What is Complement Fixation?
- Complement fixation is a fundamental technique used to determine the presence of antigen-antibody complexes in a sample. It is a classical method that has been widely employed in immunological medical tests. This technique relies on the interaction between antigens, antibodies, and complement proteins.
- In complement fixation, the antigen-antibody complex formed within a solution becomes fixed with complement proteins. This fixation process is crucial because neither the antigen nor the antibody alone is capable of fixing the complement. Fixing the complement indicates that the complement protein is actively participating in the immune response.
- Complement fixation can occur both in vivo, within the human body, and in vitro, in artificial conditions such as laboratory tests. It has been an important tool in diagnosing infections, especially those caused by microbes that are challenging to detect using traditional culture methods. Additionally, it has been utilized in the diagnosis of rheumatic diseases.
- However, in modern clinical diagnostic laboratories, complement fixation has been largely replaced by other serological techniques like Enzyme-Linked Immunosorbent Assay (ELISA) and DNA-based methods for pathogen detection, such as Polymerase Chain Reaction (PCR). These newer methods offer increased sensitivity, specificity, and efficiency, making them more suitable for routine diagnostic use.
- Although complement fixation may not be as commonly employed today, its historical significance and contribution to the field of immunology cannot be overlooked. It played a crucial role in advancing our understanding of antigen-antibody interactions and paved the way for the development of more advanced diagnostic techniques.
Principle of Complement Fixation Test
The principle of the complement fixation test is based on the interaction between antigens, antibodies, and complement proteins. When an antigen and antibody come into contact, they form an antigen-antibody (Ag-Ab) complex. This complex then interacts with complement proteins and becomes fixed with them. As a result, the complement protein undergoes degradation or cleavage, producing smaller and larger fragments.
For example, C2, one of the complement proteins, gets fragmented into C2a and C2b. The larger fragments remain attached to the Ag-Ab complex, while the smaller fragments act as signaling molecules. These signaling molecules provide a signal to macrophages, triggering the engulfment and destruction of the antigen. This process represents a biological or in vivo mechanism.
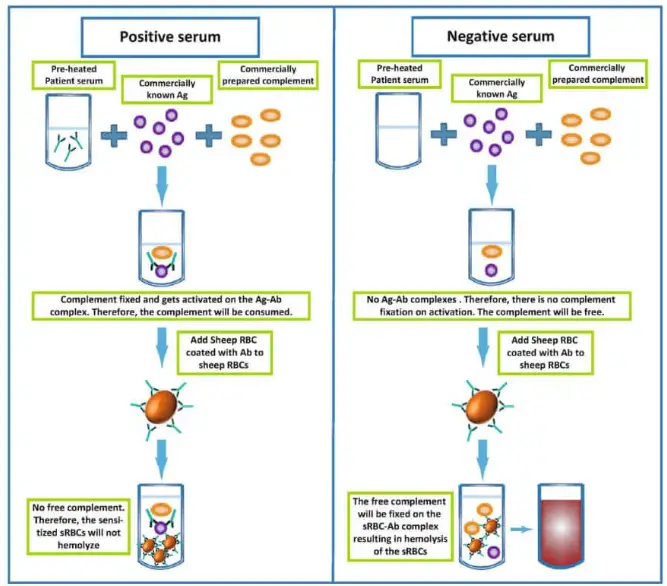
In the complement fixation test, the principle is that only the Ag-Ab complex can fix the complement and have an effect on the hemolysis of red blood cells (RBCs) used in the indicator system.
If the sample being tested contains the desired antibodies or antigens, the Ag-Ab complex will form when a complementary reactant (either antigen or antibody, depending on the component being detected) is added. In this case, the indicator system will not be able to react with the added complement because it has already been fixed with the Ag-Ab complex. As a result, there will be no change in the indicator system.
No change in the indicator system refers to no lysis of RBCs, meaning that there is no hemolysis observed.
In a positive test result, the presence of antibodies in the sample, along with the addition of the corresponding antigen and complement, leads to the formation of the Ag-Ab complex that fixes the complement. When the indicator system is added, no change is observed, indicating no hemolysis.
