What is the Electron Transport Chain?
- The Electron Transport Chain (ETC) is a critical biochemical pathway that drives the production of adenosine triphosphate (ATP), the primary energy currency in cells. It operates as the final stage of cellular respiration and involves a sequence of redox reactions, where electrons are transferred from donors like NADH and FADH₂ to acceptors, ultimately generating energy.
- In eukaryotes, the ETC resides within the inner mitochondrial membrane. It comprises four large protein complexes, often referred to as Complexes I to IV, along with associated molecules. These complexes facilitate the flow of electrons derived from the oxidation of nutrients such as carbohydrates, fats, and proteins. The energy released during these redox reactions is used to pump protons (H⁺ ions) across the inner mitochondrial membrane, creating an electrochemical gradient.
- This proton gradient, known as the proton-motive force, is essential for ATP synthesis. The protons flow back into the mitochondrial matrix through ATP synthase, a specialized enzyme that utilizes this movement to convert ADP and inorganic phosphate (Pi) into ATP. This process is termed oxidative phosphorylation.
- Oxygen plays a vital role in aerobic respiration as the terminal electron acceptor, combining with electrons and protons to form water. In anaerobic conditions, other molecules like sulfate may serve as the final electron acceptors, though the efficiency of ATP production is reduced.
- The ETC’s function is not confined to cellular respiration. In photosynthetic organisms, a similar process occurs within the thylakoid membranes of chloroplasts. Here, light energy excites electrons, which then travel through a specialized ETC, generating a proton gradient used for ATP synthesis. Unlike mitochondrial ETC, this energy is primarily used to build carbohydrates rather than release it.
- The ETC is indispensable for energy metabolism, linking the oxidation of biomolecules to ATP production. Its efficient operation supports vital cellular activities, making it a cornerstone of both aerobic and photosynthetic energy systems.
Electron transport chain Definition
The electron transport chain is a series of protein complexes and molecules located in membranes, where electrons are transferred through redox reactions to generate a proton gradient that drives ATP synthesis via oxidative phosphorylation.
Electron Transport Chain Animation Video
Fundamentals of the Electron Transport Chain
The Electron Transport Chain (ETC) plays a key role in energy production within cells, both in cellular respiration and photosynthesis. It involves a series of protein complexes that transfer electrons through redox reactions, creating an electrochemical gradient that drives ATP synthesis.
- Location of the ETC
The electron transport chain is located in the inner mitochondrial membrane in eukaryotic cells. In photosynthetic organisms, it can be found in the thylakoid membrane of chloroplasts. - Role in Cellular Respiration
The ETC is the final stage of aerobic cellular respiration, following glycolysis and the citric acid cycle. During these earlier steps, glucose is broken down into pyruvate, which is then converted into acetyl-CoA. The resulting NADH and FADH₂ molecules are key electron donors for the ETC. - Electron Transport and Redox Reactions
The chain is composed of four main protein complexes (I-IV), which facilitate the transfer of electrons from NADH and FADH₂ to oxygen. These redox reactions release energy, which is harnessed to pump protons (H⁺) across the inner mitochondrial membrane, creating an electrochemical gradient. - Chemiosmosis and ATP Synthesis
The proton gradient created by the electron transport chain generates potential energy. This proton-motive force is used by the ATP synthase complex to convert ADP and inorganic phosphate (Pi) into ATP, a process known as chemiosmosis. - Role of Oxygen
Oxygen acts as the final electron acceptor in aerobic respiration. When electrons reach the end of the chain, they combine with protons and oxygen to form water. This is why oxygen is essential for the production of ATP in aerobic organisms. - Energy Production Efficiency
The energy released by the redox reactions is used to pump protons into the intermembrane space, establishing a proton gradient. This proton-motive force is critical for the synthesis of a large amount of ATP, which powers various cellular activities. - Function in Photosynthesis
In photosynthetic organisms, a similar electron transport chain operates within the thylakoid membrane of chloroplasts. Here, light energy excites electrons, which travel through the ETC to generate a proton gradient. This gradient is then used to produce ATP, which is essential for the synthesis of sugars in the light-independent reactions.
Electron Transport Chain Location
The electron transport chain (ETC) operates within the mitochondria, the powerhouse of eukaryotic cells. Its precise location within this organelle is vital for its function in energy production. The unique structure of the mitochondrion enables efficient electron transfer and ATP synthesis.
- Mitochondrion Structure:
- Enclosed by a double membrane: an outer membrane and an inner membrane.
- The inner membrane is folded into ridges called cristae, maximizing surface area for the ETC.
- Inside, two compartments are formed: the matrix and the intermembrane space.
- Membrane Characteristics:
- The outer membrane: Highly permeable to ions and houses enzymes crucial for the citric acid cycle.
- The inner membrane: Impermeable to most ions, contains the ETC components, and is equipped with ATP-synthesizing enzymes.
- Cell-Specific Adaptations:
- The number of ETC complexes varies by cell type and function.
- In liver mitochondria, approximately 10,000 ETC sets are present.
- Heart mitochondria contain three times as many ETC complexes as liver mitochondria, reflecting the heart’s higher energy demand.
- Compartments of the Mitochondrion:
- Intermembrane space: Contains enzymes like adenylate kinase.
- Matrix: Rich in molecules such as ATP, ADP, AMP, NAD, NADP, and essential ions like calcium (Ca²⁺) and magnesium (Mg²⁺).
Electron Transport Chain Components/Electron Carriers
Electron carriers play a central role in the electron transport chain by transferring electrons step by step from substrates to oxygen, releasing energy in the process. These carriers consist of chemical groups and proteins that facilitate redox reactions essential for ATP production.
- Flavin Mononucleotide (FMN)
- The chain begins with the transfer of electrons from NADH to flavin mononucleotide (FMN).
- FMN is reduced to FMNH₂ during this process:
NADH + H⁺ + FMN → NAD⁺ + FMNH₂ - This reaction is catalyzed by NADH dehydrogenase.
- The electrons are then passed to iron-sulfur complexes (Fe-S), which have a higher electron affinity.
