The DNase test is a differential biochemical test which is used to detect the ability of microorganism to produce an extracellular enzyme called deoxyribonuclease (DNase). It is the process where polymerized DNA present in the medium is hydrolyzed into smaller nucleotide fragments by the action of this enzyme. These degraded products can be absorbed by the bacterial cell and used as source of carbon nitrogen and energy.
It is considered that DNase enzyme helps in pathogenicity of certain bacteria. This enzyme helps in breaking down the DNA structures released by host immune cells and thus assisting the organism to escape immune defense. Due to this reason DNase production is often associated with virulent strains.
The test is performed by inoculating the test organism on DNase agar medium which contains DNA as a substrate. After incubation the medium is treated depending on the type of DNase agar used. In case of plain DNase agar hydrochloric acid is added which precipitates intact DNA and produces a cloudy background while clear zone appears around DNase positive colonies. In dye based media such as methyl green or toluidine blue the breakdown of DNA is indicated by colour change around the colonies.
Thus DNase test is an important laboratory test used mainly to differentiate closely related bacterial species based on their ability to hydrolyze DNA.
Principle of DNase Test
The principle of DNase test is based on the ability of certain microorganisms to produce an extracellular enzyme known as deoxyribonuclease (DNase). DNA is a large polymer molecule which cannot pass through bacterial cell membrane due to its size and negative charge. To utilize DNA as a nutrient source the organism secretes DNase enzyme which hydrolyzes the phosphodiester bonds of DNA. As a result the long DNA molecules is broken down into smaller soluble fragments such as oligonucleotides and nucleotides which can be taken up by the cell and used as source of carbon nitrogen and phosphate.
In laboratory this enzymatic activity is demonstrated on a DNase agar medium containing polymerized DNA. When a DNase producing organism grows on the medium it degrades the DNA present around the colonies. This breakdown of DNA is detected either by adding hydrochloric acid which precipitates intact DNA leaving a clear zone around positive colonies or by using indicator dyes such as methyl green which loses its colour when DNA is hydrolyzed. Thus appearance of a clear or discoloured zone around bacterial growth indicates a positive DNase reaction.
Objectives of DNase Test
- To detect the production of deoxyribonuclease (DNase) enzyme by the microorganism.
- To determine the ability of organism to hydrolyze polymerized DNA into smaller soluble fragments.
- To differentiate DNase producing bacteria from non-DNase producing bacteria.
- To identify Staphylococcus aureus and differentiate it from coagulase negative staphylococci.
- To use as a supplementary test when coagulase test gives doubtful results.
- To differentiate Serratia species from other members of Enterobacteriaceae such as Klebsiella and Enterobacter.
- To distinguish Moraxella catarrhalis from Neisseria species.
- To help in screening of certain pathogenic organisms based on DNase activity.
- To assess the association of DNase production with bacterial virulence.
Requirements for DNase Test
Media
- DNase agar medium containing polymerized DNA as substrate.
- DNase agar with methyl green indicator dye.
- DNase agar with toluidine blue indicator.
- Nutrient broth or Brain heart infusion broth for preparing culture.
Reagents
- Hydrochloric acid (1N HCl) for detection of DNA precipitation.
- Methyl green dye (when incorporated in medium).
- Toluidine blue dye (when used as indicator).
Supplies and Equipment
- Sterile inoculating loop or wire.
- Incubator for incubation at 35–37°C.
- Sterile petri plates.
- Measuring cylinder and conical flasks.
- Bunsen burner or spirit lamp.
- Dark background for observing clear zone.
