What is Corynebacterium diphtheriae?
- Corynebacterium diphtheriae is a Gram-positive bacterium known primarily for causing diphtheria, a potentially life-threatening infection. Discovered in 1884 by German bacteriologists Edwin Klebs and Friedrich Löffler, the bacterium is also called the Klebs–Löffler bacillus. While typically harmless, it becomes pathogenic when infected by a bacteriophage that introduces a gene capable of producing a potent exotoxin. This toxin is responsible for the clinical manifestations of diphtheria, affecting various mucosal layers, particularly in the respiratory tract, and can lead to systemic damage in severe cases.
- The disease begins when C. diphtheriae adheres to and invades the mucosal surfaces of the throat or nose, releasing the exotoxin locally. This toxin can cause tissue destruction at the site of infection, leading to symptoms such as a characteristic pseudomembrane in the throat. Additionally, the exotoxin can enter the bloodstream, causing damage to other organs such as the heart, kidneys, and nerves, a phenomenon known as systemic toxicity. This makes diphtheria a disease with both local and distant effects on the body.
- Diphtheria was once a major cause of childhood mortality, but its impact has been significantly reduced due to the widespread use of the DTaP vaccine, introduced in the early 20th century. Despite this, diphtheria still occurs in regions where vaccination coverage is low, primarily in developing countries. The bacterium has four recognized subspecies: C. d. mitis, C. d. intermedius, C. d. gravis, and C. d. belfanti. These subspecies differ slightly in their biochemical properties, including their ability to metabolize specific nutrients and their colonial morphology. However, all subspecies can be either toxigenic, capable of causing diphtheria, or nontoxigenic.
- Strain subtyping of C. diphtheriae is important for tracking outbreaks and understanding the bacteria’s epidemiology. It involves categorizing different strains based on genetic and phenotypic characteristics. While this process is essential for pinpointing the source of outbreaks, it is often hindered by limited resources for strain identification, which complicates effective monitoring and response efforts. Despite these challenges, the ongoing development and application of molecular techniques hold promise for improving strain classification and outbreak management.
Scientific classification of Corynebacterium diphtheriae
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Mycobacteriales |
| Family: | Corynebacteriaceae |
| Genus: | Corynebacterium |
| Species: | C. diphtheriae |
Geographical Distribution and Habitat of Corynebacterium diphtheriae
Corynebacterium diphtheriae is the causative agent of diphtheria, and its distribution is heavily influenced by geographic, environmental, and immunization factors. Here’s a breakdown of where it’s found and how it thrives:
Geographical Distribution
- Endemic Regions:
In the early 1990s, diphtheria was still endemic in many parts of the world, including:- The Indian subcontinent
- Indonesia
- Philippines
- Brazil
- Nigeria
- Various republics of the former Soviet Union
- The 1990s Outbreak:
The most significant diphtheria outbreak in the developed world occurred between 1990 and 1996 in the states of the former Soviet Union.- This outbreak was unusual because it primarily affected adolescents and adults rather than children.
- Over 110,000 cases were reported, with 2,900 fatalities.
- The outbreak decreased by 1996, likely due to immunization campaigns and early detection measures that followed.
- Other Notable Outbreaks:
Outbreaks have also been seen in areas like:- Central Asia
- Algeria
- Ecuador
- Recent Trends:
In developed countries like the United States, Europe, and Eastern Europe, outbreaks have mostly occurred among alcohol and drug abusers, particularly in populations with low immunization rates.- Since the introduction of widespread immunization procedures in 1994, the case fatality rate has dropped significantly.
Habitat of Corynebacterium diphtheriae
- The upper respiratory tract is the primary habitat for C. diphtheriae.
- It colonizes areas like the throat, pharynx, tonsils, and larynx, where it can cause symptoms of respiratory diphtheria.
- C. diphtheriae can also inhabit the superficial layers of skin lesions.
- This is particularly relevant in cutaneous diphtheria, where the bacteria infect superficial wounds or abrasions.
Morphology of Corynebacterium diphtheriae
Corynebacterium diphtheriae is a Gram-positive bacillus that exhibits notable pleomorphism. Its distinct shape and arrangement contribute to its identification under the microscope.
- Shape and Gram Staining:
- The bacterium is a Gram-positive bacillus.
- It shows maximum pleomorphism, meaning its shape can vary depending on growth conditions.
- Cell Arrangement:
- C. diphtheriae cells commonly appear in a palisade formation or as individual cells arranged at sharp angles to each other.
- The arrangement forms V-shaped or L-shaped patterns.
- This Chinese letter formation occurs due to incomplete cell division when grown on certain media, like coagulated egg medium or Loeffler’s serum.
