What is Competitive ELISA?
- Competitive ELISA, also known as the blocking ELISA or inhibition ELISA, is a complex technique used to measure the concentration of an antigen or antibody in a sample by detecting interference in an expected signal output. It is a variation of the standard ELISA assay and can be adapted from various assay types, including direct, indirect, and sandwich ELISA formats.
- The principle behind competitive ELISA involves the competition between a sample antigen or antibody and a reference molecule for binding to a limited amount of labeled antibody or antigen, respectively. The higher the concentration of the sample antigen, the weaker the output signal, indicating an inverse correlation between the signal output and the amount of antigen present in the sample.
- Let’s take an example of a competitive ELISA based on the direct detection method. In this example, a known antigen is used to coat a multiwell plate. After blocking and washing steps, samples containing unknown antigens are added to the wells. A labeled detection antibody is then applied for detection, along with relevant substrates such as 3,3′,5,5′-Tetramethylbenzidine (TMB). If the sample contains a high concentration of antigen, there will be a significant reduction in the signal output. On the other hand, if the sample has very little antigen, there will be minimal reduction in the expected signal output.
- The competitive ELISA technique is particularly useful for determining the concentration of small-molecule antigens in complex sample mixtures. It provides a quantitative measurement by comparing the signal output of the sample with that of a known reference. The competitive format can be applied to different ELISA formats, such as the direct competition ELISA, which incorporates labeled antigen, or the indirect competition ELISA, which uses reporter-labeled antibody.
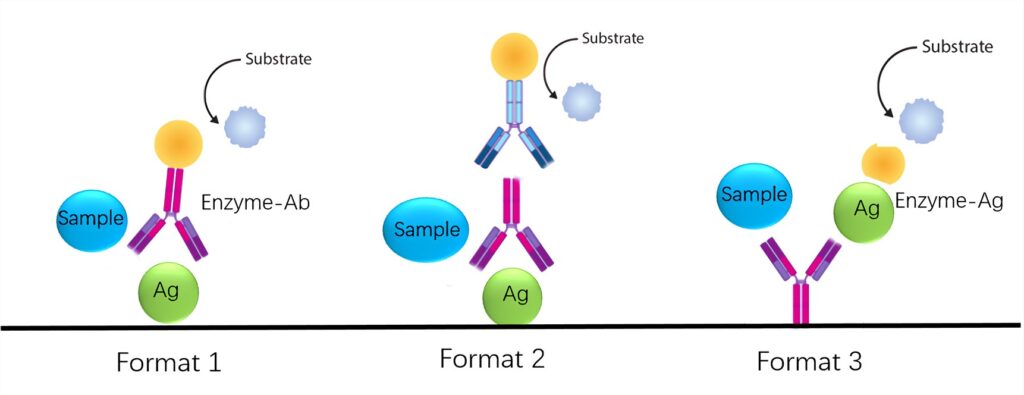
- In the indirect competition ELISA (Format 1), a known antigen standard is adsorbed onto the microtiter plate before incubation with the sample and labeled antibody. Unbound antigen in the sample and labeled antibody are washed away, and a substrate is added. The amount of antigen in the sample determines the amount of labeled antibody bound to the solid antigen, resulting in a signal that is inversely proportional to the antigen concentration in the sample. Thus, higher antigen concentrations in the test sample lead to less binding of solid antigen to the enzyme-labeled antibody and a weaker resultant signal.
- In the indirect competition ELISA (Format 2), known antigen standards are adsorbed onto the microtiter plate before incubation with both primary antibody and unknown test samples. After washing away unbound primary antibody and sample antigen, a secondary antibody and substrate are added. The amount of antigen in the sample determines the amount of reporter-labeled secondary antibody bound to the primary antibody, which directly binds to the solid antigen. This yields a signal that is inversely proportional to the antigen concentration in the sample, with higher antigen concentrations resulting in a weaker signal.