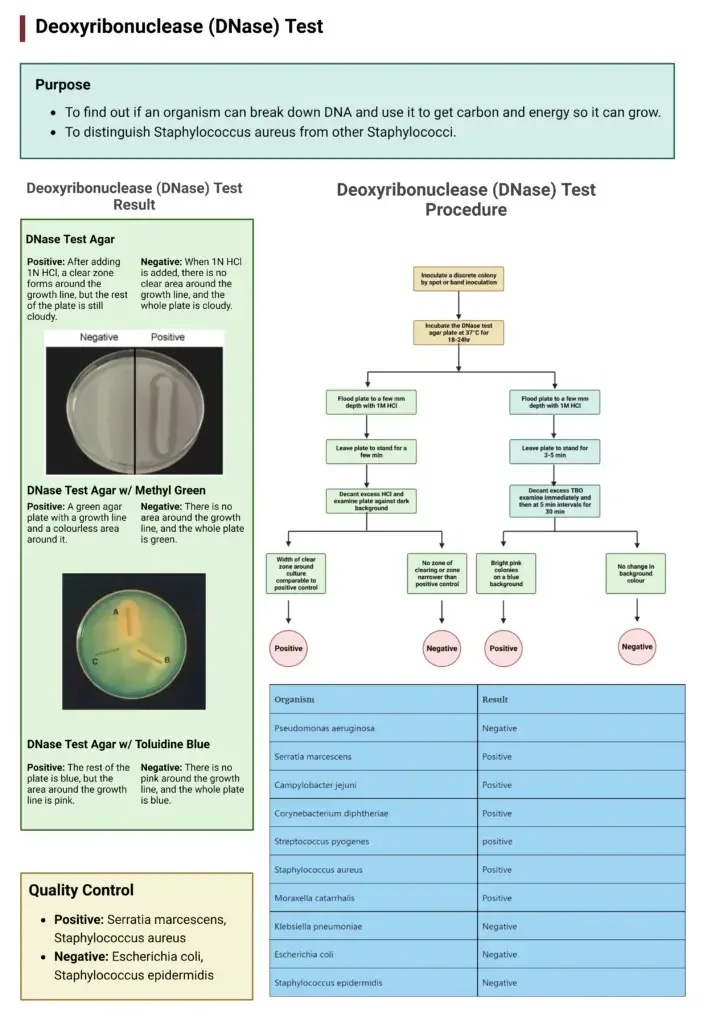
Procedure of DNase Test
Standard DNase Agar Plate Method
- Take a DNase agar plate and allow the surface of medium to dry properly.
- Using a sterile inoculating loop pick a well isolated colony from an 18–24 hours old pure culture.
- Inoculate the organism on the DNase agar by spot inoculation or by drawing a single straight streak on the surface of medium.
- If required divide the plate into sections and inoculate different organisms separately.
- Incubate the inoculated plates in inverted position at 35–37°C for 18–24 hours.
- After incubation observe the plate depending on the type of medium used.
- For plain DNase agar flood the surface with 1N hydrochloric acid and allow it to react for few minutes.
- Pour off excess acid and examine the plate against a dark background for clear zone around growth.
- For DNase agar with methyl green or toluidine blue do not add acid and directly observe the colour change or clear zone around colonies.
Thermonuclease Test (Special Method)
- Inoculate the test organism into brain heart infusion broth and incubate for 18–24 hours.
- Heat the broth at 100°C for 15 minutes to destroy bacteria and heat labile enzymes.
- Allow the broth to cool and dispense it into wells cut on toluidine blue DNase agar.
- Incubate at 35–37°C for 2–3 hours and observe for pink coloured zone around the well.
DNase Tube Test
- Inoculate a heavy growth of organism into broth containing DNA.
- Incubate at 37°C for specific time period.
- Detect degradation of DNA by appropriate method to confirm DNase activity.
