exhibit flat, dark colonies with a green metallic sheen. Others produce larger, mucoid colonies that are typically purple only in the center.
In the case of E. coli, most strains display a characteristic green sheen. This is attributed to their rapid fermentation of lactose, leading to the production of strong acids, which lowers the pH of the medium. The formation of the green metallic sheen in E. coli is dependent on the rapid fermentation of lactose and the subsequent acid production.
On the other hand, lactose non-fermenters may increase the pH of the medium through deamination of proteins, preventing the absorption of the dyes. As a result, colonies of lactose non-fermenters appear either colorless or light lavender.
The medium also contains peptone, which serves as a source of carbon, nitrogen, and other essential growth nutrients. Lactose and sucrose are included as fermentable carbohydrates, providing energy for bacterial growth. Eosin Y and methylene blue function as differential indicators, while phosphate buffers the medium.
EMB agar has both selective and differential properties. It is selective for gram-negative bacteria, with methylene blue inhibiting the growth of gram-positive bacteria. The medium is particularly effective against most gram-positive bacteria due to the inhibitory effects of small amounts of methylene blue. Eosin, being pH-sensitive, changes from colorless to black under acidic conditions, aiding in differentiation.
The presence of lactose and sucrose in EMB agar allows for the differentiation of enteric bacteria based on their ability to ferment lactose. Lactose-fermenting gram-negative bacteria, including fecal coliforms, acidify the medium through lactose fermentation. Under acidic conditions, the dyes in the medium produce a dark purple complex, often accompanied by a green metallic sheen. This metallic green sheen is indicative of vigorous lactose and/or sucrose fermentation typical of fecal coliforms. Slower lactose fermentation results in a brown-pink coloration of growth. Non-lactose fermenters appear translucent or pink.
Composition of Eosin Methylene Blue (EMB) Agar
| Ingredients | Gms/liter |
| Peptic digest of animal tissue | 10.000 |
| Dipotassium phosphate | 2.000 |
| Lactose | 5.000 |
| Sucrose | 5.000 |
| Eosin – Y | 0.400 |
| Methylene blue | 0.065 |
| Agar | 13.500 |
Final pH (at 25°C): 7.2±0.2
Preparation of Eosin Methylene Blue (EMB) Agar
To prepare Eosin Methylene Blue (EMB) agar, the following steps can be followed:
- Weigh out 35.96 grams of EMB agar powder and add it to 1000 ml of distilled water. Ensure that the powder is evenly suspended in the water.
- Heat the mixture to boiling, allowing the medium to dissolve completely. It is important to heat the medium adequately to ensure proper dissolution.
- Sterilize the medium by autoclaving at 15 pounds of pressure (121°C) for 15 minutes. Be cautious not to overheat the medium during sterilization.
- After autoclaving, allow the medium to cool down to a temperature of 45-50°C. This temperature range is suitable for the subsequent steps.
- Shake the medium vigorously to facilitate the oxidation of methylene blue, which restores its blue color. This step also helps to suspend any flocculent precipitate present in the medium.
- If the EMB agar is going to be inoculated on the same day of preparation, it can be used without autoclave sterilization. However, if it will be used later, it should be properly stored and sterilized prior to use.
Precaution: Store the prepared EMB agar away from light to prevent photooxidation, which can affect the quality and performance of the medium.
Following these steps ensures the proper preparation and sterilization of EMB agar, making it ready for use in microbiological applications.
Inoculation Method on Eosin Methylene Blue (EMB) Agar
When inoculating Eosin Methylene Blue (EMB) agar, the following methods and guidelines can be followed:
- Allow the EMB agar plates to reach room temperature before proceeding with inoculation. This helps to prevent condensation on the agar surface.
- Ensure that the agar surface is dry before inoculating. Excess moisture can interfere with the growth and observation of colonies.
- Inoculate and streak the specimen onto the agar surface as soon as possible after collection. This helps to maintain the viability and integrity of the microorganisms being cultured.