In a negative test result, a sample without antibodies is mixed with the antigen and complement. In this case, the complement remains free and unbound. However, when the antigen is mixed with antibodies present in the indicator system (on the surface of RBCs), an Ag-Ab complex forms. This complex then interacts with the complement, fixing the complement system, and resulting in hemolysis of the RBCs in the indicator system.
The complement fixation test allows for the detection of specific antibodies or antigens in a sample based on their ability to fix the complement and influence the hemolysis of RBCs in the indicator system.
Requirements for Complement Fixation Test
The complement fixation test requires several specific components and reagents to be conducted effectively. These requirements include:
- Samples: The test typically utilizes samples such as serum (the clear, cell-free liquid portion of blood) or cerebrospinal fluid (CSF). These samples may or may not contain the specific antigens or antibodies of interest that are being detected.
- Known Complementary Antigens: The complement fixation test requires the use of known complementary antigens. These antigens are selected based on the specific component being targeted for detection in the test. The presence or absence of the complementary antigen in the sample will determine the formation of the antigen-antibody complex and subsequent complement fixation.
- Complement Proteins: The native complement proteins present in the sample are typically inactivated or depleted to prevent interference with the test. Complement proteins from other sources, such as the serum of guinea pigs, are added to the sample during the test. These exogenous complement proteins are used to facilitate the complement fixation process.
- Indicator System: The indicator system consists of red blood cells (RBCs) that are used to visualize the presence or absence of hemolysis, indicating complement fixation. In the complement fixation test, sheep erythrocytes or sensitized RBCs are commonly employed as the indicator system. Sensitized RBCs are obtained by coating the surface of RBCs with antibodies, typically derived from rabbit serum. The sensitized RBCs react with the complement system to produce visible changes in the indicator system.
By combining these essential components—samples, known complementary antigens, complement proteins, and the indicator system—the complement fixation test allows for the detection of specific antigens or antibodies based on their ability to fix the complement and induce hemolysis of the indicator RBCs.
Complement Fixation Test Protocol/Procedure
The complement fixation test follows a specific protocol or procedure to ensure accurate and reliable results. The general steps involved in conducting the complement fixation test are as follows:
- Collection of Serum Sample: A serum sample is obtained from the patient or individual being tested. The serum is the liquid component of blood obtained after the blood has clotted and the clot has been removed.
- Heating the Serum: The collected serum sample is heated at approximately 56 °C. This heating step is performed to inactivate or remove any complement proteins that may already be present in the sample. By removing the native complement, it helps prevent interference during the test and ensures that complement fixation is solely due to the interaction of the added complement and the antigen-antibody complex formed.
- Adsorption with Washed Sheep RBC: The heated serum is then adsorbed with washed sheep red blood cells (RBCs). This step is necessary to prevent interference caused by anti-RBC antibodies that may be present in the serum. By adsorbing the serum with sheep RBCs, any cross-reactive antibodies against the sheep RBCs are removed, minimizing potential false-positive results.
- Addition of Antigen and Complement: Known complementary antigens, specific to the target being detected, are added to the adsorbed serum sample. Following the addition of the antigen, the complement proteins obtained from a different source (such as guinea pig serum) are added. The antigen and complement are mixed thoroughly with the sample.
- Incubation: The sample is then incubated at a temperature of 37 °C for a specified duration, typically around 30 minutes. This incubation period provides optimal conditions and sufficient time for the formation of the antigen-antibody (Ag-Ab) complex within the sample.
- Addition of the Indicator System: After the incubation period, the indicator system is added to the sample. The indicator system consists of red blood cells (RBCs), often sensitized sheep erythrocytes or RBCs coated with antibodies derived from rabbit serum. The sensitized RBCs or sheep erythrocytes are used to visualize any changes resulting from the occurrence or non-occurrence of hemolysis.
- Observation of Hemolysis: The sample is carefully observed for any changes in the indicator system. Hemolysis, the rupture or lysis of RBCs, indicates the occurrence of complement fixation. If the complement has been fixed by the antigen-antibody complex, there will be no hemolysis observed, resulting in no change in the indicator system. Conversely, if the complement is not fixed, it remains free, leading to hemolysis of the indicator RBCs and a visible change in the indicator system.