Result Interpretation Of Deoxyribonuclease (DNase) Test
Standard DNase Agar (HCl Method)
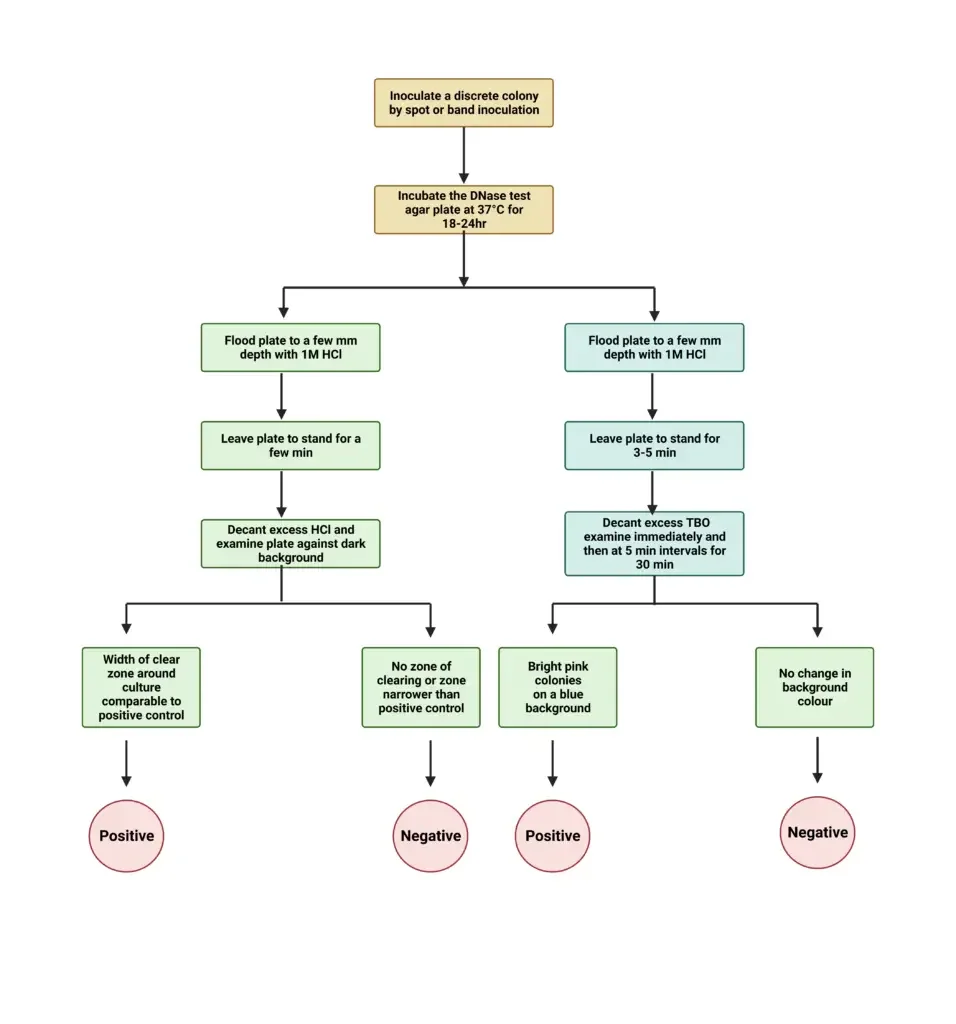
- Positive result – A clear zone is seen around the bacterial growth after flooding with hydrochloric acid. The remaining medium appears cloudy due to precipitation of intact DNA.
- Negative result – No clear zone is observed around the growth and the entire medium becomes cloudy.
DNase Agar with Methyl Green
- Positive result – A clear or colourless zone is formed around the colony due to breakdown of DNA and loss of dye.
- Negative result – The medium remains uniformly green with no clear zone around growth.
DNase Agar with Toluidine Blue
- Positive result – A pink or rose red zone appears around the colony or streak.
- Negative result – The medium remains blue and no colour change is observed.
Thermonuclease Test
- Positive result – A pink coloured halo is seen around the well indicating presence of heat stable DNase.
- Negative result – No pink zone is formed and the medium remains blue.
Common Organism Reaction
- DNase positive organisms – Staphylococcus aureus, Serratia species, Moraxella catarrhalis.
- DNase negative organisms – Staphylococcus epidermidis, Escherichia coli, Klebsiella species.