- Size and Structure:
- The bacterium measures 3-6 micrometers in length and 0.6-0.8 micrometers in width.
- The cells are slender with occasionally swollen ends.
- Metachromatic Granules:
- Most cells contain 2-3 granules at the swollen ends.
- These granules stain reddish-purple with Loeffler alkaline methylene blue.
- The rest of the cell appears unevenly stained with the dye.
- These granules are also called volutin granules, Babe-Ernest’s granules, or metachromatic granules.
- These are accumulations of polymerized phosphates, contributing to the bacterium’s characteristic beaded appearance.
- Special Staining:
- The metachromatic granules can be visualized using special stains such as Albert stain, Neisser stain, and Ponder stain.
- Other Features:
- C. diphtheriae is non-motile and does not form spores.
Genome of Corynebacterium diphtheriae
The genome of Corynebacterium diphtheriae, the bacterium responsible for diphtheria, is both compact and complex, providing key insights into its pathogenic potential and mechanisms.
- Genome Size:
- The total length of the genome is 2,488,635 nucleotides. This compact genome is packed with essential genetic material required for survival and infection.
- Gene Count:
- The genome contains a total of 2389 genes, with 2272 of these encoding proteins that contribute to the bacterium’s metabolic and structural functions.
- Structural RNA Genes:
- There are 69 structural RNA genes, which are critical in regulating various cellular processes, including protein synthesis and stress responses.
- Gene Coding Efficiency:
- An impressive 87% of the genome consists of coding sequences, meaning that a significant portion of the genome is dedicated to functions directly involved in the bacterium’s survival and pathogenicity.
- G+C Content:
- The genome has a G+C content of 53%, indicating a relatively balanced proportion of guanine (G) and cytosine (C) bases, which can affect the stability and mutation rate of the genome.
- Absence of Plasmids:
- Corynebacterium diphtheriae lacks plasmids, meaning its genetic material is contained entirely within the chromosomal DNA, with no additional extrachromosomal DNA.
- Pathogenicity Islands (PAIs):
- The bacterium’s genome contains pathogenicity islands (PAIs), which are clusters of genes that contribute to its virulence. These islands play a crucial role in the bacterium’s ability to cause disease, including the production of toxins like the diphtheria toxin.
Culture and Biochemical Reactions of Corynebacterium diphtheriae
Corynebacterium diphtheriae is an aerobic and facultative anaerobic bacterium, but it grows optimally in aerobic conditions. The bacterium’s growth requirements and its behavior on different culture media provide key insights into its identification and pathogenic potential.
- Growth Conditions:
- C. diphtheriae thrives at 37°C and a pH range of 7.2–7.4.
- The bacterium grows best on media enriched with blood, serum, or egg.
- Loeffler’s serum slope is an enriched medium that enhances the growth of C. diphtheriae. This medium allows the bacteria to produce luxuriant growth within 6-8 hours at 37°C.
- On this medium, colonies start small, circular, opaque, and white, but after prolonged incubation, they become larger with a distinct yellow tint.
- Loeffler’s serum slope is selective, inhibiting the growth of certain bacteria like streptococci and pneumococci.
- Selective Media:
- MacLeod’s or Hoyle’s tellurite blood agar are examples of selective media used to culture C. diphtheriae.
- These media contain tellurite (0.04%), which inhibits the growth of contaminant bacteria, helping to isolate the target organism.
- C. diphtheriae on tellurite agar forms gray or black colonies after 48 hours of incubation. The bacteria reduce tellurite to metallic tellurium, which incorporates into the colonies, giving them a characteristic black color.
- Growth on this medium also requires nicotinic acid, pantothenic acid, and, in some cases, other vitamins like thiamine or biotin.
- For optimal toxin production, the medium needs to be supplemented with amino acids and a source of iron.
- Biotypes of C. diphtheriae:
C. diphtheriae is classified into three biotypes based on the colony morphology seen on tellurite agar:- Mitis: Small, round, convex, and black colonies.
- Intermedius: Small, flat, and gray colonies.
- Gravis: Large, irregular, and gray colonies.
These distinctions, once thought to correlate with disease severity, are now considered less reliable for determining the disease outcome, though they remain useful for epidemiological classification.
- Biochemical Reactions:
- C. diphtheriae ferments various sugars, including glucose, galactose, maltose, and dextrin, producing acid but no gas.
- It does not ferment lactose, mannitol, or sucrose, although some virulent strains can ferment sucrose.
- The bacterium lacks proteolytic activity, does not hydrolyze urea, and does not produce phosphatase.
- Testing for sugar fermentation is typically done using Hiss’s serum water.
