What is Diffusion?
- Diffusion is a fundamental process involving the movement of particles, such as atoms, ions, or molecules, from an area of higher concentration to one of lower concentration. This movement continues until the concentration is uniform throughout the medium, reaching equilibrium. It occurs naturally in all types of substances—solids, liquids, and gases—due to the inherent random motion of molecules.
- In biological contexts, diffusion plays a crucial role in processes such as the transport of gases (like oxygen and carbon dioxide) and nutrients across cell membranes. It is a passive process, meaning it does not require energy input from molecules like ATP. Instead, it relies solely on the concentration gradient, the difference in concentration between two areas.
- For example, if copper sulfate is added to water, the crystals initially create a high concentration in one part of the beaker. Over time, the copper sulfate molecules spread out through the water, and the solution becomes uniformly colored. This illustrates how diffusion works: substances move from areas of higher concentration to areas of lower concentration, eventually achieving uniform distribution.
- The rate of diffusion varies depending on the medium and the type of substance involved. For instance, gases diffuse more rapidly than liquids or solids due to the greater freedom of movement of their molecules. An example is the rapid spreading of the ammonia smell in the air or the diffusion of nitrogen gas from a leaking container into the atmosphere. Liquids, such as water, diffuse more slowly, while solids exhibit the slowest diffusion due to the limited movement of their particles.
- Additionally, diffusion can occur between liquids, as seen when water and glycerol are mixed. Over time, the two liquids diffuse into each other, forming a uniform solution. However, when immiscible liquids like water and oil are combined, diffusion occurs only at the small interface between the two, as they do not mix thoroughly.
- In physics, diffusion is often described mathematically using Fick’s laws, which quantify the rate at which particles spread. These laws apply to normal, or Fickian, diffusion, where the movement follows predictable patterns based on the concentration gradient. In certain cases, the diffusion process may not follow these predictable patterns, resulting in what is called anomalous diffusion.
- In addition to its biological significance, the concept of diffusion is widely applicable across various scientific fields. In economics, for instance, diffusion models describe how innovations or information spread through populations. Similarly, in data science and marketing, diffusion models are used to understand how ideas, trends, or products gain popularity.
Definition of Diffusion
Diffusion is the process by which molecules move from an area of higher concentration to an area of lower concentration, driven by random molecular motion, until equilibrium is reached.
Types of Diffusion
Diffusion is a critical process in biology, physics, and chemistry, where molecules move from an area of higher concentration to lower concentration. It happens in two primary ways: simple diffusion and facilitated diffusion. Here’s a breakdown of each type and their subcategories:
1. Simple Diffusion
- Definition: Simple diffusion involves the direct movement of molecules across a semi-permeable membrane without the help of transport proteins.
- Conditions: The solute should be non-polar and have a low molecular weight (under 10,000 kDa).
- Examples:
- Movement of gases like oxygen and carbon dioxide in and out of cells.
- Transport of water molecules and nutrients in bacteria through the cell membrane.
2. Facilitated Diffusion
- Definition: Facilitated diffusion involves molecules moving across the membrane with the help of transport proteins. These proteins form channels or carriers that aid in the passage of molecules.
- Characteristics: Like simple diffusion, facilitated diffusion moves molecules from high to low concentration but requires a helper protein.
- Examples: Movement of larger or charged molecules like glucose or ions into the cell.
Subtypes of Facilitated Diffusion:
- Dialysis
- Definition: Dialysis refers to the diffusion of solutes through a selectively permeable membrane, allowing only specific molecules or ions to pass through.
- Example: The process in kidney dialysis, where waste products are filtered from the blood.
- Osmosis
- Definition: Osmosis is the movement of solvent molecules (usually water) through a semi-permeable membrane from an area of lower solute concentration to an area of higher solute concentration.
- Example: Water movement in plant roots, or the absorption of water in human cells.
- Types of Osmosis:
- Endosmosis: Movement of solutions into the cell.
- Exosmosis: Movement of solutions out of the cell.
Each type of diffusion plays a key role in maintaining cellular functions, whether it’s the movement of gases in respiration or water regulation in plants.
Causes of Diffusion
Diffusion is a natural and self-driven process that occurs without the need for external force or stirring. It’s driven by several underlying causes.