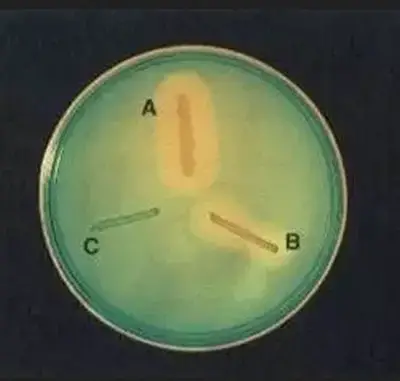
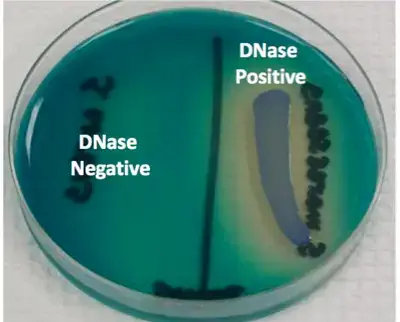

Organisms Showing DNase Test Reaction
DNase Positive Organisms
- Staphylococcus aureus
- Streptococcus pyogenes
- Staphylococcus intermedius
- Staphylococcus schleiferi
- Serratia marcescens
- Moraxella catarrhalis
- Stenotrophomonas maltophilia
- Aeromonas species
- Vibrio species
- Proteus vulgaris
- Helicobacter pylori
- Corynebacterium diphtheriae
- Corynebacterium ulcerans
- Bacillus cereus
DNase Negative Organisms
- Staphylococcus epidermidis
- Staphylococcus haemolyticus
- Escherichia coli
- Klebsiella pneumoniae
- Enterobacter species
- Proteus mirabilis
- Plesiomonas shigelloides
- Burkholderia cepacia
- Neisseria gonorrhoeae
- Neisseria meningitidis
- Corynebacterium pseudotuberculosis
Precautions of DNase Test
- Use a heavy inoculum for inoculation to ensure sufficient production of DNase enzyme.
- Always use a fresh and pure culture of 18–24 hours old organism.
- Ensure the surface of DNase agar medium is properly dried before inoculation.
- Incubate the plates at recommended temperature and for proper duration only.
- While using hydrochloric acid method observe the results immediately after adding acid.
- Do not re-incubate the plates after flooding with hydrochloric acid.
- Avoid using excessive inoculum on methyl green agar as it may cause false positive result.
- Check proper growth of organism on toluidine blue agar as the dye may inhibit some bacteria.
- Use freshly prepared and properly stored media to avoid degradation of DNA.
- Handle hydrochloric acid carefully as it is corrosive.
Uses of DNase Test
- To differentiate Staphylococcus aureus from coagulase negative staphylococci.
- To use as a supplementary or alternative test to coagulase test for identification of Staphylococcus species.
- To identify Serratia species and differentiate them from Klebsiella and Enterobacter species.
- To differentiate Moraxella catarrhalis from Neisseria species in respiratory specimens.
- To screen pathogenic Corynebacterium such as Corynebacterium diphtheriae and Corynebacterium ulcerans.
- To differentiate Aeromonas species from Plesiomonas shigelloides.
- To help in identification of Stenotrophomonas maltophilia.
- To assist in characterization of certain Campylobacter and Helicobacter species.
- To assess DNase production as an indicator of bacterial virulence.
Advantages of DNase Test
- It helps in differentiation of pathogenic bacteria from non pathogenic related species.
- It is useful in identification of Staphylococcus aureus from coagulase negative staphylococci.
- It can be used as an alternative or supplementary test to coagulase test.
- It helps in differentiation of Serratia species from other Enterobacteriaceae members.
- It is useful in distinguishing Moraxella catarrhalis from Neisseria species.
- It serves as a simple screening test for pathogenic Corynebacterium species.
- Indicator based DNase agar allows colonies to remain viable for further testing.
- Thermonuclease test gives rapid and specific identification of Staphylococcus aureus.
- The test is simple to perform and does not require complex equipment.
- It is cost effective and suitable for routine laboratory diagnosis.
Limitations of DNase Test
- DNase test alone is not sufficient for complete identification of organism.
- Positive result may be shown by different genera therefore other biochemical tests are required.
- Use of light inoculum may give false negative result due to insufficient enzyme production.
- Very heavy inoculum may cause false positive result especially on methyl green agar.
- Standard DNase agar method requires 18–24 hours incubation which is time consuming.