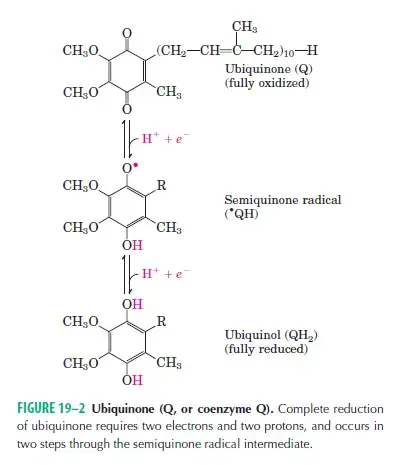
- Ubiquinone (Coenzyme Q)
- Ubiquinone (UQ) bridges flavoproteins and cytochromes within the chain.
- Unlike other carriers, ubiquinone is not bound to a protein, allowing it to move freely between different complexes.
- Electrons from FMNH₂ are transferred to ubiquinone via Fe-S centers, forming UQH₂ and regenerating FMN:
FMNH₂ + UQ → FMN + UQH₂
- Cytochromes
- Cytochromes are heme-containing proteins that transport electrons sequentially from ubiquinone to oxygen.
- Each cytochrome transfers only one electron, unlike FMN and ubiquinone, which can transfer two.
- There are five types of cytochromes, labeled a, b, c, and so on, based on their light absorption properties.
- These cytochromes are organized in a specific order, enabling efficient electron flow to molecular oxygen.
Electron Transport Chain Components/Electron Carriers
| Component | Role | Reaction/Process | Unique Characteristics |
|---|---|---|---|
| Flavin Mononucleotide (FMN) | Initial electron acceptor from NADH. | NADH + H⁺ + FMN → NAD⁺ + FMNH₂ | Reduces to FMNH₂; transfers electrons to Fe-S clusters. |
| Iron-Sulfur Complexes (Fe-S) | Intermediate electron carriers. | Accepts electrons from FMNH₂ and passes them to ubiquinone. | High electron affinity; tightly bound to proteins. |
| Ubiquinone (Coenzyme Q) | Mobile carrier between complexes. | FMNH₂ + UQ → FMN + UQH₂ | Lipid-soluble; freely moves between protein complexes. |
| Cytochromes | Sequential electron transfer to oxygen. | Transfers single electrons from ubiquinone to oxygen. | Heme-containing; categorized as a, b, c, etc. |
Electron Transport Chain Complexes
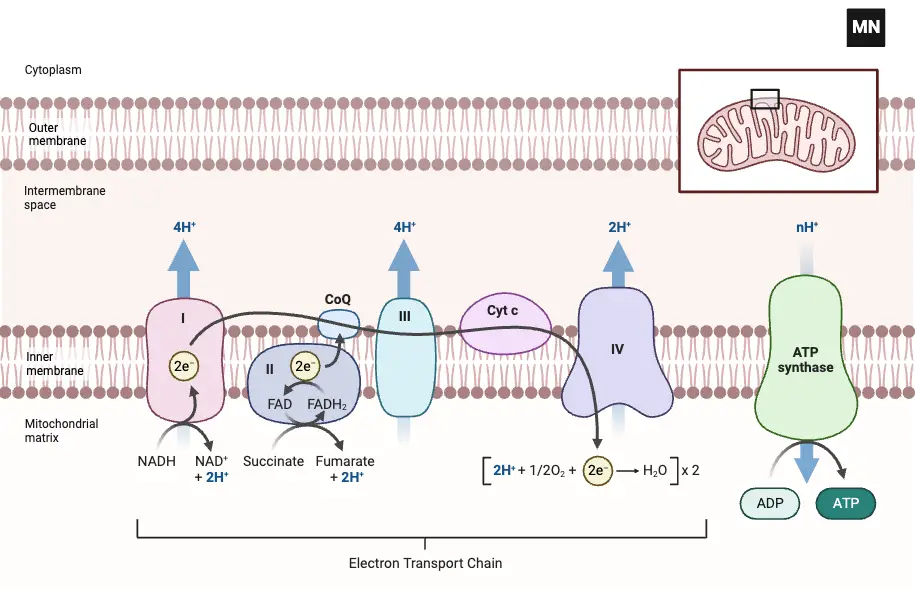
The electron transport chain (ETC) is a critical component of cellular respiration. It consists of four major protein complexes embedded in the inner mitochondrial membrane, which transfer electrons and create a proton gradient essential for ATP production.
Each complex plays a specific role in electron transfer, and together, they work to transport electrons from NADH and FADH2 to molecular oxygen, producing water. Here’s how each complex functions:
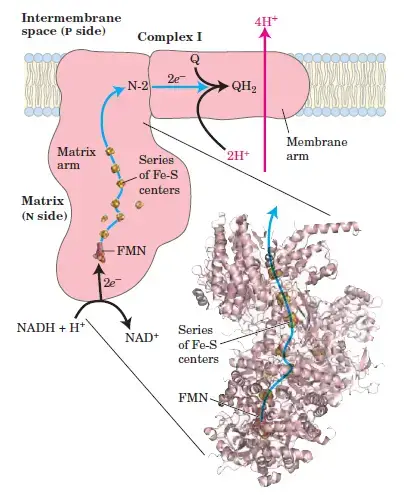
1. Complex I: NADH-Coenzyme Q Oxidoreductase
- Role: Complex I is the first step in the electron transport chain, accepting electrons from NADH and transferring them to ubiquinone (CoQ).
- Structure: It consists of NADH dehydrogenase, flavin mononucleotide (FMN), iron-sulfur (Fe-S) clusters, and coenzyme Q (CoQ).
- Electron Transfer Process:
- NADH donates two electrons to FMN, reducing it to FMNH2.
- FMNH2 then transfers the electrons to Fe-S clusters, which pass them to CoQ, reducing it to ubiquinol (CoQH2).
- Proton Pumping: As electrons are transferred, four protons (H+) are pumped across the membrane into the intermembrane space, contributing to the proton gradient used to synthesize ATP.