Virulence Factors of Corynebacterium diphtheriae
The primary virulence factor of Corynebacterium diphtheriae is its ability to produce diphtheria toxin, a powerful exotoxin that plays a central role in the pathogenicity of the bacteria. Here’s a breakdown of how the bacteria produce and regulate this toxin, along with its implications:
- Diphtheria Toxin Production:
- The diphtheria toxin is not produced by all strains of C. diphtheriae. Only those strains that are lysogenized with bacteriophages carrying the tox gene can produce the toxin.
- When the phage DNA integrates into the bacterium’s genome, the bacteria gain the ability to synthesize the toxin. This integration results in the formation of tox strains.
- Genetic Control of Toxin Synthesis:
- The gene for toxin production is located on the chromosome of the prophage (the viral DNA integrated into the bacterial genome).
- The expression of this gene is tightly controlled by a repressor protein produced by the bacteria. The repressor inhibits toxin production under normal conditions.
- The repressor’s activity is influenced by iron levels. It is activated when iron is abundant, leading to the suppression of toxin production.
- When iron levels are low, the repressor protein is deactivated, triggering the bacteria to produce high levels of the diphtheria toxin.
- Impact of Toxin:
- The diphtheria toxin is a polypeptide exotoxin that interferes with protein synthesis in host cells, leading to cell death and the formation of a characteristic pseudomembrane in the throat, a hallmark of diphtheria.
- The production of this toxin is a crucial factor in the severity of the disease, as it leads to widespread tissue damage, especially in the respiratory and cardiovascular systems.
Pathogenesis of Corynebacterium diphtheriae
Corynebacterium diphtheriae primarily causes diphtheria through its ability to produce diphtheria toxin, which plays a central role in both local tissue damage and systemic spread. Here’s how the infection progresses step-by-step:
- Entry and Initial Infection:
- C. diphtheriae typically enters the body through the upper respiratory tract, but it can also infect through the skin, genital tract, or even the eye.
- The bacteria initially adhere to the infected site, commonly starting in the tonsils or oropharynx.
- The infection can then spread to the nasopharynx, larynx, and trachea.
- Local Tissue Damage:
- After attachment, the bacteria rapidly multiply in the epithelial cells, forming a local lesion.
- The bacteria secrete exotoxins, which lead to necrosis (death) of the cells in that area.