- If the specimen to be cultured is on a swab, roll the swab over a small area of the agar surface. Then, using a sterile loop, streak the agar surface for isolation. This method helps to separate individual bacterial colonies for further analysis.
- Incubate the inoculated plates aerobically at a temperature of 35-37°C for 18-24 hours. This temperature range is optimal for the growth of many microorganisms. It is important to protect the plates from direct light during incubation to avoid potential interference with the dye reactions.
- After the incubation period, examine the plates for colonial morphology. Observe the size, shape, color, and other characteristics of the colonies that have grown on the agar surface. These characteristics can provide valuable information for the identification and classification of the microorganisms.
- If there are no visible colonies after 24 hours of incubation, reincubate the plates for an additional 24 hours. This extended incubation period allows for the detection of slower-growing or fastidious microorganisms that may require more time to grow and form visible colonies.
Result Interpretation on EMB Agar – Colony Morphology
Interpreting the results of EMB agar based on colony morphology and color changes can provide valuable information about the bacteria present. Here is a general guide to result interpretation on EMB agar:
- Absence of growth:
- No bacterial growth may indicate the absence of bacteria in the sample.
- It could also suggest the presence of gram-positive bacteria, as they are inhibited by the selective components of the medium.
- Certain bacteria with specific growth requirements may not grow on EMB agar.
- Growth on EMB agar:
- If there is growth on EMB agar, observe the color of the colonies for further interpretation.
- Strong lactose fermenters (e.g., E. coli):
- These bacteria typically produce colonies with an intense green metallic sheen.
- The colonies appear blue-black with a greenish sheen, which is a characteristic feature of E. coli on EMB agar.
- Lesser lactose fermenters:
- Colonies exhibiting a pink color indicate a lesser degree of lactose fermentation compared to strong fermenters.
- The pink color may suggest the presence of bacteria with lower lactose fermenting abilities.