- Reaction:
- NADH + UQ + 5H⁺ → NAD⁺ + UQH₂ + 4H⁺
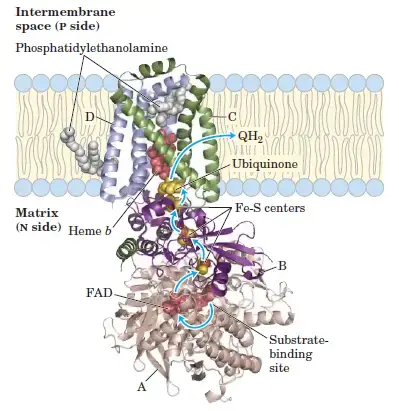
2. Complex II: Succinate-Coenzyme Q Oxidoreductase
- Role: Complex II functions parallel to Complex I but is connected to the Krebs cycle. It does not contribute to proton pumping, unlike the other complexes.
- Structure: It consists of succinate dehydrogenase, FAD, and iron-sulfur centers.
- Electron Transfer Process:
- Succinate, an intermediate in the Krebs cycle, is oxidized to fumarate, while FAD is reduced to FADH₂.
- The electrons from FADH₂ are transferred to Fe-S centers, then to CoQ, reducing it to CoQH₂.
- Proton Pumping: Complex II does not pump protons, meaning it has a lower ATP yield than Complex I.
- Reaction:
- Succinate + FADH₂ + CoQ → Fumarate + FAD⁺ + CoQH₂
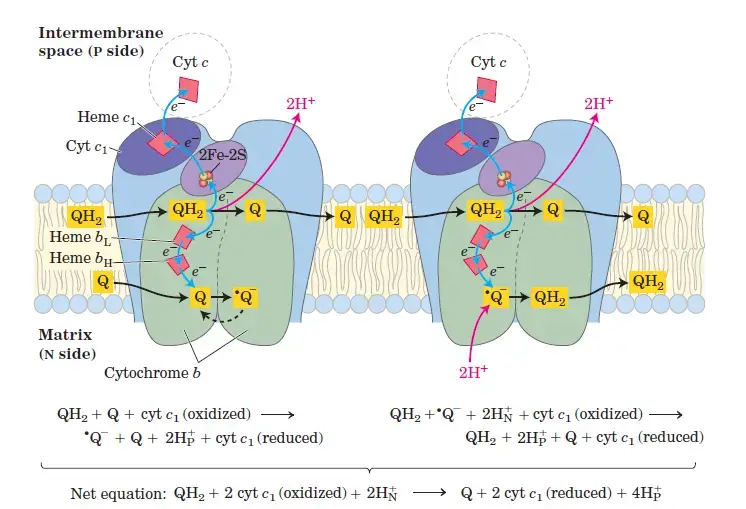
3. Complex III: Cytochrome bc1 Oxidoreductase
- Role: Complex III is responsible for transferring electrons from ubiquinol (CoQH₂) to cytochrome c.
- Structure: It contains cytochrome b, cytochrome c₁, a Rieske center (Fe-S), and heme prosthetic groups.
- Electron Transfer Process:
- Ubiquinol (CoQH₂) donates two electrons. One electron is transferred to cytochrome c₁, which passes it to cytochrome c. The second electron is transferred to cytochrome b, which passes it back to CoQ, regenerating ubiquinol.
- This occurs through the Q cycle, where one ubiquinol molecule is oxidized, and one is reduced, maintaining electron flow.
- Proton Pumping: Four protons are pumped across the membrane for each Q cycle, helping establish the proton gradient.
- Reaction:
- CoQH₂ + 2 Cyt c (Fe³⁺) → CoQ + 2 Cyt c (Fe²⁺) + 4H⁺
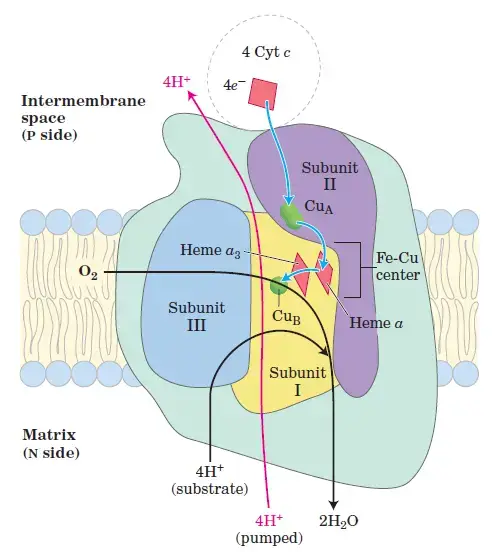
4. Complex IV: Cytochrome c Oxidase
- Role: Complex IV is the final step in the electron transport chain, where electrons are transferred to molecular oxygen, reducing it to water.
- Structure: It contains cytochrome a, cytochrome a₃, copper centers CuA and CuB.
- Electron Transfer Process:
- Electrons from cytochrome c (Fe²⁺) are transferred to cytochrome a and a₃, ultimately reducing oxygen (O₂) to water (H₂O).
- Four electrons are required to fully reduce one oxygen molecule to two water molecules.
- Proton Pumping: Four protons are pumped across the membrane for every oxygen molecule reduced.
- Reaction:
- 4 Cyt c (Fe²⁺) + O₂ → 4 Cyt c (Fe³⁺) + 2 H₂O
Complexes of the Electron Transport Chain
| Complex | Name | Key Components | Function | Proton Transport | Reaction |
|---|---|---|---|---|---|
| Complex I | NADH-Coenzyme Q Oxidoreductase | NADH dehydrogenase, FMN, Fe-S clusters, CoQ | Transfers electrons from NADH to CoQ, reducing it to UQH₂. | Pumps 4 protons into the intermembrane space. | NADH + UQ + 5H⁺ → NAD⁺ + UQH₂ + 4H⁺ |
| Complex II | Succinate-Coenzyme Q Oxidoreductase | Succinate dehydrogenase, FADH₂, Fe-S clusters, CoQ | Transfers electrons from FADH₂ to CoQ via Fe-S clusters. | No protons pumped. | Succinate + UQ → Fumarate + UQH₂ |
| Complex III | Cytochrome bc₁ Oxidoreductase | Cytochrome b, cytochrome c₁, Rieske iron-sulfur center | Transfers electrons from UQH₂ to cytochrome c via the Q-cycle, pumping protons. | Pumps 4 protons into the intermembrane space. | UQH₂ + 2Cytc + 2H⁺ → UQ + 2Cytc + 4H⁺ |
| Complex IV | Cytochrome c Oxidase | Cytochromes a and a₃, copper centers CuA and CuB | Transfers electrons from cytochrome c to oxygen, reducing it to water, and pumps protons. | Pumps 2 protons into the intermembrane space per electron pair. | 4Cytc + O₂ + 4H⁺ → 4Cytc + 2H₂O + 2H⁺ |
Electron Transport Chain Diagram
Electron Transport Chain Equation
The electron transport chain involves a sequence of oxidation-reduction reactions that release energy. The energy released during these reactions is harnessed to synthesize ATP, a crucial process for cellular functions.