- As the cells die, an inflammatory response occurs, with red blood cells, necrosed cells, bacteria, fibrin, and lymphocytes accumulating at the site.
- This results in the formation of the characteristic pseudomembrane, a thick, leathery, grayish-blue or white membrane that adheres tightly to the underlying mucosa.
- If the pseudomembrane is forcibly removed, it exposes a raw, bleeding surface.
- The membrane can spread down the bronchial tree, leading to respiratory tract obstruction and difficulty breathing (dyspnea).
- Toxin Absorption and Systemic Spread:
- While C. diphtheriae growth is confined to the oral cavity, the toxin is absorbed into the bloodstream, leading to toxemia (toxins circulating in the blood).
- This absorption results in systemic manifestations of diphtheria, such as demyelinating peripheral neuritis and myocarditis.
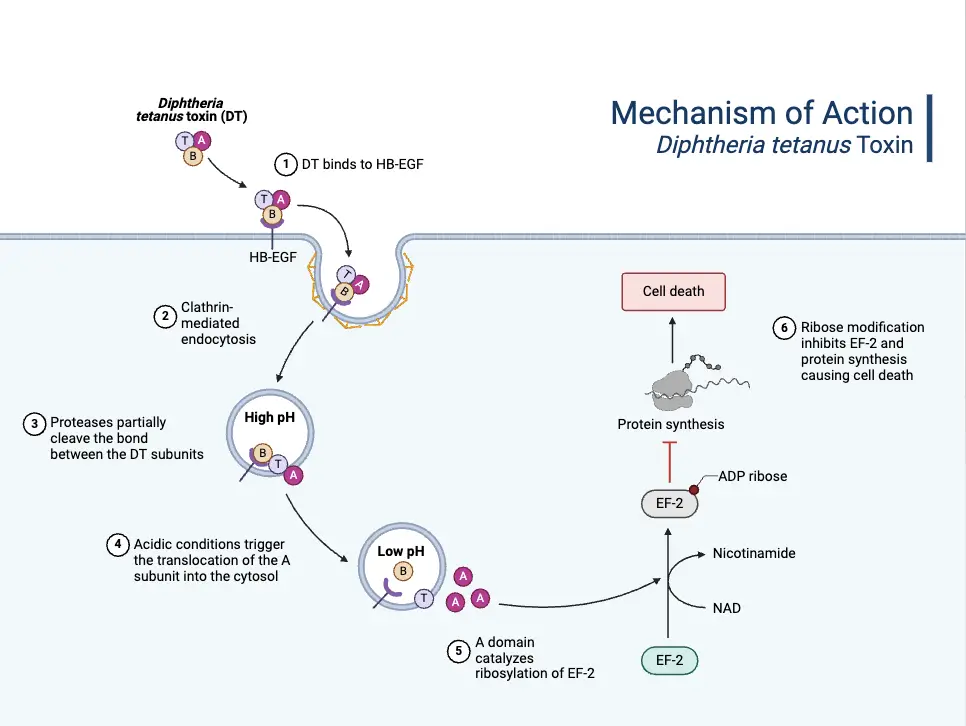
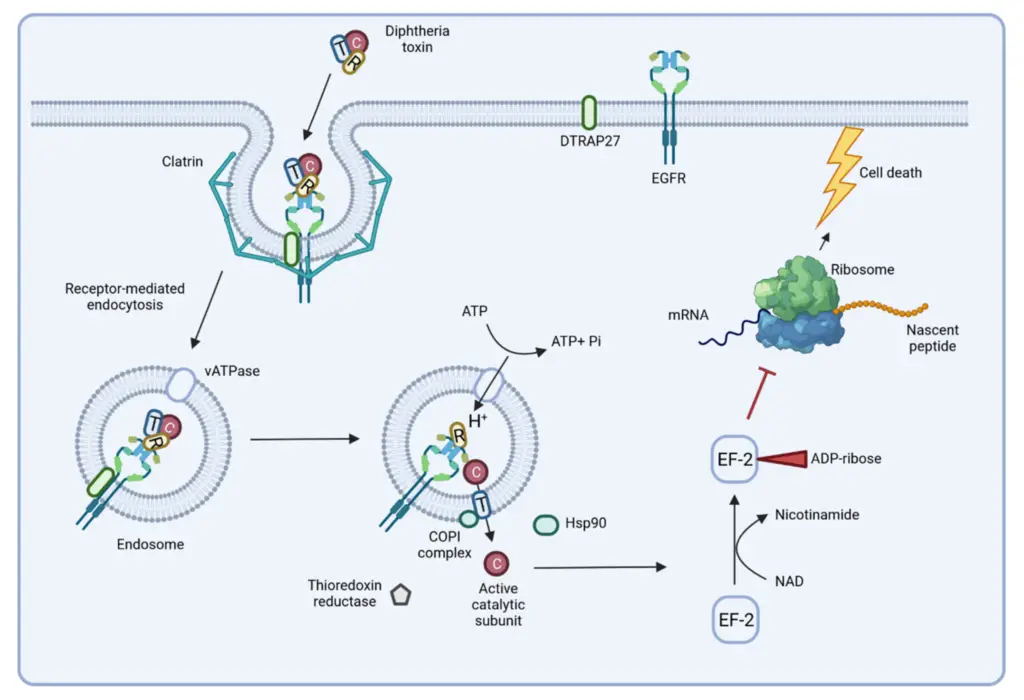
- Mechanism of Toxin Action:
- The diphtheria toxin binds to a specific receptor on susceptible cells, known as the HB-EGF receptor, and enters the cell through receptor-mediated endocytosis.
- Inside the cell, the toxin’s A subunit is cleaved and released from the B subunit.
- The A subunit then enters the cell’s interior, where it catalyzes ADP ribosylation of NAD+, leading to the inhibition of protein synthesis by inactivating EF-2 (elongation factor 2).
- This disruption of protein synthesis causes cell death, which clinically manifests as the necrotic lesions seen in diphtheria.
- Impact of Diphtheria Toxin:
- Locally, the toxin causes tissue destruction at the site of membrane formation (primarily in the upper respiratory tract), aiding the bacteria in replication and transmission.
- Systemically, the toxin can cause serious complications, including damage to nerves (peripheral neuritis) and the heart (myocarditis).
Host Immunity Against Diphtheria Infection
Host immunity to diphtheria relies heavily on the presence of antitoxin in the bloodstream, which can either be developed through natural exposure to the bacteria or through active immunization. Here’s how immunity works in response to Corynebacterium diphtheriae infection:
- Immunity in Infants:
- Infants younger than 6 months carry IgG antibodies derived from the mother. These antibodies are passed either transplacentally during pregnancy or through breastfeeding.
- As the child grows, they start to produce IgG and IgA antibodies of their own in response to infections or immunizations.
- Immunity in Endemic Areas:
- In regions where diphtheria is still common and where mass immunization isn’t practiced, young children remain highly vulnerable to infection.