- Non-lactose fermenters:
- Colorless colonies on EMB agar generally indicate non-lactose fermenting bacteria.
- Organisms such as Salmonella, Shigella, and Pseudomonas typically produce colorless colonies on EMB agar.
- Gram-positive bacteria:
- Gram-positive bacteria may show inhibition or partial to complete absence of growth on EMB agar.
- The selective components of the medium suppress the growth of gram-positive bacteria.
It is important to note that these observations provide initial indications and should be followed by confirmatory tests or additional identification methods to accurately identify the specific bacterial species present.
By interpreting the colony morphology and color changes on EMB agar, microbiologists can gather preliminary information about the potential lactose fermenting abilities and microbial composition of the sample.
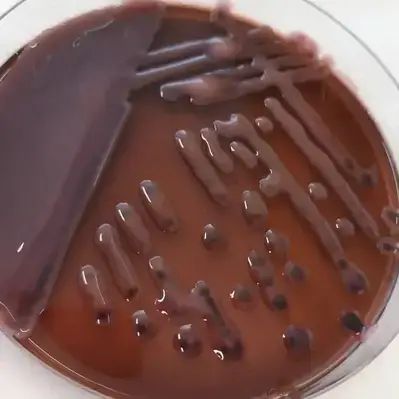
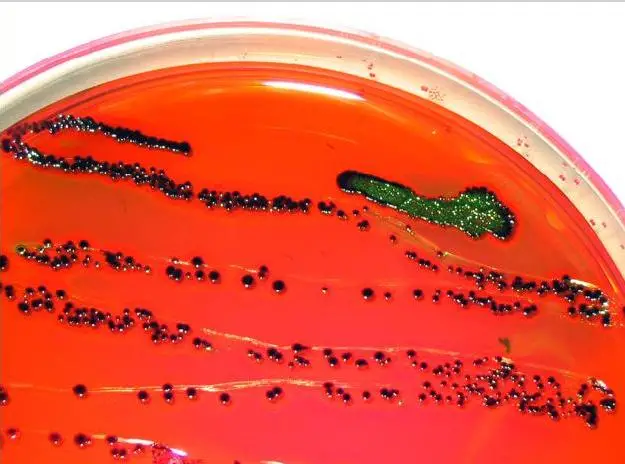
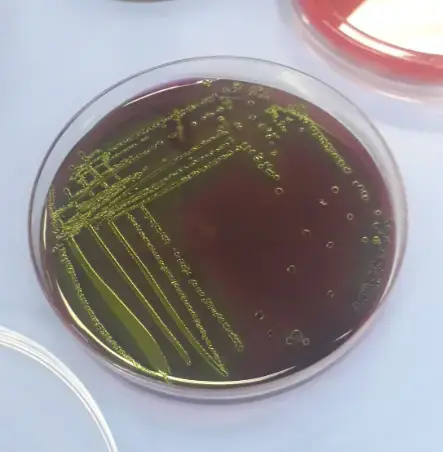
| Organisms | Growth |
| Escherichia coli | Blue-black bull’s eye; may have a green metallic sheen |
| Pseudomonas aeruginosa | Colorless |
| Enterobacter aerogenes | Good growth; pink, without sheen |
| Klebsiella pneumoniae | Pink, mucoid colonies |
| Proteus mirabilis | Luxuriant growth; colorless colonies |
| Salmonella Typhimurium | Luxuriant growth; colorless colonies |
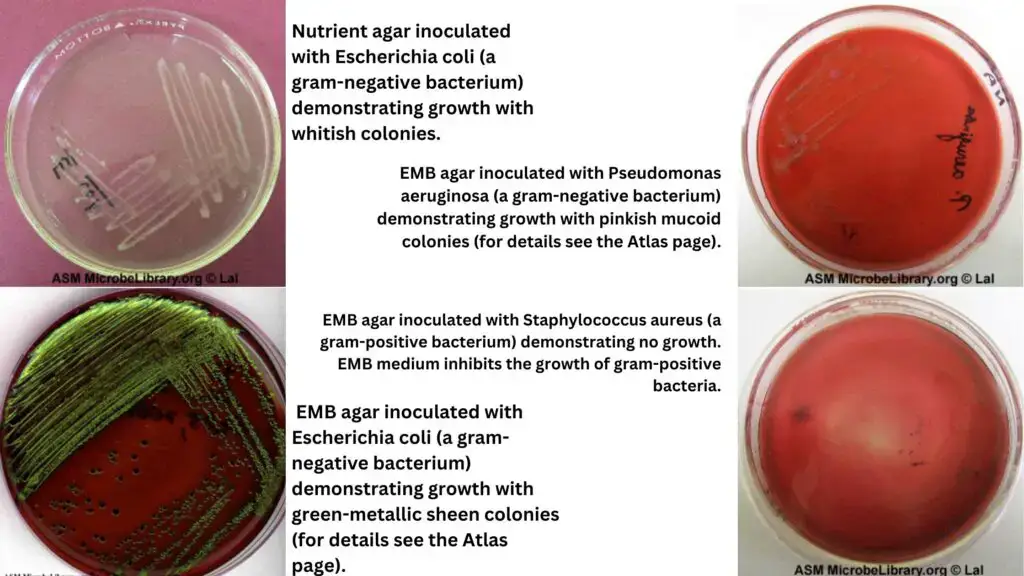
Quality Control of EMB agar
Quality control of Eosin Methylene Blue (EMB) agar involves assessing various parameters to ensure its performance and reliability. Here are some aspects of quality control for EMB agar:
- Appearance: EMB agar should have a light pink to purple homogeneous free-flowing powder appearance. Any deviation from this may indicate a potential quality issue.
- Gelling: The agar should gel firmly and have a comparable consistency to a 1.35% Agar gel. This ensures that the medium solidifies properly and provides a suitable surface for bacterial growth.
- Colour and clarity of prepared medium: The prepared EMB agar should have a reddish-purple color with an opalescent gel appearance. It may also have a greenish cast and a finely dispersed precipitate in Petri plates. These visual characteristics are indicative of a properly prepared medium.
