- Based on genomic investigations of this microbe, Chlamydia’s taxonomy has undergone substantial revisions in recent years.
- Chlamydia is a member of the Chlamydiales order, which encompasses the Chlamydiaceae family.
- Previously, the family had a single genus of four species, Chlamydia (Chlamydia trachomatis, Chlamydia psittaci, Chlamydia pneumoniae, and Chlamydia pecorum).
- Now the family is divided into the genera Chlamydia and Chlamydophila. C. trachomatis is classified within the genus Chlamydia, but C. psittaci and C. pneumoniae are classified inside the new genus Chlamydophila.
- Additionally, there are additional species of unusual human infections that have been assigned to either of the two genera.
Chlamydia
- Chlamydiae are human and animal intracellular parasites having a remarkable preference for the squamous epithelial cells of the gastrointestinal and respiratory systems.
- Chlamydiae were originally thought to be viruses due to their ability to pass through a 0.45-micron filter and their inability to proliferate on cell-free media.
- Previously, these viruses were known as Psittacosis lymphogranuloma trachoma (PLT) viruses or PLT agents.
- However, Chlamydia exhibits the following bacterial characteristics that distinguish them from viruses:
- They both contain DNA and RNA.
- They have the same cell wall as Gram-negative bacteria.
- They contain ribosomes from prokaryotes.
- They reproduce via binary fission.
- They manufacture and synthesis nucleic acid, lipids, and proteins on their own.
- They are sensitive to numerous antibiotics, including tetracyclines, erythromycin, macrolides, and rifampin.
Human infections caused by Chlamydia species
| Bacteria | Diseases |
| Chlamydia trachomatis | Lymphogranuloma venereum, ocular lymphogranuloma venereum, trachoma, adult inclusion conjunctivitis, neonatal conjunctivitis, infant pneumonia, and urogenital infections |
| Chlamydophila pneumoniae | Pharyngitis, sinusitis, bronchitis, and pneumonia |
| Chlamydophila psittac | Psittacosis |
General Properties
There are two morphologically distinct forms of chlamydiae: elementary body and reticulate body.
Elementary body
- The elementary body (EB) is a tiny, infective, extracellular form. It is a circular particle between 300 and 400 nm in diameter.
- The cell wall has a hard trilaminar structure, similar to that of Gram-negative bacterial cell walls.
- Other bacteria have a peptidoglycan coating, however these bacteria do not. However, their outer membrane proteins confer stiffness to the cell wall due to the substantial cross-linking of outer membrane proteins.
- Chlamydiae in the EB type do not reproduce, but are contagious.
- They induce infections by attaching to receptors on epithelial cells and enhance bacterial uptake through infiltration.
Reticulate body
- The reticulate body (RB) is a big type of Chlamydia that is not contagious. It is between 500 and 1000 nm in size.
- It is a metabolically active and replicative Chlamydia strain.
- RBs lack the extensively cross-linked proteins that impart stiffness. This strain of Chlamydia is therefore osmotically unstable and friable.
- However, this form is safeguarded due to its intracellular position. Chlamydia contains a genus-specific lipopolysaccharide (LPS) in its cell wall.
- This LPS can be detected by a complement fixation test (CFT) and outer membrane proteins that are species- and strain-specific.
Growth and Multiplication
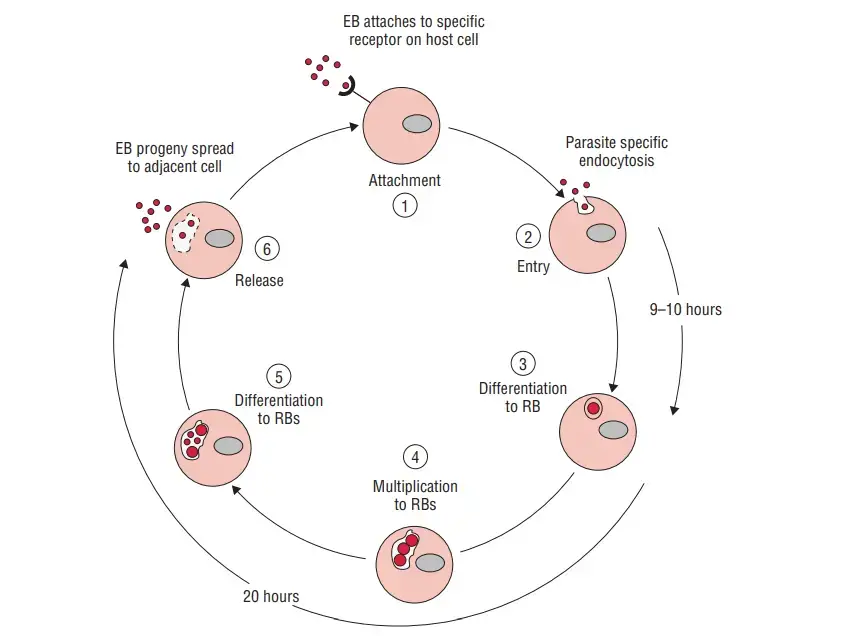
- Within susceptible host cells, Chlamydia multiplies according to a particular growth cycle.