- Those who recover from diphtheria may continue to carry the bacteria in the throat or nose for weeks, or even months, acting as carriers of the bacteria.
- Healthy carriers have historically played a key role in spreading diphtheria, ensuring that toxigenic bacteria remain circulating in the population.
- Assessing Immunity:
- The presence of antitoxin in the blood can determine an individual’s immunity to diphtheria.
- Schick’s test, introduced in 1913, is a method to assess immunity in children. It involves injecting small amounts of highly purified diphtheria toxin into the skin and observing the reaction.
Schick’s Test Results and Reactions:
- Positive Reaction:
- In this case, a local inflammatory response appears at the injection site within 4–7 days.
- This suggests the individual has no immunity to C. diphtheriae and has not developed sufficient antitoxin levels.
- Negative Reaction:
- If no inflammation occurs, it indicates the presence of antitoxin in the individual’s blood.
- This person is immune to diphtheria, as their immune system can neutralize the toxin.
- Pseudoimmune Reaction:
- In endemic areas, individuals may show an allergic response to the toxin, even if they are immune.
- In this case, both the test and control injections will cause an inflammatory reaction.
- The inflammation peaks within 36 hours and subsides by 72 hours, indicating hypersensitivity but no active infection.
- Combined Reaction:
- This occurs when a person shows inflammation in both the test and control arms, but the inflammation in the control arm is milder and resolves sooner.
- This reaction suggests the individual is not immune and has hypersensitivity to the toxin, requiring attention and possible immunization.
Clinical Syndromes of Diphtheria
The clinical presentation of diphtheria varies widely based on several factors, including the patient’s immune status, the virulence of the infecting bacteria, and the site of infection. Here’s a breakdown of the common clinical syndromes:
- Factors Influencing Disease Severity:
- Immune status: Nonimmune patients experience severe disease, while partially immune individuals have milder symptoms. Fully immune individuals may show no symptoms at all.
- Virulence of the bacteria: Toxigenic strains cause more severe disease, while nontoxigenic strains lead to milder conditions.
- Site of infection: The location of infection determines the severity of symptoms and complications.
Respiratory Diphtheria
- Incubation period: Typically between 2 to 5 days after exposure.
- Initial symptoms:
- Often starts with a sore throat without significant systemic symptoms.
- Fever, malaise, dysphagia (difficulty swallowing), and headache are common.
- Characteristics:
- A fibrinous pseudomembrane forms in the upper respiratory tract (palate, pharynx, epiglottis, larynx, or trachea).
- The membrane is thick, gray-brown, and firmly adherent, and it may cause bleeding if disturbed.
- Respiratory distress can occur if the membrane detaches and blocks the airway.
- The bull neck sign, marked edema (swelling) of the tonsils, uvula, submandibular area, and anterior neck, is characteristic.
- Complications:
- Myocarditis is the most common and can occur in up to two-thirds of cases.
- Circulatory collapse, heart failure, atrioventricular blocks, and dysrhythmias are potential complications.
- Cranial nerve involvement can lead to paralysis of the soft palate, causing difficulty swallowing and nasal regurgitation.
- Polyneuritis can affect the lower extremities, but recovery is typically complete in both cases.
Cutaneous Diphtheria
- Cause: Often caused by nontoxigenic strains of C. diphtheriae.
- Symptoms:
- Presents as a superficial ulcer with a gray-brown membrane that does not heal easily.
- Typically occurs at sites of mild trauma or bruising, with pain, tenderness, erythema (redness), and exudate.
- The condition often affects the extremities more than the trunk or head.
- Course of Disease:
- The infection is generally indolent and nonprogressive, lasting for weeks to months.
- Toxic complications and symptomatic respiratory tract infection occur rarely.
- Cutaneous diphtheria usually causes no toxicity and may lead to natural immunity.
- Epidemiology:
- It can cause epidemics in populations with low immunization rates, even though it is less severe than respiratory diphtheria.
Diphtheria of Other Sites
- External ear, eye, and genital mucosa:
- Eye infections often affect the palpebral conjunctivae, leading to local irritation.
- Genital diphtheria can involve mucosal membranes, though it’s a rare manifestation.
- Endocarditis:
- Rare cases of endocarditis have been reported, mostly caused by nontoxigenic strains.
- Septicemia:
- Septicemia from C. diphtheriae is an extremely rare but fatal condition.