- In the direct competition ELISA (Format 3), an antigen-specific capture antibody is adsorbed onto the microtiter plate before incubation with either a known labeled standard antigen or unknown test samples. After washing away unbound labeled standard antigen and test antigen, a substrate is added. The amount of antigen in the standard or test sample determines the amount of reporter-labeled antigen bound to the capture antibody, resulting in a signal that is inversely proportional to the antigen concentration in the sample. Higher antigen concentrations in the test sample lead to less labeled antigen binding to the capture antibody and a weaker resultant signal.
- In summary, competitive ELISA is a versatile technique used to measure the concentration of antigens or antibodies in a sample. It operates on the principle of competition between a sample molecule and a reference molecule for binding to a limited amount of labeled molecules. The inverse relationship between the signal output and the amount of analyte in the sample allows for quantitative analysis using competitive ELISA.
Principle of Competitive ELISA
The principle of competitive ELISA is based on the competitive binding process that occurs between two specific antibodies and an antigen of interest. In this assay, two antibodies are utilized: one is conjugated with an enzyme, and the other is present in the test serum (if the serum contains the antibodies being tested for).
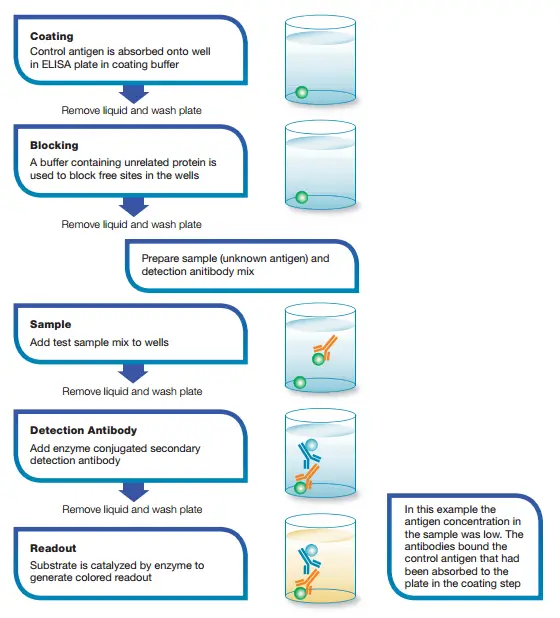
The competitive ELISA begins by coating a solid surface, such as a microtiter plate, with the antigen of interest. This antigen serves as a binding site for the antibodies. Next, the test serum, which may contain the antibodies being detected, is mixed with a known amount of enzyme-conjugated antibodies.
During the incubation step, a competitive binding process takes place. The sample antigen competes with the enzyme-conjugated antibodies for binding to the antibodies present in the test serum. If the sample antigen is present in the test serum, it will bind to the antibodies, leaving fewer binding sites available for the enzyme-conjugated antibodies.
After the incubation period, the unbound components are washed away to remove any excess antibodies or antigens. Then, a substrate specific to the enzyme conjugated to the antibodies is added. If the test serum does not contain the antibodies being detected (negative test), the enzyme-conjugated antibodies will bind to the coated antigen on the solid surface. This results in the conversion of the substrate into a colored product, generating a detectable signal.
Conversely, if the test serum contains the antibodies being detected (positive test), the sample antigen competes with the enzyme-conjugated antibodies for binding to the antibodies in the test serum. As a result, fewer enzyme-conjugated antibodies bind to the coated antigen, leading to a reduced signal or the absence of color.
Therefore, in competitive ELISA, the appearance of color indicates a negative test, meaning the absence of the antibodies being detected. On the other hand, the absence of color indicates a positive test, indicating the presence of the antibodies in the test serum.
It is worth noting that the procedures for performing a competitive ELISA may differ in some aspects compared to other forms of ELISA. The competitive format specifically involves the competition between the sample antigen and the enzyme-conjugated antibodies for binding to the antibodies in the test serum. This competitive binding process is the central event that allows for the detection and quantification of the antibodies in the sample.