- Reaction: A 3.6% w/v aqueous solution of EMB agar should exhibit a pH of 7.2±0.2 at 25°C. This pH range is essential for maintaining the optimal conditions for bacterial growth and differentiation on the medium.
- pH: The pH of the prepared EMB agar should fall within the range of 7.00-7.40. Deviations from this range may affect the performance and interpretation of bacterial growth.
- Cultural response: EMB agar should be tested with specific bacterial strains to assess its ability to support their growth and exhibit characteristic colony appearances. The cultural response should be observed after incubation at 35-37°C for 18-24 hours.
- Organism inoculum: Specific bacterial strains, such as Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Salmonella Typhimurium, and Staphylococcus aureus, should be inoculated onto EMB agar with defined inoculum sizes (CFU).
- Growth recovery: The medium should support good growth recovery for the tested strains. The observed colony growth should align with the expected characteristics of the organisms being tested.
- Colour of colony: The colonies formed by the tested strains should exhibit the appropriate colors associated with their respective characteristics on EMB agar. For example, Escherichia coli should display purple colonies with a black center and a green metallic sheen.
- Inhibition: EMB agar should effectively inhibit the growth of certain organisms, such as Staphylococcus aureus, as indicated by minimal or no visible growth.
By evaluating these quality control parameters, laboratories can ensure the consistency, reliability, and performance of EMB agar, enabling accurate and reliable results in microbiological testing.
Uses of Eosin Methylene Blue (EMB) Agar
EMB agar (Eosin Methylene Blue agar) has several uses in microbiology, as outlined below:
- Isolation and differentiation of gram-negative enteric bacteria: EMB agar is recommended for the isolation and differentiation of gram-negative enteric bacteria from various clinical and nonclinical specimens. It allows for the selective growth of these bacteria and helps distinguish them from other types of microorganisms.
- Differentiation between gram-positive and gram-negative bacteria: EMB agar can be utilized to differentiate between gram-positive and gram-negative bacteria. The medium selectively supports the growth of gram-negative bacteria while inhibiting the growth of gram-positive bacteria.
- Isolation and differentiation of enteric and gram-negative bacilli: EMB agar is particularly useful in isolating and differentiating enteric bacilli and other gram-negative bacilli. It allows for the identification of specific bacterial species and aids in their classification.
- Water quality testing: EMB agar is employed in testing the quality of water, specifically for determining if the water is contaminated by harmful microorganisms. By inoculating water samples on EMB agar plates and observing the growth and characteristics of colonies, microbiologists can assess the presence of coliforms and fecal coliforms, which indicate potential contamination.
- Differentiation within the colon-typhoid-dysentery group: EMB agar assists in the differentiation of microorganisms within the colon-typhoid-dysentery group. This includes distinguishing between Escherichia coli, nonpathogenic lactose-fermenting enteric gram-negative rods, and bacteria from the Salmonella and Shigella genera. The medium provides distinctive colony appearances and growth characteristics for each group, aiding in their identification.
- Differentiation of lactose fermenters: EMB agar is valuable in the isolation and differentiation of lactose fermenting and non-lactose fermenting enteric bacilli. It allows for the identification of bacteria based on their ability to ferment lactose, providing useful information in the study of specific microbial groups.
In summary, EMB agar finds wide application in microbiology laboratories. Its uses include the isolation and differentiation of gram-negative enteric bacteria, differentiation between gram-positive and gram-negative bacteria, testing water quality, differentiation within specific bacterial groups, and differentiation of lactose fermenters. This versatile medium aids in the identification and classification of various microorganisms, contributing to our understanding of their characteristics and potential pathogenicity.
Limitations of Eosin Methylene Blue (EMB) Agar
Despite its many uses, EMB agar has certain limitations that should be considered. These limitations are discussed below:
- Non-selectivity: EMB agar is not a selective medium on its own. Therefore, it is recommended to inoculate a non-selective medium alongside EMB agar to ensure the growth of a wide range of microorganisms and avoid missing potential pathogens or non-lactose-fermenting organisms.