Reservoir, Source, and Transmission of Infection of Corynebacterium diphtheriae
Corynebacterium diphtheriae is a bacterium that infects humans, and humans are its sole natural host. Understanding how it spreads is key to controlling its transmission. Here’s a breakdown of its transmission dynamics and sources of infection:
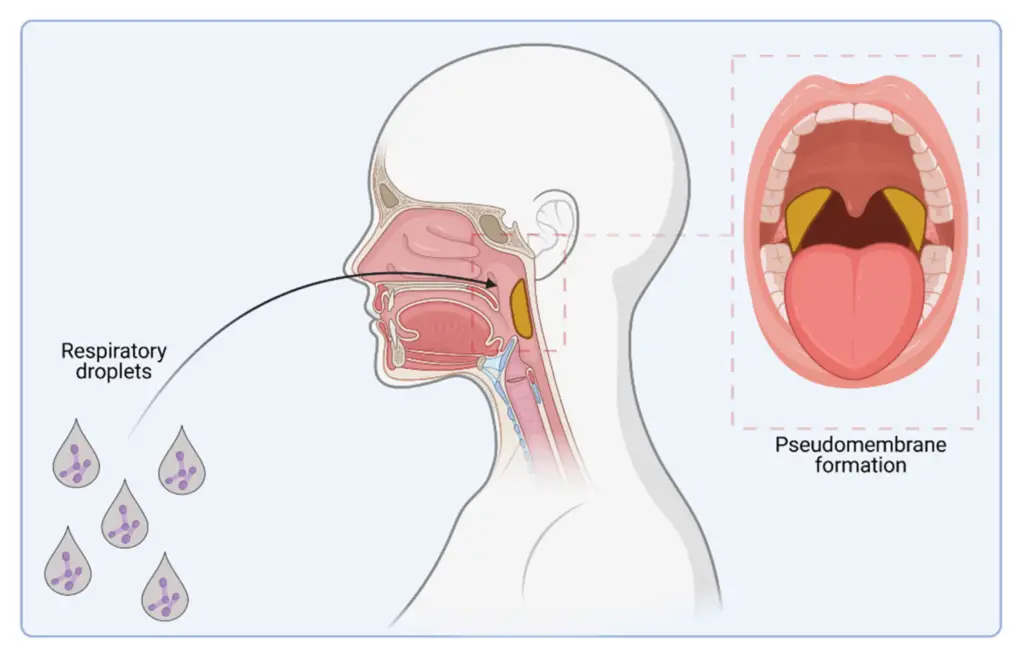
Reservoir and Source of Infection
- Humans as the Only Natural Host:
- C. diphtheriae relies exclusively on humans for its survival and transmission.
- There are no animal reservoirs for the bacteria.
- Common Sources of Infection:
- The primary sources are infective droplets from respiratory secretions or nasopharyngeal excretions.
- Infected skin lesions can also serve as a source.
Transmission of Infection
- Direct Human Contact:
- Close contact with an infected person is the most frequent mode of transmission.
- People with active infections are the main spreaders of diphtheria.
- Respiratory Droplets:
- Transmission occurs most often through droplets from the nose or throat of an infected person, especially when they cough, sneeze, or talk.
- Skin Lesions:
- In addition to respiratory droplets, infection can spread from skin lesions, particularly when the lesions are infected.
- These skin lesions are more commonly associated with cutaneous diphtheria, which is less severe but still transmissible.
- Asymptomatic Carriers:
- People who do not show symptoms of diphtheria but carry the bacteria in their respiratory tract can spread the infection.
- These asymptomatic carriers play a significant role in the transmission, especially in communities where immunization is low.
- Immunization appears to reduce the likelihood of carrying the bacteria asymptomatically.
- Other Routes of Transmission:
- Dust and fomites (objects or materials likely to carry infection) can carry the bacteria.
- C. diphtheriae can survive for up to 6 months in these environments, increasing the potential for indirect transmission.
Shifts in Epidemiology
- Shifting to Adults:
- While children under 15 years used to be the primary demographic for diphtheria infections, this has changed in recent years.
- Adults, especially those who did not receive the full series of booster doses or were not naturally exposed during childhood, have become more susceptible.
- A serosurvey in the U.S. and other developed countries (such as Sweden, Italy, and Denmark) found that 25% to 60% of adults lacked protective antitoxin levels.
- The elderly population tends to have particularly low levels of protection.
Strain Typing
- C. diphtheriae strains can be classified into various serotypes based on their reactions to agglutination.
- Mitis strains: 40 types.
- Intermedius strains: 4 types.
- Gravis strains: 13 types.
- Gravis Type II strains are found worldwide, while Type IV is mainly seen in Egypt and Type V in the United States.
- Strain typing can also be done through:
- Biotyping
- Lysotyping
- Molecular biology techniques, including restriction enzyme digestion patterns and genetic probes for bacterial sequences.