Competitive ELISA Animated Video

Reagents
In a competitive ELISA, various reagents are used to facilitate the different steps of the assay. Here are some key reagents commonly employed:
- Coating Buffer: The coating buffer is used to prepare the antigen-coated wells on the microtiter plate. It typically consists of the following components:
- Na2CO3: 1.5 g
- NaHCO3: 2.93 g
- Distilled water: 1 liter, adjusted to a pH of 9.6
- Blocking Buffer: The blocking buffer is used to prevent non-specific binding of antibodies to the solid surface of the microtiter plate. It usually contains phosphate-buffered saline (PBS) with 1% w/v bovine serum albumin (BSA).
- Wash Buffer: The wash buffer is used to wash away unbound antibodies, antigens, and other components during the assay. It typically consists of PBS with a detergent such as Tween-20 at a concentration of 0.05% v/v.
- Substrates and Stop Solution:
- TMB (3,3′,5,5′-Tetramethylbenzidine): This substrate is used with horseradish peroxidase (HRP)-conjugated antibodies. It undergoes a colorimetric reaction in the presence of the HRP enzyme. The reaction is stopped by adding 2M H2SO4.
- pNPP (p-Nitrophenyl phosphate): This substrate is used with alkaline phosphatase (AP)-conjugated antibodies. It undergoes a colorimetric reaction in the presence of the AP enzyme. The reaction is stopped by adding 1M NaOH.
Note: Sodium azide should not be used in any buffers or solutions as it can inactivate the HRP enzyme.
It is important to ensure that all solutions and reagents are at ambient temperature before using them in the assay. This helps to maintain consistency and accuracy during the experimental procedure.
These reagents play crucial roles in the competitive ELISA, from coating the antigen, blocking non-specific binding, washing away unbound components, to generating a detectable signal through substrate-enzyme reactions. By utilizing appropriate reagents and following the recommended protocols, competitive ELISA can provide reliable and quantitative measurements of antigen or antibody concentrations in a sample.
Steps/Process of Competitive ELISA
The process of performing a competitive ELISA involves several key steps:
- Incubation with Primary Antibody: The primary antibody, which is unlabeled, is incubated with the sample antigen. This allows the formation of antibody-antigen complexes.
- Addition to Coated Well Plates: The antibody-antigen complexes are added to well plates that have been pre-coated with the same antigen. The antigen in the well competes with the sample antigen for binding to the primary antibody.
- Removal of Unbound Antibody: The plate is then washed to remove any unbound primary antibody. The amount of unbound antibody is inversely related to the concentration of antigen in the sample. The more antigen present, the less antibody will be able to bind to the antigen in the well, resulting in a competitive binding process.
- Addition of Secondary Antibody: A secondary antibody, specific to the primary antibody and conjugated with an enzyme, is added. This secondary antibody recognizes and binds to the primary antibody-antigen complexes that have remained bound to the coated well.
- Enzymatic Reaction: A substrate that can be acted upon by the enzyme conjugated to the secondary antibody is added. If the secondary antibody has successfully bound to the primary antibody-antigen complexes, the enzyme will act on the substrate and produce a chromogenic or fluorescent signal.
- Signal Detection: The presence or absence of a signal is observed. In a positive test, where antibodies are present in the test serum, the antigen-antibody reaction occurs, and the primary antibody is prevented from binding to the coated antigen. As a result, when the substrate is added, there is no enzyme available to act on it, leading to the absence of a color reaction. In a negative test, where no antibodies are present in the serum, the antigen in the coated wells is available to combine with the enzyme-conjugated antibodies. The enzyme acts on the substrate, producing a color reaction.
By evaluating the presence or absence of a color reaction, the competitive ELISA can determine whether the sample contains the antibodies being tested for.
Protocol of Competitive ELISA
Direct Competitive ELISA
The direct competitive ELISA is a variation of competitive ELISA where the labeled antigen competes with the unlabeled antigen from the sample for binding to the limited amount of specific antibody. Here are the steps involved in conducting a direct competitive ELISA:
- Coat the microtiter plate wells: Add 100 µl of the antibody solution, typically at a concentration of 1-10 µg/ml, to each well of the microtiter plate coated with the specific antibody. Ensure the plate is covered and incubate it overnight at 4°C or for 2 hours at 37°C to allow the antibody to bind to the plate. Wash the plate three times using the wash buffer to remove any unbound antibody.