- Reactants:
- NADH: Donates electrons to the chain.
- Oxygen (O₂): Final electron acceptor.
- Protons (H⁺): Contribute to the electrochemical gradient.
- ADP: Converted to ATP.
- Inorganic phosphate (Pi): Combines with ADP to form ATP.
- Products:
- NAD⁺: Regenerated form of NADH.
- ATP: Synthesized through oxidative phosphorylation.
- Water (H₂O): Produced when oxygen accepts electrons and combines with protons.
The General Equation:
NADH + 1/2O₂ + H⁺ + ADP + Pi → NAD⁺ + ATP + H₂O
This equation captures the essential chemical process of electron transfer, where NADH and oxygen are involved, ATP is produced, and water is formed. The energy released during electron transfer is used to generate a proton gradient across the mitochondrial membrane, which drives ATP synthesis.
Electron Transport Chain Steps
The electron transport chain (ETC) consists of a series of reactions that transfer electrons from NADH and FADH2 to molecular oxygen. This process helps generate ATP through oxidative phosphorylation.
- Step 1: NADH to Ubiquinone (CoQ)
- NADH is produced in several metabolic pathways like the TCA cycle, β-oxidation, and pyruvate dehydrogenase.
- NADH moves from the mitochondrial matrix to the intermembrane space.
- Complex I (NADH dehydrogenase) facilitates electron transfer from NADH to FMN in the intermembrane space.
- FMN transfers the electrons to Fe-S centers, one electron at a time.
- The electrons are eventually passed to ubiquinone (CoQ), forming semiquinone and then ubiquinol (CoQH2).
- This electron transfer generates energy, which is used to pump protons (H+) across the membrane.
- The proton gradient is utilized by ATP synthase to generate ATP as protons flow back into the matrix.
- Step 2: FADH2 to CoQ
- FADH2 is generated by the oxidation of succinate to fumarate in the TCA cycle.
- FADH2 transfers its electrons to CoQ through Complex II (succinic dehydrogenase), which contains Fe-S centers.
- Unlike Complex I, Complex II does not pump protons across the membrane.
- Electrons from FADH2 reach CoQ, contributing to the electron flow through the chain.
- Step 3: CoQH2 to Cytochrome c
- The reduced CoQH2 donates electrons to Complex III (cytochrome reductase).
- The electrons pass through cytochrome b and c1, and eventually to cytochrome c.
- During this transfer, Fe3+ in cytochrome is reduced to Fe2+.
- For each NADH molecule oxidized, two cytochromes are reduced.
- Energy released during electron transfer pumps protons across the membrane, contributing to the proton gradient.
- Protons then flow back through ATP synthase, producing ATP.
- Step 4: Cytochrome c to Molecular Oxygen
- In the final step, Complex IV (cytochrome oxidase) catalyzes the transfer of electrons from cytochrome c to molecular oxygen.
- Oxygen is reduced to water as it accepts two electrons, with half a molecule of oxygen reduced for each NADH oxidized.
- The Fe2+ in cytochrome c is oxidized back to Fe3+.
- Energy released during this step is used to pump protons across the membrane.
- As protons flow back to the matrix through ATP synthase, ATP is produced.
The transport of electrons from cytoplasmic NADH to the mitochondrial electron transport chain (ETC) is a critical step in cellular respiration. Since intact mitochondrial membranes are impermeable to NADH and NAD+, the cell has developed mechanisms to shuttle electrons into the mitochondria for ATP production.
- Cytoplasmic NAD+ Regeneration
In order to maintain glycolysis, which relies on the continuous regeneration of NAD+ in the cytoplasm, electrons from NADH must be transferred into the mitochondria. This is accomplished through two main shuttle systems: the malate-aspartate shuttle and the glycerol 3-phosphate shuttle. - Malate-Aspartate Shuttle
- Process Overview: In this system, electrons from NADH are first transferred to oxaloacetate (OAA) in the cytoplasm, forming malate (Mal).
- Crossing the Mitochondrial Membrane: Malate then crosses the mitochondrial membrane via an antiporter that exchanges malate for α-ketoglutarate (α-KG).
- Inside the Mitochondria: Once inside, malate donates its electrons to NAD+, regenerating NADH in the mitochondria. OAA, which cannot cross the mitochondrial membrane, is then converted to aspartate (Asp) inside the mitochondria.
- Aspartate Transfer: Aspartate crosses the membrane back to the cytoplasm, where it is converted to α-KG, completing the cycle.
- Glycerol 3-Phosphate Shuttle
- Process Overview: The glycerol 3-phosphate shuttle operates similarly but involves the reduction of dihydroxyacetone phosphate (DHAP) to glycerol 3-phosphate (glycerol 3-P) in the cytoplasm.
- Electrons Transfer: Glycerol 3-P then enters the mitochondria, where it is reoxidized to DHAP by glycerol 3-phosphate dehydrogenase, with FAD serving as the electron acceptor. This process generates FADH2, which enters the electron transport chain at complex II.
- Completion: DHAP then exits the mitochondria, ready to participate in further rounds of electron transport.
- Key Differences Between Shuttles
- The malate-aspartate shuttle is more widely utilized across various tissues, providing a more efficient method for electron transfer, as it yields 3 ATP molecules per NADH.
- The glycerol 3-phosphate shuttle, while functional in several tissues, is less efficient, as it only contributes 2 ATP molecules per NADH.
- Tissue-Specific Activity
- Certain tissues show preferences for one shuttle system over the other. For example, the malate-aspartate shuttle is more dominant in the heart and liver, while the glycerol 3-phosphate shuttle is prominent in tissues like the brain, brown adipose tissue, and insect flight muscle.
The efficient movement of electrons from NADH into the mitochondrial ETC ensures proper ATP generation and supports various cellular functions.