- Further testing required for identification: While EMB agar provides valuable information about the morphology and characteristics of colonies, additional biochemical, immunological, molecular, or mass spectrometry testing is necessary to obtain complete identification of the isolated organisms.
- Some strains of Salmonella and Shigella may not grow: Certain strains of Salmonella and Shigella may fail to grow on EMB agar, potentially leading to false-negative results. Therefore, alternative media or tests may be required for the detection of these organisms.
- Growth of gram-positive bacteria: EMB agar may support the growth of gram-positive bacteria such as enterococci, staphylococci, and yeast, which can form pinpoint colonies. This can make the differentiation of these organisms from gram-negative pathogens more challenging and necessitates additional tests for accurate identification.
- Non-pathogenic, non-lactose-fermenting organisms: Non-pathogenic organisms that do not ferment lactose can still grow on EMB agar, making it necessary to perform additional biochemical tests to distinguish them from potentially pathogenic strains.
- Serial inoculation for adequate isolation: In cases where mixed flora samples are present, serial inoculation may be required to ensure proper isolation of individual microbial species. This step helps prevent the overgrowth of dominant organisms and increases the chance of isolating rarer or slower-growing species.
- Lack of characteristic green metallic sheen in some strains of E. coli: While a characteristic green metallic sheen is often associated with E. coli colonies on EMB agar, some strains may not produce this sheen. Therefore, the absence of the green metallic sheen should not be solely relied upon as a diagnostic feature for E. coli identification.
Understanding these limitations is important for accurate interpretation of results when using EMB agar and to ensure that appropriate supplementary tests are performed for comprehensive microbial identification.
FAQ
What is EMB Agar?
EMB agar is a selective and differential microbiological medium commonly used for the isolation and differentiation of gram-negative enteric bacteria.
How does EMB Agar differentiate lactose fermenters?
Lactose fermenters produce colonies with dark centers and transparent outer margins on EMB agar. Some may exhibit a green metallic sheen, while others have larger mucoid colonies.
How does EMB Agar differentiate non-lactose fermenters?
Non-lactose fermenters on EMB agar do not produce acid from lactose fermentation. They may appear colorless, light lavender, or have different colony characteristics depending on the species.
Is EMB Agar selective?
EMB agar is selective for gram-negative bacteria. The inclusion of dyes like methylene blue inhibits the growth of gram-positive bacteria to a limited extent.
What is the purpose of using EMB Agar?
EMB agar helps differentiate between lactose fermenting and non-fermenting bacteria and aids in the identification and classification of gram-negative bacteria, particularly enteric bacteria.
Can EMB Agar be used for water testing?
Yes, EMB agar is commonly used in water quality testing to detect and differentiate coliforms and fecal coliforms, which may indicate the presence of harmful microorganisms.
Are there any limitations to using EMB Agar?
Yes, some limitations include the need for additional tests for complete identification, the potential for non-lactose fermenters and gram-positive bacteria to grow, and the variability in colony characteristics among different bacterial species.
How should EMB Agar be inoculated?
It is recommended to streak the specimen on EMB agar as soon as possible after collection using a swab or loop. Plates should be incubated aerobically at the appropriate temperature.
What does EMB Agar contain?
EMB agar contains peptone, lactose, sucrose, eosin Y, methylene blue, and phosphate. These components serve as sources of nutrients, energy, and differential indicators.
How long should EMB Agar be incubated?
Typically, EMB agar plates are incubated for 18-24 hours at 35-37°C. If no colonies are observed after 24 hours, an additional 24-hour incubation may be necessary.
References
- https://asm.org/ASM/media/Protocol-Images/Eosin-Methylene-Blue-Agar-Plates-Protocol.pdf?ext=.pdf
- https://exodocientifica.com.br/_technical-data/M317.pdf
- https://microbeonline.com/eosin-methylene-blue-emb-agar-composition-uses-colony-characteristics/
- https://www.austincc.edu/microbugz/eosin_methylene_blue_agar.php
- https://microbiologie-clinique.com/emb-agar.html
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.