- Block the plate: Add 150 µl of the blocking solution, commonly a blocking buffer such as BSA (bovine serum albumin) or milk, to each well. Incubate the plate for 1 hour at 37°C to prevent any nonspecific binding. Wash the plate four times with the wash buffer to remove the blocking solution.
- Prepare the antigen mixture: Mix 50 µl of the sample containing the unlabeled antigen with 50 µl of the enzyme-conjugated antigen in a separate tube for each well. Incubate the mixture for 1 hour at 37°C to allow competition between the labeled and unlabeled antigens.
- Add the antigen mixture to the plate: Add 100 µl of the antigen mixture to each well of the microtiter plate. Incubate the plate for 1 hour at 37°C, allowing the competition between the labeled and unlabeled antigens to occur. Wash the plate three times using the wash buffer to remove any unbound antigens.
- Add the substrate solution: Add 100 µl of the appropriate substrate solution to each well. The substrate solution will undergo a chemical reaction with the enzyme conjugated to the labeled antigen, resulting in a color change or the generation of a fluorescent or luminescent signal. Incubate the plate at room temperature, and if necessary, protect it from light, for a specific time (usually 30 minutes) or until the desired color change is achieved.
- Measure absorbance or stop the reaction: Immediately after the incubation period, measure the absorbance of each well at the appropriate wavelength using a microplate reader. Alternatively, if a stop solution is used, add 50 µl of the stop solution to each well, gently tap the plate to mix the contents, and then measure the absorbance within 30 minutes.
- Data analysis: Analyze the obtained absorbance values using appropriate statistical or data analysis methods to determine the concentration or presence of the target antigen in the samples based on a standard curve or comparison with control samples.
The direct competitive ELISA provides a quantitative or qualitative assessment of the target antigen’s concentration or presence in the samples based on the competitive binding between the labeled and unlabeled antigens.
Indirect Competitive ELISA
Method 1
The indirect competitive ELISA is a variation of competitive ELISA where the primary antibody competes with the labeled antigen for binding to the limited amount of specific antigen. Here are the steps involved in conducting an indirect competitive ELISA using Method 1:
- Coat the microtiter plate wells: Add 100 µl of the antigen solution, typically at a concentration of 1-10 µg/ml, to each well of the microtiter plate. Cover the plate and incubate it overnight at 4°C or for 2 hours at 37°C to allow the antigen to bind to the plate. Wash the plate three times using the wash buffer to remove any unbound antigen.
- Block the plate: Add 150 µl of the blocking solution, commonly a blocking buffer such as BSA (bovine serum albumin) or milk, to each well. Incubate the plate for 1 hour at 37°C to prevent any nonspecific binding. Wash the plate four times with the wash buffer to remove the blocking solution.
- Prepare the antigen-antibody mixture: Mix 50 µl of the antigen with 50 µl of the primary antibody in a separate tube for each well in the assay. Incubate the mixture for 1 hour at 37°C to allow the primary antibody to bind to the antigen.
- Add the antigen-antibody mixture to the plate: Add 100 µl of the antigen-antibody mixture to each well of the microtiter plate. Incubate the plate for 1 hour at 37°C, allowing the competition between the labeled antigen and the primary antibody to occur. Wash the plate three times using the wash buffer to remove any unbound antigen-antibody complexes.
- Add the enzyme-conjugated secondary antibody: Add 100 µl of the appropriately diluted enzyme-conjugated secondary antibody in the wash buffer to each well. The secondary antibody will bind to any unoccupied binding sites on the primary antibody. Incubate the plate for 1 hour at 37°C. Wash the plate three times using the wash buffer to remove any unbound secondary antibody.