In biological systems, oxidation and reduction are fundamental processes involving the transfer of electrons. Oxidation refers to the removal of electrons, while reduction is the gain of electrons. These reactions always occur together; when one compound is oxidized, another must be reduced. This interplay of oxidation and reduction is crucial for the operation of the electron transport chain (ETC), where electrons are passed through a series of protein complexes to ultimately produce ATP.
- Electron Flow in the ETC
Electrons are transferred through the ETC from compounds with lower electron affinity, such as NADH and FADH2, to molecules with higher electron affinity like coenzyme Q (CoQ) and cytochrome c (Cyt c). The final electron acceptor is molecular oxygen (O2), which combines with electrons and hydrogen to form water (H2O). - Structure of the ETC
The ETC consists of four main protein complexes and two mobile electron carriers:- Complex I: NADH-CoQ Reductase
This complex accepts two electrons from NADH. It contains 25 different proteins, including nonheme iron proteins and flavin mononucleotide (FMN), which is derived from vitamin B2. FMN accepts the electrons from NADH and passes them through iron-sulfur clusters (FeS), which transfer them to CoQ. - Complex II: Succinate-CoQ Reductase
Complex II accepts electrons from FADH2, which are either generated from the succinate to fumarate reaction or the glycerol 3-phosphate shuttle. The electrons are transferred through iron-sulfur proteins to CoQ. Since this pathway bypasses the first ATP-generating step, it produces only 2 ATP per FADH2 rather than the 3 ATP produced from NADH. - Complex III: Cytochrome c Reductase
In complex III, electrons are transferred from CoQ to cytochrome c (Cyt c), a small heme-containing protein. The transfer is mediated by heme proteins and iron-sulfur proteins. During this process, the iron in the heme changes from the ferric (+3) to the ferrous (+2) state, a key step in electron transfer. - Complex IV: Cytochrome c Oxidase
The final complex in the ETC, cytochrome c oxidase, transfers electrons from cytochrome c to molecular oxygen (O2). It involves copper-containing enzymes that also undergo redox changes during the electron transfer, changing from Cu²⁺ to Cu⁺.
- Complex I: NADH-CoQ Reductase
- Mobile Electron Carriers
In addition to the large protein complexes, there are two important mobile electron carriers in the ETC:- Coenzyme Q (CoQ): A lipid-soluble carrier that shuttles electrons between complexes I and II to complex III.
- Cytochrome c (Cyt c): A small heme-containing protein that carries electrons from complex III to complex IV.
- Electron Transfer and Energy Production
The movement of electrons through these complexes contributes to the creation of a proton gradient across the mitochondrial membrane. This gradient drives the synthesis of ATP through oxidative phosphorylation, a process crucial for cellular energy production.
The tightly regulated flow of electrons from NADH and FADH2 to oxygen is essential for maintaining cellular energy levels, ensuring that the processes of oxidation and reduction are effectively coupled in the mitochondria.
Phosphorylation is the process by which a phosphate group is added to a molecule, typically to a protein or nucleotide, altering its activity. In cells, this modification plays a pivotal role in energy transfer and cellular regulation. One of the most well-known types of phosphorylation occurs in the mitochondria, specifically during oxidative phosphorylation, which is integral to ATP production.
- ATP Synthesis and Phosphorylation
Phosphorylation is key to the production of ATP, the cell’s main energy carrier. This process takes place in the inner mitochondrial membrane, where protons (H⁺) are pumped out of the matrix as electrons are transferred through the electron transport chain (ETC). The flow of protons back into the matrix powers ATP synthase, an enzyme complex located in the membrane.- The ATP synthase consists of two components: F0 and F1.
- F0: Spans the inner membrane, acting as a channel for protons to flow back into the mitochondrial matrix.
- F1: Projects into the matrix, where it catalyzes the phosphorylation of ADP and inorganic phosphate (Pi), forming ATP.
- The ATP synthase consists of two components: F0 and F1.
- The Role of the Electron Transport Chain
As electrons move through the complexes of the ETC, energy is released, which is used to pump protons out of the matrix into the intermembrane space. This creates an electrochemical gradient, or proton gradient, across the inner mitochondrial membrane. The energy stored in this gradient drives the ATP synthesis process.- Electrons pass from NADH to CoQ, then to cytochrome c (Cyt c), and ultimately to oxygen (O₂), which combines with protons to form water. During these electron transfers, protons are actively transported across the membrane, contributing to the gradient necessary for ATP production.
- ATP Transport and Utilization
Once ATP is synthesized in the mitochondrial matrix, it is transported across the inner mitochondrial membrane via a membrane ADP/ATP antiporter. This exchange ensures that ADP is brought into the matrix, where it can be phosphorylated again into ATP.
The entire process of oxidative phosphorylation and the associated phosphorylation of ADP to ATP is critical for the cell’s energy supply, driving various cellular functions.
Cellular Level of Electron Transport Chain
The electron transport chain (ETC) is crucial for energy production at the cellular level. It is embedded within the inner mitochondrial membrane and serves as the final stage in the process of cellular respiration, converting energy from electrons into usable ATP.
- Electron Transfer Through Protein Complexes
- Electrons from NADH and FADH2 are transferred through a series of protein complexes, ultimately leading to the formation of water and the generation of a proton gradient.
- The flow of electrons increases the reduction potential of each protein in the chain, releasing energy that is primarily used to pump hydrogen ions (H+) across the mitochondrial membrane.
- This proton gradient plays a crucial role in ATP synthesis, as protons flow back through ATP synthase to generate ATP.