- Random Movement of Molecules
- Molecules in liquids and gases move randomly due to their inherent kinetic energy.
- This random motion leads them to collide with one another and change direction, facilitating diffusion.
- Concentration Gradient
- The difference in concentration between two regions is a key cause of diffusion.
- Molecules tend to move from areas of higher concentration to lower concentration, seeking equilibrium.
- Molecular Collisions
- Molecules constantly collide with each other in any given medium.
- These collisions contribute to the movement of particles from a densely packed area to a less dense area, aiding the diffusion process.
- Kinetic Energy
- The energy within molecules makes them move in random directions.
- This movement from one area to another is driven by the molecules’ kinetic energy, which is constantly in play.
Factors Affecting Diffusion
Diffusion is the movement of particles from an area of higher concentration to an area of lower concentration. Several factors influence the speed and extent of this process, shaping how quickly substances spread in various environments. These factors operate together, either speeding up or slowing down diffusion.
- Particle Size
- Smaller particles diffuse more quickly than larger ones.
- Larger particles face more resistance as they move, slowing down the process.
- Temperature
- Higher temperatures cause particles to move faster, which speeds up diffusion.
- As the temperature increases, the energy of the particles increases, leading to faster movement.
- Viscosity and Density of the Medium
- The viscosity (thickness) of the medium impacts how easily particles can move through it.
- A denser medium creates more resistance to particle movement, slowing diffusion.
- Surface Area
- A larger surface area allows for more particles to interact at once, speeding up diffusion.
- More contact points for particles to spread out means a faster diffusion process.
- Distance to Travel
- The further particles need to travel, the longer the diffusion process takes.
- Shorter distances between the high and low concentration areas allow for quicker diffusion.
- Concentration Gradient
- A steeper concentration gradient (a larger difference in concentration) leads to faster diffusion.
- The greater the disparity between areas, the more quickly particles will move toward equilibrium.
- Availability of Transport Proteins
- Some particles, especially in biological systems, rely on transport proteins to move across membranes.
- If these proteins are abundant, diffusion can happen more efficiently.
- Solubility of the Particle
- Solubility in the medium plays a role in diffusion speed.
- More soluble substances diffuse faster because they interact more readily with the medium.
Examples of Diffusion in Daily Life
Diffusion is at play all around us, from the movement of gases in the air to the spreading of flavors in our food. Here are several clear examples of diffusion in action:
- Perfume Scent in the Air
- When a bottle of perfume is opened, the scent gradually spreads across the room.
- The perfume molecules move from an area of high concentration (the bottle) to areas of lower concentration (the room).
- Sugar in Tea or Coffee
- When sugar is added to a hot drink, it dissolves and spreads throughout the liquid.
- The sugar molecules disperse without needing to be stirred, due to the diffusion process.
- Tea Bag in Hot Water
- A tea bag immersed in hot water slowly releases its flavor and color as the tea particles diffuse into the water.
- The water molecules interact with the tea leaves, spreading the flavor evenly.
- Incense Smoke
- When an incense stick is lit, the smoke diffuses into the air, filling the room with fragrance.
- The smoke particles spread from the area near the incense stick to other parts of the room.
- Salt in Water
- When salt is added to water, the salt particles spread evenly throughout the liquid through diffusion.
- No stirring is needed as the salt dissolves and disperses naturally in the water.
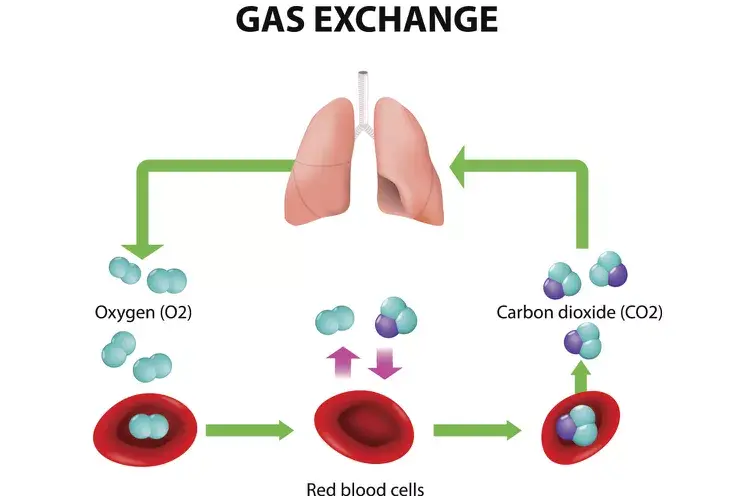
- Respiration in Cells
- During respiration, oxygen diffuses into cells for energy production.