- Flooding with hydrochloric acid kills the organism and further testing cannot be done.
- Results must be observed immediately after adding hydrochloric acid otherwise interpretation becomes difficult.
- Toluidine blue may inhibit growth of some Gram positive organisms.
- Improper or degraded DNA in medium affects accuracy of methyl green method.
- Some strains of Staphylococcus aureus may give weak or negative reaction.
- Few coagulase negative staphylococci may show positive DNase reaction leading to confusion.
- Acharya, T. (n.d.). DNase Test: Principle, Procedure, Results. Microbe Online.
- BD. (n.d.). DNase Test Agars [Difco & BBL Manual]. Becton, Dickinson and Company.
- Biolife Italiana. (2023). Deoxyribonuclease Test Medium (Rev. 3) [Instructions for Use].
- Canning, B., Mohamed, I., Wickramasinghe, N., Swindells, J., & O’Shea, M. K. (2020). Thermonuclease test accuracy is preserved in methicillin-resistant Staphylococcus aureus isolates. Journal of Medical Microbiology, 69(4), 548. https://doi.org/10.1099/jmm.0.001166
- Comprehensive Diagnostic Analysis of Microbial Deoxyribonuclease Activity: Biochemical Principles, Laboratory Methodologies, and Clinical Applications. (n.d.). [Source provided in prompt].
- Dalynn Biologicals. (2006). DNase Test Agar [Catalogue No. PD60 & PD61].
- Gerceker, D., Karasartova, D., Elyürek, E., Barkar, S., Kiyan, M., Özsan, T. M., Calgin, M. K., & Sahin, F. (2009). A new, simple, rapid test for detection of DNase activity of microorganisms: DNase Tube test. The Journal of General and Applied Microbiology, 55(4), 291–294. https://doi.org/10.2323/jgam.55.291
- HiMedia Laboratories. (2015). DNase Test Agar Base w/ methyl green [Technical Data M1419].
- HiMedia Laboratories. (2024). DNase Test Agar w/ Methyl Green [Technical Data M1419].
- Madison, B. M., & Baselski, V. S. (1983). Rapid identification of Staphylococcus aureus in blood cultures by thermonuclease testing. Journal of Clinical Microbiology, 18(3), 722–724. https://doi.org/10.1128/jcm.18.3.722-724.1983
- Merck Millipore. (n.d.). DNase Test Agar. In Merck Microbiology Manual (12th ed., pp. 270–271).
- Pimenta, F. P., Souza, M. C., Pereira, G. A., Hirata, R., Camello, T. C., & Mattos-Guaraldi, A. L. (2008). DNase test as a novel approach for the routine screening of Corynebacterium diphtheriae. Letters in Applied Microbiology, 46(3), 307–311. https://doi.org/10.1111/j.1472-765X.2007.02310.x
- Pokhrel, P. (2015, July 19). Deoxyribonuclease (DNase) Test- Principle, Uses, Procedure, Result Interpretation, Quality Control, Examples and Limitations. Microbiology Notes.
- Remel. (2010). DNase Test Agar w/ and w/o Additives [Instructions for Use]. Thermo Fisher Scientific.
- Sapkota, A. (2022, January 27). DNase Test- Definition, Principle, Procedure, Result, Uses. Microbe Notes.
- Sigma-Aldrich. (n.d.). DNase test agar with toluidine blue, NutriSelect® Basic [Product Information].
- Sunarno, S., Puspandari, N., Febriyana, D., Febrianti, T., Saraswati, R. D., Sulistyaningrum, N., & Pracoyo, N. E. (2021). Application of Polymerase Chain Reaction in Diphtheria Laboratory Examination: A Field Need. Jundishapur Journal of Microbiology, 14(7), e117884. https://doi.org/10.5812/jjm.117884
- UK Health Security Agency. (2025). Deoxyribonuclease test (UK Standards for Microbiology Investigations TP 12, Issue 4.1).
- Virtual Microbiology Lab Simulator (VUMIE). (2025). Deoxyribonuclease (DNase) test.