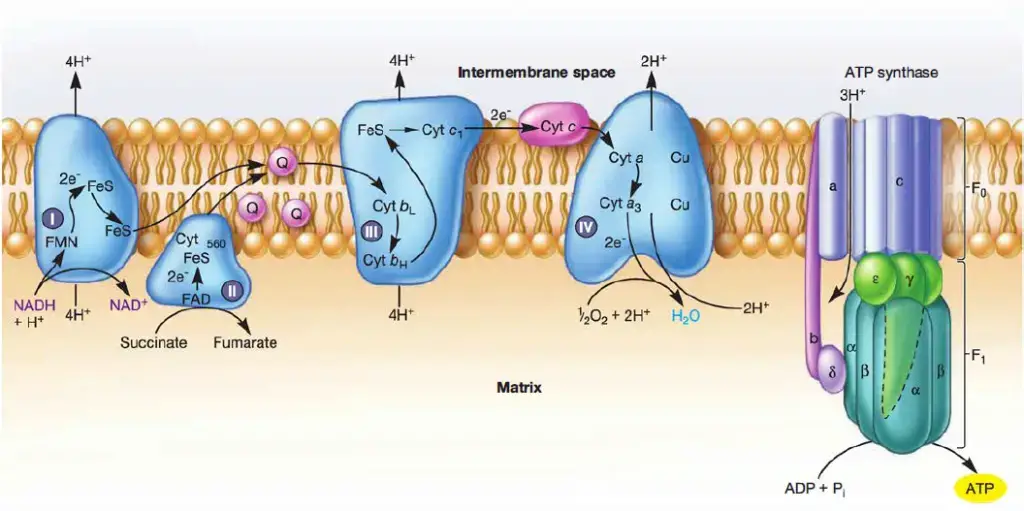
- Complex I: NADH Dehydrogenase
- Complex I, or ubiquinone oxidoreductase, is where NADH, derived from glycolysis and the citric acid cycle, donates electrons.
- These electrons first pass to flavin mononucleotide (FMN), then to iron-sulfur (Fe-S) clusters, and finally to coenzyme Q.
- During this process, four protons are transported from the mitochondrial matrix into the intermembrane space, contributing to the electrochemical gradient.
- The chemical reaction at Complex I is as follows:
(NADH + H+) + CoQ + 4 H+ (matrix) → NAD+ + CoQH2 + 4 H+ (intermembrane).
- Complex II: Succinate Dehydrogenase
- Complex II acts as a second entry point into the ETC, accepting electrons from succinate.
- Succinate is oxidized to fumarate, and the resulting electrons are transferred to FAD within Complex II, which then passes them to Fe-S clusters and subsequently to coenzyme Q.
- Unlike Complex I, Complex II does not pump protons across the membrane, thus contributing less to the proton gradient and producing fewer ATP.
- Coenzyme Q (Ubiquinone)
- Coenzyme Q functions as an electron carrier between Complex I, Complex II, and Complex III.
- It undergoes reduction to form semiquinone (CoQH-) and ubiquinol (CoQH2), which transfer electrons to Complex III, playing an essential role in the electron transfer process.
- The Q cycle elaborates on how coenzyme Q facilitates the transfer of electrons, moving from one state to another during this process.
- Complex III: Cytochrome c Reductase
- Complex III consists of cytochrome b, Rieske subunits, and cytochrome c proteins, and is responsible for transferring electrons from coenzyme Q to cytochrome c.
- Electrons are transferred in a two-step process, called the Q cycle, which helps release four protons into the intermembrane space.
- This contributes to the proton gradient that drives ATP production. Cytochrome c then carries the electrons to Complex IV.
- Complex IV: Cytochrome c Oxidase
- Complex IV, also known as cytochrome c oxidase, is the final complex in the electron transport chain.
- It receives electrons from cytochrome c and transfers them to oxygen, the final electron acceptor in the chain.
- The oxygen molecule is reduced to form water, and in this process, four protons are moved into the intermembrane space, further enhancing the proton gradient.
- ATP Synthase: ATP Production
- ATP synthase, also referred to as Complex V, uses the proton gradient generated by the electron transport chain to synthesize ATP.
- It operates through a rotational mechanism, where protons flow from the intermembrane space through the F0 subunit, causing it to rotate.
- This rotation drives conformational changes in the F1 subunit, catalyzing the conversion of ADP and inorganic phosphate (Pi) into ATP.
- For every four protons that flow through ATP synthase, one molecule of ATP is produced.
Molecular Level of Electron Transport Chain
The electron transport chain (ETC) operates at the molecular level through a series of redox reactions involving electron carriers and protein complexes that transfer electrons and pump protons, ultimately generating a proton gradient essential for ATP production.
- Nicotinamide Adenine Dinucleotide (NAD)
- NAD exists in two forms: NAD+ (oxidized) and NADH (reduced), which participate in metabolic redox reactions.
- In the reduction reaction, NAD+ accepts two electrons and one proton, becoming NADH.
- The reaction is as follows:
RH2 + NAD+ → R + H+ + NADH
where RH2 is the electron donor, such as sugar. - NADH then enters the ETC at Complex I, transferring its electrons to the electron transport system. This generates a total of 10 protons (H+), which are pumped across the mitochondrial membrane.
- For every 4 protons pumped through ATP synthase, one ATP molecule is synthesized, resulting in 2.5 ATP per NADH.
- The oxidation of NADH releases two electrons and a proton, reverting it back to NAD+:
NADH → NAD+ + H+ + 2 e-.
- Flavin Adenine Dinucleotide (FAD)
- FAD, similar to NAD, has four redox states: FAD (oxidized), FADH- (semiquinone), and FADH2 (reduced).
- FAD is made of an adenine nucleotide and flavin mononucleotide (FMN), and it plays a crucial role in electron transfer.
- The reduction of FAD to FADH2 is critical in several metabolic processes, including fatty acid oxidation and DNA repair.
- When FADH2 is oxidized in the ETC, it releases electrons, and its aromatic structure changes, releasing energy. This results in a more positive reduction potential compared to NADH.
- FADH2 enters the ETC at Complex II, producing 1.5 ATP per FADH2. This is due to the proton pumping at Complex III and Complex IV, where 6 protons are pumped, and for every 4 protons, one ATP is produced:
FADH2 → FAD + 2 H+ + 2 e-.
- Energy Yield from NADH and FADH2
- NADH and FADH2 are key electron donors in the ETC. The electrons they carry pass through multiple protein complexes, contributing to the generation of the proton gradient used to drive ATP synthesis.
- NADH results in the production of 10 H+ ions, which are pumped across the mitochondrial membrane and ultimately lead to the synthesis of 2.5 ATP.
- In contrast, FADH2 contributes 6 H+ ions, yielding 1.5 ATP due to its entry into the ETC at a later point (Complex II).
These molecular steps highlight the critical roles of NADH and FADH2 in driving the electron transport chain, producing a proton gradient across the mitochondrial membrane, and generating ATP in the cell.