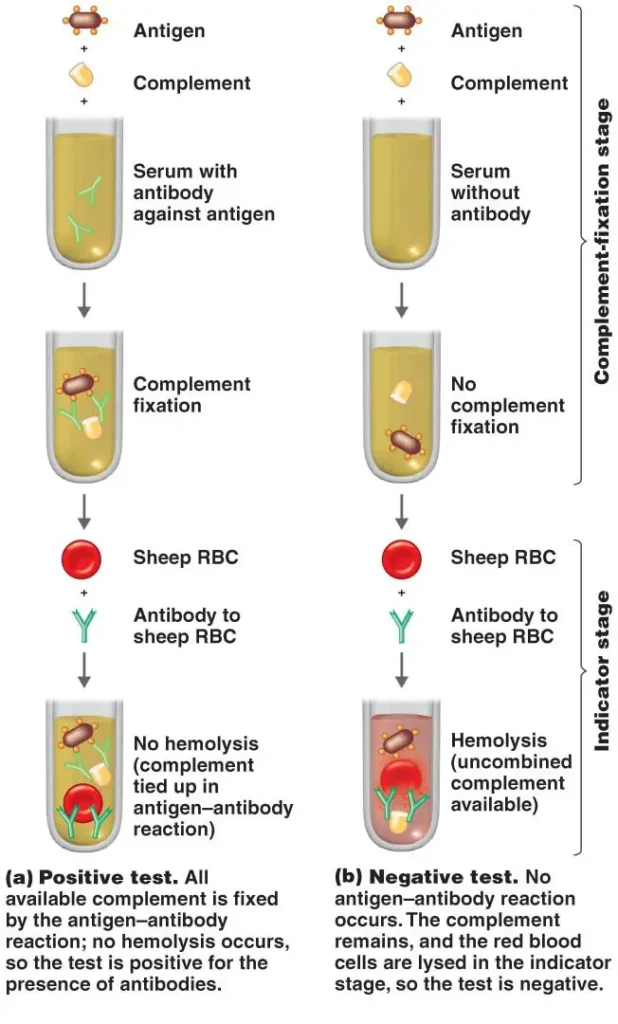
By following this protocol, the complement fixation test allows for the detection and determination of the presence or absence of specific antigens or antibodies in a sample, based on the occurrence or non-occurrence of hemolysis in the indicator system.
Result Interpretation of Complement Fixation Test
The interpretation of the results obtained from the complement fixation test depends on the presence or absence of hemolysis, indicating complement fixation. The result interpretation is as follows:
- Positive Test: If the sample being tested contains the specific antibody or antigen of interest, there will be no change in the appearance of the solution after the test. The sensitized red blood cells (RBCs) used in the indicator system will remain intact and settle down at the bottom of the sample. This lack of hemolysis or change in the solution indicates a positive test result. It suggests that the antigen-antibody complex formed in the sample has fixed the complement proteins, preventing hemolysis.
- Negative Test: Conversely, if there is any change in the appearance of the sample or solution during the test, such as the occurrence of hemolysis, it indicates a negative test result. Hemolysis refers to the rupture or lysis of red blood cells, resulting in a visible change in the solution. This indicates that the complement proteins were not fixed by the antigen-antibody complex, allowing the complement to remain active and induce hemolysis of the sensitized RBCs.
Types of Complement Fixation Test
The complement fixation test encompasses various types that are utilized depending on the specific requirements of the diagnostic investigation. These types include:
- Indirect Complement Fixation Test: This type of complement fixation test is employed when certain serums, such as avian (Parrot, Duck) and mammalian (Cat, Horse) serums, are unable to fix guinea pig complement. In this test, the procedure is carried out in duplicate. After the initial steps, a standard antiserum to an antigen known to fix complement is added to one set. If antibodies are absent in the test serum, the antigen reacts with the standard antiserum, resulting in complement fixation and subsequent hemolysis of sheep red blood cells. Conversely, if antibodies are present in the test serum, the antigen is utilized in the initial steps, preventing any reaction between the standard antiserum and the antigen, leading to a lack of complement fixation. In this case, hemolysis of the indicator system indicates a positive result.