- Add the substrate solution: Add 100 µl of the appropriate substrate solution to each well. The substrate solution will undergo a chemical reaction with the enzyme conjugated to the secondary antibody, resulting in a color change or the generation of a fluorescent or luminescent signal. Incubate the plate at room temperature, and if necessary, protect it from light, for a specific time (usually 30 minutes) or until the desired color change is achieved.
- Measure absorbance or stop the reaction: Immediately after the incubation period, measure the absorbance of each well at the appropriate wavelength using a microplate reader. Alternatively, if a stop solution is used, add 50 µl of the stop solution to each well, gently tap the plate to mix the contents, and then measure the absorbance within 30 minutes.
- Data analysis: Analyze the obtained absorbance values using appropriate statistical or data analysis methods to determine the concentration or presence of the target antigen in the samples based on a standard curve or comparison with control samples.
The indirect competitive ELISA provides a quantitative or qualitative assessment of the target antigen’s concentration or presence in the samples based on the competitive binding between the primary antibody and the labeled antigen.
Method 2
Indirect competitive ELISA can be performed using different methods. Here are the steps involved in conducting an indirect competitive ELISA using Method 2:
- Coat the microtiter plate wells: Add 100 µl of the antigen solution, typically at a concentration of 1-10 µg/ml, to each well of the microtiter plate. Cover the plate and incubate it overnight at 4°C or for 2 hours at 37°C to allow the antigen to bind to the plate. Wash the plate three times using the wash buffer to remove any unbound antigen.
- Block the plate: Add 150 µl of the blocking solution, commonly a blocking buffer such as BSA (bovine serum albumin) or milk, to each well. Incubate the plate for 1 hour at 37°C to prevent any nonspecific binding. Wash the plate four times with the wash buffer to remove the blocking solution.
- Prepare the antigen-enzyme conjugate mixture: Mix 50 µl of the antigen with 50 µl of the enzyme-conjugated antibody in a separate tube for each well in the assay. Incubate the mixture for 1 hour at 37°C to allow the enzyme-conjugated antibody to bind to the antigen.
- Add the antigen-enzyme conjugate mixture to the plate: Add 100 µl of the antigen-enzyme conjugate mixture to each well of the microtiter plate. Incubate the plate for 1 hour at 37°C, allowing the competition between the unlabeled antigen in the sample and the labeled antigen on the enzyme-conjugated antibody to occur. Wash the plate three times using the wash buffer to remove any unbound antigen-enzyme conjugate.
- Add the substrate solution: Add 100 µl of the appropriate substrate solution to each well. The substrate solution will undergo a chemical reaction with the enzyme conjugated to the antibody, resulting in a color change or the generation of a fluorescent or luminescent signal. Incubate the plate at room temperature, and if necessary, protect it from light, for a specific time (usually 30 minutes) or until the desired color change is achieved.
- Measure absorbance or stop the reaction: Immediately after the incubation period, measure the absorbance of each well at the appropriate wavelength using a microplate reader. Alternatively, if a stop solution is used, add 50 µl of the stop solution to each well, gently tap the plate to mix the contents, and then measure the absorbance within 30 minutes.
- Data analysis: Analyze the obtained absorbance values using appropriate statistical or data analysis methods to determine the concentration or presence of the target antigen in the samples based on a standard curve or comparison with control samples.
The indirect competitive ELISA using Method 2 allows for the detection and quantification of the target antigen in the samples by competing the unlabeled antigen in the sample with the labeled antigen on the enzyme-conjugated antibody. The signal generated from the substrate reaction is proportional to the amount of unlabeled antigen present in the sample, providing valuable information about the antigen’s concentration or presence.
Applications of Competitive ELISA
Competitive ELISA finds numerous applications across various fields due to its specific characteristics and advantages. Here are some key applications of competitive ELISA:
- Detection of Small Molecules: Competitive ELISA is particularly useful for detecting small antigens or molecules that cannot be bound by two different antibodies, as required in the sandwich ELISA technique. In competitive ELISA, a single antibody is employed to compete with the antigen of interest for binding to a limited amount of labeled antigen or antibody. This makes it a preferred choice when only one specific antibody is available for the target antigen or when working with small molecular targets.