Products of Electron Transport Chain
The electron transport chain (ETC) is the final phase of cellular respiration. Its main goal is to convert the energy stored in electrons to ATP, the cell’s primary energy currency. The reactions within the ETC result in the production of several key products.
- ATP Production
- The main product of the electron transport chain is ATP. As electrons pass through the various complexes, energy is released and used to pump protons across the membrane.
- The proton gradient created by this process powers ATP synthase, which synthesizes ATP as protons flow back into the matrix.
- The overall net ATP yield from oxidative phosphorylation (ETC and chemiosmosis) is typically between 30 and 32 ATP molecules per molecule of glucose.
- Water Formation
- The final step of the electron transport chain involves the transfer of electrons to molecular oxygen, which is reduced to water.
- For every two electrons transferred, one molecule of water (H2O) is produced.
- This process also helps remove excess electrons and protons from the system, maintaining a balanced charge.
- Electron and Proton Flow
- The electron flow from NADH and FADH2 through the various complexes generates energy.
- This energy is used to pump protons (H+) across the inner mitochondrial membrane, creating an electrochemical gradient.
- The protons then move back into the matrix through ATP synthase, where their movement is coupled with the production of ATP.
In total, the electron transport chain contributes to the generation of 30 to 32 ATP molecules and the formation of water as a byproduct, completing the process of oxidative phosphorylation.
Inhibitors and Uncouplers of Electron Transport Chain
The electron transport chain (ETC) is a critical component of cellular respiration, but its function can be disrupted by specific inhibitors and uncouplers, leading to various biochemical consequences. These substances act at different points in the chain, either by halting electron transfer or by dissociating electron flow from ATP synthesis.
Inhibitors of Electron Transport Chain
Inhibitors block the electron transport chain at various complexes, preventing the transfer of electrons and halting oxidative phosphorylation. These agents can target specific complexes within the chain:
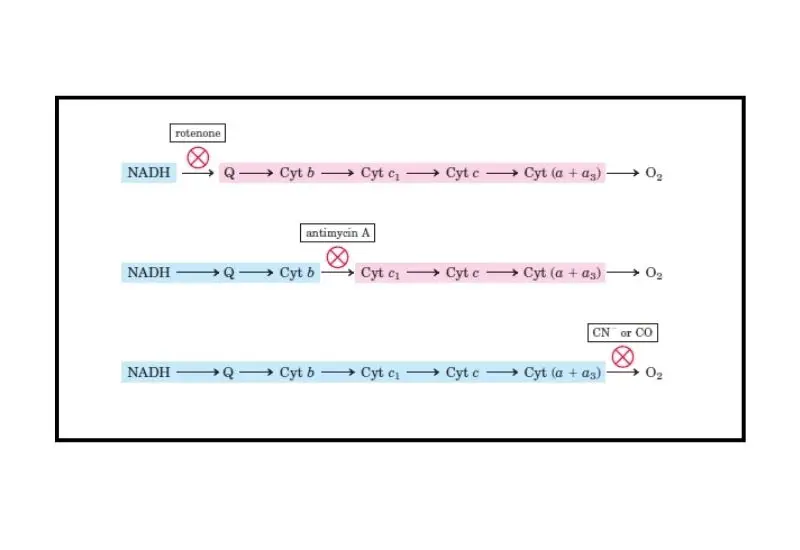
- Complex I Inhibitors
Rotenone: A pesticide that inhibits the transfer of electrons from Fe-S clusters to ubiquinone at Complex I. This results in an accumulation of electrons and an inability to pass electrons to the next components of the ETC.
Barbiturates: Similar to rotenone, barbiturates inhibit Complex I, preventing proper electron flow. - Complex II Inhibitors
Carboxin: A fungicide that interferes with electron transfer at Complex II, preventing ubiquinone from accepting electrons.
Malonate: A competitive inhibitor of succinate dehydrogenase, which is part of Complex II.
TTFA (triphenyltetrazolium chloride): Another agent that inhibits Complex II by chelating iron. - Complex III Inhibitors
Antimycin A: A piscicide that inhibits cytochrome c reductase at the Qi binding site of Complex III, preventing the recycling of ubiquinol and halting electron flow.
BAL (dimercaprol): A chelating agent that can also inhibit Complex III. - Complex IV Inhibitors
Cyanide (CN): Binds to cytochrome c oxidase, inhibiting electron transfer to oxygen and causing tissue hypoxia.
Carbon Monoxide (CO): Similar to cyanide, CO binds to cytochrome c oxidase, preventing oxygen from being reduced and blocking the ETC.
Hydrogen Sulfide (H2S): Inhibits Complex IV by binding to the cytochrome c oxidase, preventing oxygen reduction. - ATP Synthase Inhibitors
Oligomycin: An antibiotic that inhibits ATP synthase (Complex V), blocking the passage of protons through the F0 subunit. This prevents ATP synthesis despite electron transfer.
Uncouplers of Electron Transport Chain
Uncouplers disrupt the coupling of electron transport and ATP synthesis by allowing protons to leak across the inner mitochondrial membrane. This results in the dissipation of the proton gradient without the production of ATP.
- General Mechanism
Uncouplers allow protons to bypass ATP synthase, leading to the consumption of electrons without ATP production. The energy is instead released as heat. This can be compared to an engine revving without being connected to any productive work, wasting fuel and producing excess heat. - Examples of Exogenous Uncouplers
- 2,4-Dinitrophenol: A chemical that allows protons to cross the mitochondrial membrane, dissipating the proton gradient and generating heat instead of ATP.
- Dinitrocresol
- Pentachlorophenol
- M-chlorocarbonyl cyanide phenylhydrazone (CCCP)
- Valinomycin
- Gramicidin
These compounds disrupt oxidative phosphorylation by facilitating proton leakage into the mitochondrial matrix, preventing ATP synthesis.
- Endogenous Uncoupler
Thermogenin (UCP1): Found in brown adipose tissue, thermogenin is a natural uncoupler that increases proton leakage across the mitochondrial membrane. This process helps generate heat, particularly in hibernating animals and newborns. Brown adipose tissue, rich in mitochondria, allows for rapid thermogenesis without the need for ATP production.- Fatty acids: When released, fatty acids can activate thermogenin, further promoting the uncoupling process and heat generation, especially in response to cold or hormonal signals like norepinephrine.