- Conglutinating Complement Absorption Test: In this type of complement fixation test, horse complement, which is non-hemolytic, is used. The indicator system consists of sensitized sheep red blood cells mixed with bovine serum. The bovine serum contains a beta globulin called conglutinin, which can cause agglutination (conglutination) of sheep red blood cells when combined with complement. This agglutination indicates a negative result. However, if horse complement is utilized in the antigen-antibody reaction during the initial steps, agglutination of sensitized cells does not occur, suggesting a positive result.
- Immune Adherence: Certain bacteria, like Vibrio cholera or Treponema pallidum, when combined with their specific antibody in the presence of complement, can adhere to erythrocytes or platelets. This phenomenon is known as immune adherence. Immune adherence facilitates the phagocytosis of bacteria and is utilized in specific diagnostic tests.
- Immobilization Test: In the immobilization test, the antigen is incubated with the patient’s serum in the presence of complement. If a specific antibody is present, it immobilizes the antigen. For example, the Treponema pallidum immobilization test is considered the gold standard for serodiagnosis of syphilis. A positive test result indicates the presence of treponemal antibodies in the serum.
- Cytolytic Tests: In cytolytic tests, live bacteria are incubated with their specific antibodies in the presence of complement, resulting in the lysis of bacterial cells. The Vibriocidal antibody test, which measures anti-cholera antibodies, is an example of a cytolytic test.
These different types of complement fixation tests provide specific insights into the presence of antibodies, antigens, or bacterial interactions in a patient’s sample, contributing to the diagnosis of various infectious diseases.
Applications of Complement Fixation Test
The complement fixation test has various applications in the field of medical diagnostics. Some of the key applications include:
- Syphilis Detection: The Wasserman’s test, which is a type of complement fixation test, is widely used for the detection of syphilis. This test helps in detecting the presence of antibodies against Treponema pallidum, the bacterium responsible for syphilis infection. By measuring complement fixation, the test provides valuable information about the immune response to the infection, aiding in the diagnosis and monitoring of syphilis.
- Bacterial Disease Diagnosis: The complement fixation test can be employed for the diagnosis of bacterial diseases caused by microorganisms such as Mycobacterium pneumoniae (causative agent of atypical pneumonia) and Bordetella pertussis (causative agent of whooping cough). By detecting the presence of specific antibodies against these bacteria, the test assists in confirming the diagnosis and determining the immune status of the patient.
- Viral Infection Detection: The complement fixation test can be utilized for the detection of viral infections. It helps in identifying the presence of specific antibodies against viruses such as influenza, measles, mumps, and rubella. This information is valuable for diagnosing viral illnesses and assessing the immune response of individuals to these viral pathogens.
- Fungal Infection Detection: The complement fixation test is also applicable in the detection of certain fungal infections. It can be used for the diagnosis of diseases caused by fungi like Histoplasma capsulatum (causative agent of histoplasmosis) and Cryptococcus neoformans (causative agent of cryptococcosis). By detecting the presence of specific antibodies against these fungal pathogens, the test aids in confirming the diagnosis and assessing the immune response of the patient.
Advantages of Complement Fixation Test
The complement fixation test offers several advantages in the field of medical diagnostics. These advantages include:
- Easy Result Interpretation: The complement fixation test provides relatively straightforward result interpretation. The absence of hemolysis or change in the indicator system indicates a positive result, while any change or hemolysis suggests a negative result. This simplicity in result interpretation makes it easier for healthcare professionals to assess the presence or absence of specific antibodies or antigens in the tested sample.
- Detection of Low Antigen or Antibody Levels: The complement fixation test is known for its sensitivity, enabling the detection of even very small quantities of antigen or antibody components in the sample. This high sensitivity makes it a valuable tool for diagnosing infections or immune responses where the levels of antibodies or antigens may be low or present in limited quantities.
- Versatile Detection Capability: The complement fixation test has the ability to detect a wide variety of infections caused by different pathogens. It can be employed for the detection of bacterial, viral, and fungal infections, making it a versatile diagnostic tool in the field of infectious diseases. This broad applicability increases its utility and effectiveness in clinical settings.