- At the same time, carbon dioxide, a waste product, diffuses out of the cells and into the bloodstream to be removed from the body.
- Heat Transfer in a Pan
- When a pan is heated, the heat spreads from the burner through the metal of the pan.
- The heat molecules move from areas of higher temperature to cooler parts of the pan, making the entire surface warm.
- Rehydration of Dried Noodles
- When boiling water is added to dried noodles, the water diffuses into the noodles.
- This process makes the noodles plumper and hydrated as they absorb water.
Significance of Diffusion
Diffusion is a key process that drives several biological and chemical activities, essential for life as we know it. From respiration in animals to nutrient absorption in plants, diffusion is involved in countless processes that sustain life.
- Cellular Transport
- Cells rely on diffusion to move essential molecules like glucose, oxygen, and ions across their membranes.
- Without diffusion, these vital substances wouldn’t reach the areas where they’re needed for cellular functions.
- Respiration
- In respiration, carbon dioxide is diffused from the cells through the membrane into the bloodstream for removal.
- This process ensures the proper exchange of gases, which is crucial for energy production in cells.
- Nutrient and Water Absorption in Plants
- In plants, diffusion is responsible for absorbing water and nutrients from the soil through root hair cells.
- This enables plants to maintain hydration and acquire the necessary elements for growth and development.
- Neuronal Activity
- The movement of ions across neurons, which is fundamental to generating electrical charges and transmitting nerve signals, is driven by diffusion.
- This process is critical for communication within the nervous system, allowing the body to respond to stimuli.
- Chemical Reactions in Labs and Industries
- Diffusion also plays a significant role in the mixing of reactants during chemical reactions.
- In both laboratories and industrial settings, diffusion helps ensure that substances interact efficiently for desired chemical changes.
Difference Between Diffusion and Osmosis
Diffusion and osmosis are both processes that involve the movement of particles, but they are distinct in their mechanics and biological roles. Understanding their differences is key to grasping how substances move within living organisms.
- Definition
- Diffusion is the movement of particles from an area of high concentration to an area of low concentration due to random motion and kinetic energy.
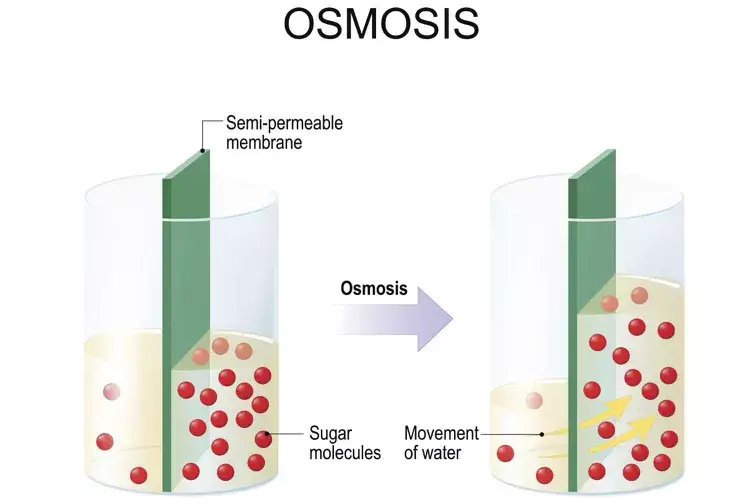
- Osmosis is the movement of solvent (usually water) across a semipermeable membrane, moving from an area of low solute concentration to an area of high solute concentration.
- Type of Movement
- Diffusion involves the movement of particles from various substances.
- Osmosis mainly involves water molecules, making it a specific type of diffusion.
- Medium
- Diffusion can occur in gases, liquids, and solids as particles move freely in these states.
- Osmosis typically occurs in liquids, such as water, where water molecules move across membranes.
- Membrane Presence
- Diffusion may or may not involve a membrane, as particles can spread in open space or across boundaries.
- Osmosis always involves a semipermeable membrane, which only allows solvent (usually water) to pass through, restricting the movement of solutes.