Physiological and Pathophysiological Effects
- Heat Generation: Uncouplers, particularly thermogenin, help organisms generate heat in conditions where it is needed, such as in newborns or animals in cold environments.
- Acidosis: Uncoupling agents can lead to metabolic changes, such as lactic acidosis, due to the shift towards anaerobic pathways when ATP production is impaired.
- Toxicological Impacts: Many inhibitors and uncouplers, particularly poisons like cyanide and carbon monoxide, can cause severe health issues by disrupting cellular respiration and leading to tissue hypoxia. Prompt treatment is often necessary, as these poisons prevent oxygen from being effectively utilized by the cells, potentially causing organ failure.
Importance of the Electron Transport Chain
The electron transport chain (ETC) plays a pivotal role in cellular respiration, acting as the final stage in the conversion of energy from food molecules into usable energy for cells.
- ATP Production
- The primary importance of the electron transport chain lies in its ability to produce ATP through oxidative phosphorylation.
- As electrons move through the chain, the energy released is harnessed to pump protons across the inner mitochondrial membrane.
- This generates a proton gradient, which ATP synthase uses to synthesize ATP as protons flow back into the mitochondrial matrix.
- Without the ETC, the cell would lack a critical mechanism to produce ATP efficiently.
- Oxygen Utilization
- The ETC is responsible for the final reduction of oxygen into water.
- Oxygen acts as the terminal electron acceptor, accepting electrons from cytochrome c and combining with protons to form water.
- This step is crucial because without oxygen to accept electrons, the entire chain would halt, preventing ATP production and leading to cell death.
- Energy Conversion
- The electron transport chain converts the energy stored in electrons, derived from NADH and FADH2, into usable cellular energy.
- This process links the oxidation of organic molecules, such as glucose and fatty acids, to the production of energy-rich molecules like ATP.
- The energy conversion also contributes to maintaining the cell’s overall metabolic functions.
- Creation of Proton Gradient
- As electrons move through the chain, protons are actively pumped across the inner mitochondrial membrane.
- This movement of protons sets up an electrochemical gradient that stores potential energy.
- The gradient is key for ATP synthesis, as protons flow back through ATP synthase, driving the production of ATP.
- Linking Metabolic Pathways
- The ETC links multiple metabolic processes, such as glycolysis, pyruvate oxidation, and the citric acid cycle, with the production of ATP.
- The NADH and FADH2 produced by these cycles feed into the ETC, continuing the flow of electrons and enabling efficient energy extraction from nutrients.
Quiz
[mcq_display ids=”66383,66384,66385,66386,66387,66388,66389,66390,66391,66392,66393,66394,66395,66396,66397,66398,66399,66400,66401,66402″]
FAQ
Where does the electron transport chain take place?
It occurs in mitochondria in both cellular respiration and photosynthesis.
What is the electron transport chain?
An electron transport chain is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions and couples this electron transfer with the transfer of protons across a membrane.
Where is the electron transport chain located?
mitochondria
Where are the proteins of the electron transport chain located
The proteins of the electron transport chain are located in the inner mitochondrial membrane of eukaryotic cells. In prokaryotic cells, such as bacteria, these proteins are found in the plasma membrane. The inner mitochondrial membrane houses the protein complexes (Complex I, II, III, IV, and ATP synthase) that are involved in the electron transport chain and oxidative phosphorylation.
What is the final electron acceptor in the electron transport chain?
The final electron acceptor is oxygen (O2). Oxygen has a high electronegativity; thus, oxygen’s high affinity for electrons makes it an ideal acceptor for low-energy electrons. With the electrons, hydrogen is added to oxygen forming water as the final product.
how many atp are produced in the electron transport chain?
34 ATP molecules are produced in the electron transport chain if we consider that one molecule of NADH produces 3 molecules of ATP and one molecule of FADH2 gives rise to 2 molecules of ATP.
what does the electron transport chain produce?
The electron transport chain produces an electrochemical gradient that drives the synthesis of ATP by chemiosmosis. The end products of the electron transport chains are ATP and water.
What is the purpose of the electron transport chain?
The main purpose of the electron transport chain is to build up a surplus of hydrogen ions (protons) in the intermembrane space sp that there will be a concentration gradient compared to the matrix of the mitochondria. This will drive ATP synthase.
Where does the electron transport chain get its electrons from?
All of the electrons that enter the transport chain come from NADH and FADH 2
in the electron transport chain the final electron acceptor is
In the electron transport chain, the final electron acceptor is oxygen (O₂). Oxygen accepts the electrons after they have passed through the chain and combines with hydrogen ions (protons) to form water (H₂O).
- Kumari, Asha (2018). Sweet Biochemistry || Electron Transport Chain. , (), 13–16. doi:10.1016/B978-0-12-814453-4.00003-0
- Ahmad M, Wolberg A, Kahwaji CI. Biochemistry, Electron Transport Chain. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526105/
- Aerts, L. (2017). Parkinson’s Disease || Electron Transport Chain. , (), 41–75. doi:10.1016/B978-0-12-803783-6.00002-X
- Nicholls, David G. (2013). Bioenergetics || Respiratory Chains. , (), 91–157. doi:10.1016/B978-0-12-388425-1.00005-1
- Engelking, Larry R. (2015). Textbook of Veterinary Physiological Chemistry || Oxidative Phosphorylation. , (), 219–224. doi:10.1016/b978-0-12-391909-0.50036-0
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition. - Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.
- Enderle, J. D. (2012). Biochemical Reactions and Enzyme Kinetics. Introduction to Biomedical Engineering, 447–508. doi:10.1016/b978-0-12-374979-6.00008-3
- Engelking, L. R. (2015). Oxidative Phosphorylation. Textbook of Veterinary Physiological Chemistry, 219–224. doi:10.1016/b978-0-12-391909-0.50036-0
- https://conductscience.com/electron-transport-chain/?srsltid=AfmBOoru_CdHU6OU6xgTgQVrfcnQDy0K6WvLzQBCzd54Bt4OZw_0aiE1
- https://courses.lumenlearning.com/wm-biology1/chapter/reading-electron-transport-chain/
- https://byjus.com/biology/electron-transport-chain/