- Good Sensitivity: The complement fixation test exhibits good sensitivity, allowing for the reliable detection of antigen-antibody complexes and complement fixation. This sensitivity is crucial in accurately diagnosing infections and assessing the immune response of individuals. By detecting even small amounts of antigen-antibody complexes, the test can provide valuable information about the presence and extent of an immune response to a specific pathogen.
Limitations of Complement Fixation Test
While the complement fixation test has its advantages, it also has certain limitations that should be considered. These limitations include:
- Decreased Usage in Current Practices: The complement fixation test is considered an older method and has been largely replaced by more modern diagnostic techniques in current clinical practices. Rapid detection tests, such as enzyme-linked immunosorbent assays (ELISA) and molecular methods like polymerase chain reaction (PCR), have gained popularity due to their faster turnaround time and simplicity.
- Slower and More Complex: Compared to newer diagnostic methods, the complement fixation test is relatively slower and more complex. It involves multiple steps, including heat inactivation, adsorption, incubation, and the addition of reagents, which require careful handling and precision. The longer incubation times and complex procedure can result in delays in obtaining results and may not be suitable for urgent or time-sensitive situations.
- Difficulty in Reagent Arrangement: Performing the complement fixation test can be challenging due to the need for various reagents, including complement proteins, indicator systems, and specific antigens or antibodies. These reagents may require specialized preparation, storage, and handling conditions, making it more cumbersome and logistically challenging to perform the test compared to other serological methods.
- Reduced Sensitivity: Although the complement fixation test is considered sensitive, it may have lower sensitivity compared to more advanced tests such as ELISA. ELISA utilizes specific antibodies labeled with enzymes or fluorophores, allowing for more sensitive and quantitative detection of antigens or antibodies. The complement fixation test, while sensitive in its own right, may have limitations in detecting low levels of antigen-antibody complexes.
FAQ
What is a complement fixation test?
The complement fixation test is an immunological test used to detect the presence of specific antigens or antibodies in a patient’s serum based on the ability to fix complement proteins.
What are the applications of the complement fixation test?
The test can be used for diagnosing infections such as syphilis, bacterial diseases, viral infections, and fungal infections. It has a wide range of applications in serological diagnostics.
Is the complement fixation test still widely used?
The complement fixation test has been largely replaced by newer serological methods such as ELISA and PCR. However, it may still be used in specific cases or research settings.
What are the advantages of the complement fixation test?
Some advantages include easy result interpretation, detection of low antigen or antibody levels, versatility in detecting different infections, and good sensitivity.
Is the complement fixation test a rapid test?
No, the complement fixation test is not a rapid test. It involves multiple steps and requires longer incubation times, making it slower compared to rapid diagnostic tests.
Are there any limitations to the complement fixation test?
Yes, limitations include reduced usage in current practices, complexity in performing the test, challenges in reagent arrangement, and potentially lower sensitivity compared to newer methods.
What is the difference between complement fixation and complement activation?
Complement fixation refers to the binding of complement proteins to antigen-antibody complexes, while complement activation involves the initiation of a cascade of enzymatic reactions leading to the destruction of the target cells.
How does the complement fixation test work?
The test involves the formation of antigen-antibody complexes, which then interact with complement proteins. If the complement is fixed, there will be no change in the indicator system, indicating a positive result.
Can the complement fixation test detect both antigens and antibodies?
Yes, the complement fixation test can detect both antigens and antibodies depending on the specific components used in the test setup.
Is the complement fixation test specific for a particular disease or pathogen?
The complement fixation test can be adapted to detect specific antigens or antibodies, making it applicable for various diseases or pathogens. The specificity depends on the choice of antigens or antibodies used in the test setup.
References
- https://www.clinisciences.com/en/buy/cat-complement-fixation-test-cft-4194.html
- https://www.biotrend.com/en/read/serological-tests-in-mycology-1190/complement-fixation-test-cft-2094.html
- https://www.biologyonline.com/dictionary/complement-fixation-test
- https://www.dshs.texas.gov/laboratory-services/programs-laboratories/serological-analysis-group/serological-analysis-group-tests
- https://link.springer.com/chapter/10.1007/978-3-319-77694-1_10
- https://laboratoryinfo.com/complement-fixation-test/