- Solute Presence
- Diffusion involves the movement of any type of particle, including solutes and gases.
- Osmosis specifically concerns the movement of the solvent, which is typically water, and not solutes.
- Direction of Movement
- In diffusion, particles move from areas of high concentration to low concentration, aiming to reach equilibrium.
- In osmosis, water moves from areas of low solute concentration to high solute concentration, driven by the difference in solute concentration on either side of the membrane.
- Biological Significance
- Diffusion is crucial for various cellular processes, including the transport of nutrients and gases, enabling cells to take in oxygen or release carbon dioxide.
- Osmosis is vital for maintaining cell turgidity and regulating fluid balance, especially in plant cells where it helps maintain their structure and hydration levels.
A Simple Diffusion Experiment
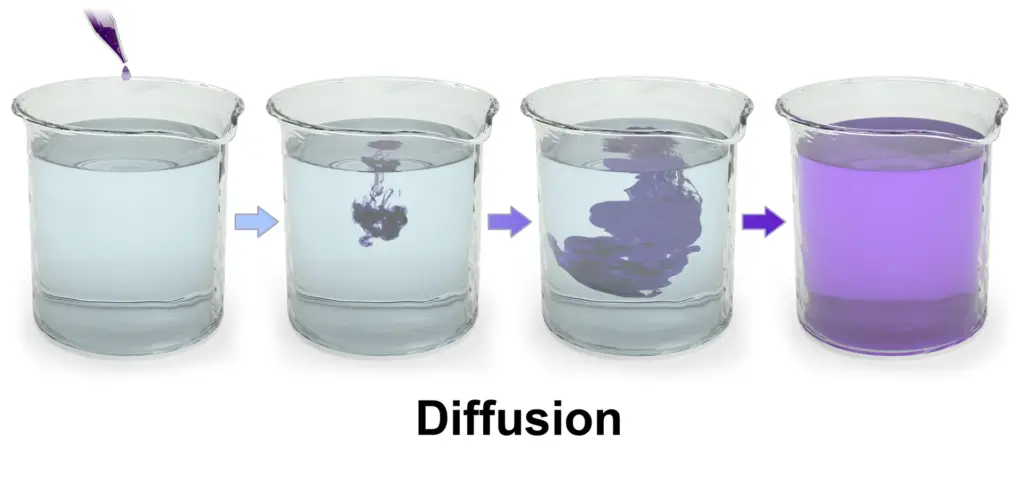
This experiment demonstrates how diffusion works in a liquid. It uses food coloring in water to visually show how particles spread from areas of high concentration to low concentration.
- Step 1: Prepare the Solution
- Fill a beaker with water.
- Add a few drops of food coloring to the water.
- Step 2: Observe the Initial Concentration
- Right after adding the food coloring, the particles are concentrated at the top of the water, where you added them. The surrounding water has a lower concentration of food coloring.
- At this point, the food coloring is denser than the water and will initially sink to the bottom of the beaker.
- Step 3: Wait and Observe the Diffusion Process
- Leave the beaker undisturbed and watch how the food coloring slowly spreads throughout the water.
- Over time, the food coloring moves from areas of high concentration (where it was first dropped) to areas of lower concentration (the rest of the water).
- Step 4: See the Even Distribution
- After a while, the food coloring will evenly distribute through the entire water, turning the solution a uniform red color.
- As the concentration equalizes, the water color will start to fade and become lighter, indicating that the food coloring has been diluted.
- Step 5: Dynamic Equilibrium
- Diffusion stops once there is no longer a difference in concentration—when the food coloring is evenly spread out throughout the water. This is known as dynamic equilibrium.
- At this point, the movement of food coloring particles continues, but there is no net movement since the concentrations are equal.
- https://www.geeksforgeeks.org/diffusion-biology/
- https://byjus.com/biology/diffusion/
- https://biologydictionary.net/diffusion/
- https://en.wikipedia.org/wiki/Diffusion
- https://www.thoughtco.com/what-is-diffusion-3967439
- https://studymind.co.uk/notes/diffusion/
- https://www.biologynotes.site/diffusion-movement-of-substances/
- https://www.sciencefacts.net/diffusion.html
- https://biologyreader.com/examples-of-diffusion-in-daily-life.html
Wow! I found this interesting