Laboratory Diagnosis of Corynebacterium diphtheriae Infection
Diagnosing diphtheria in the lab goes beyond just confirming a case—it’s about identifying the bacteria and determining its toxigenic potential for public health control. The process involves several steps from specimen collection to toxigenicity testing.
Specimens Collection
- Type of Specimens:
- Swabs are taken from the throat, nose, or directly from any pseudomembrane, if present.
- If possible, a piece of the pseudomembrane or biopsy tissue can be collected.
- At least two swabs should be taken: one for direct smear preparation and the other for culture.
- Specimens must be collected early, even if antibiotics have already been started, to ensure accurate results.
- Transport specimens to the laboratory in sterile containers or silica gel sachets to avoid contamination.
Microscopy
- Gram Staining:
- The smear of the specimen, when stained with Gram stain, shows Gram-positive bacilli, which are characteristic of Corynebacterium species.
- Albert, Neisser, or Ponder stains highlight metachromatic granules in the bacteria, which are also common in C. diphtheriae.
- Limitations:
- Smear tests may not always reveal the bacteria due to the difficulty in distinguishing them from other commensal corynebacteria found in the throat.
- While the smear can identify other bacteria like Vincent’s spirochetes (causing Vincent’s angina), it’s not conclusive for C. diphtheriae.
Culture
- Medium for Growth:
- Specimens are cultured on nonselective media (like blood agar) and selective media (such as tellurite agar).
- Tellurite agar helps isolate C. diphtheriae from other bacterial flora in clinical samples, including carriers and convalescents.
- After incubation, the bacteria on tellurite agar will produce black to grayish-black colonies within 48 hours.
- Loeffler’s Serum Slope:
- On Loeffler’s slope, the bacteria grow quickly (within 4–6 hours) at 37°C and form small, circular, white opaque colonies.
- If no colonies appear initially, the medium can be reincubated for up to 24 more hours.
Identification of Bacteria
- Biotyping:
- C. diphtheriae strains can be classified based on growth characteristics, carbohydrate fermentation patterns, and hemolysis on sheep blood agar.
- Mitis, Intermedius, and Gravis are the primary biotypes of C. diphtheriae.
- Biochemical Features:
- Toxigenic strains like C. diphtheriae, C. ulcerans, and C. pseudotuberculosis produce cystinase but lack pyrazinamidase activity.
- Modern identification techniques use 16S ribosomal RNA (rRNA) probes to identify genus and species of corynebacteria.
Toxigenicity Testing
All strains of C. diphtheriae need to be tested for their ability to produce toxins, which is key to confirming the severity and transmissibility of the infection.
In Vivo Testing
- Subcutaneous Test:
- A culture of C. diphtheriae is emulsified in broth and injected into two guinea pigs: one protected with diphtheria antitoxin and the other unprotected.
- If the strain is toxigenic, the unprotected guinea pig will die within 4 days with typical toxemia signs. The protected guinea pig remains unaffected.
- Intradermal Test:
- A smaller amount of the culture is injected into the skin of guinea pigs or rabbits.
- The test animal that is not protected shows inflammatory reactions progressing to necrosis within 48–72 hours, while the protected animal shows no such response.
- This test allows testing of a larger number of strains simultaneously without killing the animals.
In Vitro Testing
- Elek’s Gel Precipitation Test:
- This immunoprecipitation test uses a filter paper strip impregnated with diphtheria antitoxin placed across an agar plate.
- The test strain is streaked perpendicular to the strip, and after incubation, a precipitin line forms if the bacteria produce the toxin.
- Negative strains show no precipitin line.
- Tissue Culture Test:
- Tissue culture tests, using cell lines like African green monkey kidney or Chinese hamster ovary, expose eukaryotic cells to toxin produced by the bacterial strain.
- The toxin diffuses into the cells and causes cell death, confirming the strain’s toxigenic nature.
Treatment of Corynebacterium diphtheriae Infection
The treatment for diphtheria must start immediately after clinical diagnosis to prevent complications. The approach combines both antitoxin therapy and antibiotics, each serving a distinct purpose in the treatment process.
Antitoxin Therapy
- Diphtheria Antitoxin:
- This is the primary treatment for diphtheria.
- It’s a hyperimmune antiserum produced in horses and given to neutralize the toxin responsible for the disease.
- The antitoxin works by neutralizing free toxin in the bloodstream before it enters cells. It doesn’t affect toxins that have already entered the cells.
- Dosage:
- The amount of antitoxin needed depends on the severity of the infection, the site of infection, and how long the patient has been ill.
- For more severely ill patients or those with a longer illness duration, higher doses are required.