- Quantification of Analytes: Competitive ELISA is widely used for the quantitative measurement of various analytes, including hormones, drugs, metabolites, and other small molecules in biological samples. By comparing the signal output (e.g., color intensity) of the test sample with that of a standard curve, the concentration of the analyte can be determined. This makes competitive ELISA valuable in clinical diagnostics, pharmaceutical research, and environmental monitoring for assessing the levels of specific analytes in samples.
- Drug Screening: Competitive ELISA is employed in drug screening assays to determine the presence or concentration of drugs or their metabolites in biological samples. By using a drug or its derivative as the labeled antigen, competitive ELISA can accurately measure drug concentrations in samples such as urine or blood. This application is crucial in drug testing programs, forensic analysis, and therapeutic drug monitoring.
- Veterinary Diagnostics: Competitive ELISA is extensively utilized in veterinary diagnostics for the detection of various pathogens and diseases in animals. It allows for the measurement of specific antibodies or antigens associated with infectious diseases, such as viral, bacterial, or parasitic infections, in animal samples. Competitive ELISA plays a vital role in the early detection, surveillance, and control of animal diseases.
- Food Safety and Quality Control: Competitive ELISA is employed in the food industry for the detection and quantification of contaminants or allergens in food products. It enables the screening of samples for the presence of substances such as pesticides, mycotoxins, antibiotics, and food allergens, ensuring compliance with regulatory standards and ensuring food safety and quality.
- Environmental Monitoring: Competitive ELISA is used in environmental monitoring to measure the levels of specific pollutants, toxins, or environmental contaminants. It allows for the assessment of water quality, soil contamination, and the presence of harmful substances in environmental samples. Competitive ELISA aids in evaluating the impact of pollutants on ecosystems and human health.
Advantages of Competitive ELISA
The competitive ELISA technique offers several advantages that make it a valuable tool in immunoassays. Here are the main advantages of competitive ELISA:
- No Sample Processing: One of the significant advantages of competitive ELISA is that it does not require extensive sample processing. Crude or impure samples can be used directly in the assay without the need for purification or concentration steps. This simplifies the workflow and saves time in sample preparation.
- Robustness: Competitive ELISA is more robust compared to other ELISA formats, such as sandwich ELISA. It is less sensitive to sample dilution and sample matrix effects. This means that variations in sample concentration or the presence of interfering substances in the sample matrix are less likely to affect the accuracy and reliability of the assay results.
- Consistency: Competitive ELISA exhibits greater consistency and reproducibility. There is typically less variability between duplicate samples and assays, leading to more reliable and precise measurements. This consistency is advantageous in research, diagnostics, and quality control applications, where reliable and consistent results are essential.
- Flexibility: Competitive ELISA offers maximum flexibility as it can be based on different ELISA formats, such as direct, indirect, or sandwich ELISA. This adaptability allows researchers and assay developers to choose the most suitable format for their specific needs. The ability to utilize different formats expands the range of applications and increases the versatility of the assay.
Disadvantages of Competitive ELISA
While competitive ELISA offers several advantages, it also has some limitations that should be considered. Here are the main disadvantages of competitive ELISA:
- Similar Limitations as Base ELISA: Competitive ELISA inherits some of the limitations associated with the base ELISA technique. These limitations include the need for well-characterized antibodies, the potential for cross-reactivity or non-specific binding, and the requirement for optimization of various assay parameters. Therefore, the same considerations and precautions taken for base ELISA assays should be applied to competitive ELISA as well.
- Sensitivity: Competitive ELISA may have lower sensitivity compared to other ELISA formats, such as sandwich ELISA. In competitive ELISA, the signal output is inversely proportional to the analyte concentration. As the analyte competes with a labeled reference for binding to limited antibody sites, high analyte concentrations may result in weaker signal outputs, making it more challenging to accurately quantify higher concentrations of the analyte. This can be a limitation when working with samples containing low analyte levels or when precise quantification of high analyte concentrations is required.
- Complexity: Compared to other ELISA formats, competitive ELISA is generally more complex and may require additional optimization steps. The setup and interpretation of competitive ELISA assays can be more involved due to the competitive binding process and the selection of appropriate reference and labeled antigens or antibodies. This complexity may increase the risk of error and necessitate more time and resources for assay development and optimization.
- Interpretation Challenges: Analyzing and interpreting the results of competitive ELISA can be more challenging compared to other ELISA formats. The inverse relationship between signal output and analyte concentration may require careful calibration and standard curve construction to accurately quantify the analyte levels. Additionally, the presence of interfering substances or cross-reactivity with related analytes can complicate the interpretation of results and require additional validation steps.
FAQ
What is Competitive ELISA?
Competitive ELISA is an immunoassay technique used to detect and quantify the presence of an antigen in a sample. It involves competition between the labeled antigen and the unlabeled antigen in the sample for binding to a limited amount of specific antibodies.
What types of samples can be used in Competitive ELISA?
Competitive ELISA can be performed with a variety of sample types, including serum, plasma, cell culture supernatants, and other biological fluids. It is suitable for crude or impure samples, as the competition step helps mitigate interference from nonspecific binding components.
What reagents are needed for Competitive ELISA?
The reagents required for Competitive ELISA typically include coating buffer, blocking buffer, wash buffer, primary antibodies (unlabeled), labeled antigen, and substrate solution specific to the enzyme conjugated to the labeled antigen.
How is the signal detected in Competitive ELISA?
The signal in Competitive ELISA is detected through a chromogenic or fluorogenic reaction. The enzyme conjugated to the labeled antigen catalyzes the conversion of a substrate into a colored or fluorescent product, and the intensity of the signal is measured using a spectrophotometer or a fluorometer.
Can Competitive ELISA be used for quantitative analysis?
Yes, Competitive ELISA can be used for quantitative analysis. By comparing the signal obtained from the sample with a standard curve generated using known concentrations of the antigen, the concentration of the antigen in the sample can be determined.
How does Competitive ELISA work?
In Competitive ELISA, the sample antigen competes with a labeled antigen for binding to the specific antibodies immobilized on the plate. If the sample antigen is present, it will inhibit the binding of the labeled antigen, resulting in a decrease in the signal. A higher concentration of the sample antigen leads to a weaker signal.
What are the advantages of Competitive ELISA?
Some advantages of Competitive ELISA include its suitability for samples with limited or no purification, its robustness against sample dilution and matrix effects, and its ability to detect small antigens that cannot be captured by two different antibodies in the sandwich ELISA.
What are the disadvantages of Competitive ELISA?
The disadvantages of Competitive ELISA are similar to those of other ELISA techniques. It requires a known labeled antigen for competition, which may limit its application for certain antigens. Additionally, the sensitivity of Competitive ELISA may vary depending on the antibody-antigen interaction and the overall assay design.
Can Competitive ELISA be automated?
Yes, Competitive ELISA can be automated using robotic systems or ELISA platforms. Automation helps increase efficiency, reduce manual handling errors, and enable high-throughput analysis.
What are the applications of Competitive ELISA?
Competitive ELISA finds applications in various fields, including medical diagnostics, biomedical research, food safety testing, environmental monitoring, and pharmaceutical development. It is particularly useful when only one specific antibody is available for the antigen of interest or when detecting small antigens that cannot be captured by two different antibodies in a sandwich format.
References
- https://www.antibody-creativebiolabs.com/protocol-of-competition-inhibition-elisa.htm
- https://www.bio-rad-antibodies.com/elisa-types-direct-indirect-sandwich-competition-elisa-formats.html
- https://www.antibody-creativebiolabs.com/competition-inhibition-elisa.htm
- https://www.aatbio.com/resources/faq-frequently-asked-questions/What-is-a-Competition-ELISA
- https://www.abbexa.com/elisa-competitive-inhibition-standard-curve
- https://www.sinobiological.com/category/competitive-elisa-protocol