- The general dose ranges from 20,000–100,000 U.
- Antitoxin is typically administered intravenously over 30–60 minutes.
- Limitations:
- Cutaneous diphtheria (skin infection) does not benefit from antitoxin therapy.
- The antitoxin is also ineffective for treating asymptomatic carriers of the bacteria.
Antibiotic Therapy
- Purpose of Antibiotics:
- Antibiotics help limit toxin production, eliminate the bacteria from the body, and reduce the risk of transmission to others.
- They are crucial for eradicating the bacteria but cannot replace antitoxin therapy in neutralizing the toxin already present in the body.
- Recommended Antibiotics:
- Penicillin and erythromycin are the only antibiotics recommended for diphtheria treatment.
- Both are effective at resolving fever and local symptoms of diphtheria.
- Erythromycin is slightly more effective in eliminating the carrier state, making it preferable for this purpose.
- Eradication Confirmation:
- To confirm that the bacteria have been cleared from the body, two successive negative cultures from the throat, nose, or skin are required, collected 24 hours apart.
- If the cultures remain positive after the initial treatment, erythromycin therapy should be repeated.
Prevention and Control of Corynebacterium diphtheriae Infection
The prevention of diphtheria centers around vaccination, while control measures also involve both active and passive immunization to reduce disease spread and severity.
Active Immunization
- Diphtheria Toxoid Vaccine:
- This is the most effective method for preventing diphtheria.
- The vaccine works by stimulating the body to produce antibodies against the diphtheria toxin, which protects individuals from future infections.
- The vaccine contains inactivated diphtheria toxin, or toxoid, which triggers an immune response without causing the disease.
- Protective Serum Levels:
- A serum antitoxin level of 0.01 IU/mL is generally regarded as the minimum protective threshold.
- A level of 0.1 IU/mL provides more definite protection.
- Maintaining these levels ensures that individuals are prepared to fight off the infection if exposed to C. diphtheriae.
- High-Risk Groups:
- Vaccination is particularly important for those in high-risk categories, such as children, elderly individuals, and immigrants from areas where diphtheria is still common.
- By vaccinating these groups, the overall risk of outbreaks is significantly reduced.
Passive Immunization
- Antidiphtheric Serum (ADS):
- Passive immunization involves the administration of 500–1000 U of antidiphtheric serum (ADS), typically given subcutaneously.
- This serum contains antibodies that neutralize the diphtheria toxin directly.
- It’s usually administered as an emergency treatment for individuals who have been exposed to the bacteria and are at risk of developing severe illness.
- Effectiveness of ADS:
- ADS is highly effective in reducing fatality rates, especially when given before the toxin enters the cells.
- It works best when it interacts with the toxin before it is internalized by cells.
Combined Immunization
- Simultaneous Administration of ADS and Toxoid:
- In certain situations, combined immunization may be used.
- This involves giving ADS in one arm and the diphtheria toxoid in the other.
- After this, a full vaccination schedule with the toxoid is followed to ensure long-term immunity.
- This combination provides immediate protection while also preparing the immune system for future exposure.
Together, active and passive immunization strategies offer comprehensive prevention and control against Corynebacterium diphtheriae. Active vaccination helps build long-term immunity, while passive immunization can provide immediate protection in emergency situations.
- Ott L, Möller J, Burkovski A. Interactions between the Re-Emerging Pathogen Corynebacterium diphtheriae and Host Cells. International Journal of Molecular Sciences. 2022; 23(6):3298. https://doi.org/10.3390/ijms23063298
- https://www.ncbi.nlm.nih.gov/books/NBK7971/
- https://www.ncbi.nlm.nih.gov/books/NBK559015/
- https://www.mayoclinic.org/diseases-conditions/diphtheria/symptoms-causes/syc-20351897
- https://my.clevelandclinic.org/health/diseases/17870-diphtheria
- https://www.cdc.gov/diphtheria/index.html
- https://www.osmosis.org/learn/Corynebacterium_diphtheriae_(Diphtheria)
- https://www.sciencedirect.com/topics/medicine-and-dentistry/corynebacterium-diphtheriae
- https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-020-00805-7
- https://emedicine.medscape.com/article/782051-overview
- https://journals.asm.org/doi/10.1128/jb.00183-12
- https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/corynebacterium-diphtheriae.html
- https://microbewiki.kenyon.edu/index.php/Corynebacterium_diphtheriae
- https://www.ecdc.europa.eu/en/diphtheria/facts
- https://pubmlst.org/organisms/corynebacterium-diphtheriae
- https://en.wikipedia.org/wiki/Corynebacterium_diphtheriae
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.