- Primary infectious agents are elementary bodies, which commence the cycle. Attachment of the EB to the microvilli of susceptible epithelial cells, followed by penetration into the host cell, initiates the infection.
- Within the host cell, EB remains within the cytoplasmic phagosomes where it begins to proliferate.
- The fusion of phagosomes harbouring EB with cell wall lysosomes is blocked, preventing intracellular death of EB. This phagolysosomal fusion is typically inhibited in intact outer membrane-containing host cells.
- Within 6–8 hours of entering the cell, the phagosome transforms the EBs into big, metabolically active RBs.
- These RBs are capable of synthesising their own proteins and nucleic acids, but are incapable of producing their own high-energy phosphate molecule.
- The chlamydiae are referred to as energy parasites because to this shortage. Some strains of Chlamydia depend on the host for their amino acid requirements.
- The RBs will continue to undergo binary fission for the following 18–24 hours. Inclusion body refers to the growing phagosome with accumulated reticulated bodies within the host cell.
- The mature inclusion body contains between 100 and 500 EBs, which can be readily confirmed by a variety of staining techniques.
- Eventually, the host cell will break and release the EBs. Within 70–96 hours of C. trachomatis infection, the release of EBs occurs.
- The existence of a scar indicates the discharge of the host cell. In C. psittaci infections, the release of EBs occurs within 48 hours due to the lysis of the host cell, which causes significant harm to the infected host cell.
- During active growth, Chlamydiae express Chlamydia-specific LPSs on the surface of the infected host cell.
- On the outer surface of the cell, these LPSs are highly antigenic and provoke immunological and inflammatory responses.
- These species have distinct (a) growth properties, (b) antigens, (c) nucleic acid profiles, (d) plasmids, and (e) inclusion body features.

Chlamydia trachomatis
- C. trachomatis is a human-specific pathogen.
- Chlamydia trachomatis, often known as chlamydia, is a bacterium that causes chlamydia, which can appear in a variety of forms, such as trachoma, lymphogranuloma venereum, nongonococcal urethritis, cervicitis, salpingitis, and pelvic inflammatory disease. C. trachomatis is the most prevalent infectious cause of blindness and sexually transmitted bacterium.
- Different strains of C. trachomatis are responsible for various illnesses. Other strains cause disease in the eye or lymph nodes. the most prevalent strains cause disease in the genital tract.
- C. trachomatis, like other Chlamydia species, has two morphologically distinct life stages: elementary bodies and reticulate bodies.
- Elementary bodies are spore-like and contagious, but reticulate bodies are in the replicative stage and can only be observed within host cells.
Morphology of Chlamydia trachomatis
- C. trachomatis bacteria are Gram-negative. However, Giemsa, Castaneda, Machiavello, or Gimenez stains are more effective.
- Similar to other chlamydiae, C. trachomatis exists in two morphologically distinct forms: elementary body and reticulate body.
- EB is an infectious extracellular particle. It is tiny and spherical, with a diameter of 800–1200 nm.
- After staining with Giemsa, Castaneda, or Machiavello, these inclusion bodies in infected cells, including conjunctiva, urethra, and corneal smears, can be observed.
- These inclusion bodies are big particles that are easily observable under a light microscope.
- Staining with Lugol’s iodine demonstrates that these entities are composed of glycogen matrix. This type of Chlamydia is metabolically active and capable of reproduction.
Culture of Chlamydia and Chlamydophila
- C. trachomatis grows more efficiently in diverse tissue cultures including nonreplicating, stationary-phase cells.
- A few cell lines, including HeLa-229, McCoy, BHK-21, and buffalo green monkey kidney cells, support bacterial growth.
- When isolating bacteria, McCoy and HeLa cells are routinely employed. C. trachomatis can be propagated through inoculation of embryonated eggs and experimental infection of mice.
- Chlamydia spp. develop within the yolk sac of 6- to 8-day-old chick embryos.
- The presence of elementary and inclusion bodies as well as group-specific complement-fixing antigen in the yolk sac indicates the progression of chlamydia.
- Injecting intracellularly infected C. trachomatis strains (L1, L2, and L3) that differ in their infectiousness and ability to infect animals results in infection.
Susceptibility to physical and chemical agents
Chlamydiae are heat-sensitive bacteria that can be destroyed in minutes by heating at 56°C. Ethanol, ether, phenol, formaldehyde, iodine, potassium permanganate, sodium hypochlorite, silver nitrite, and chlorite are vulnerable to them. At 4°C, they are totally viable for several days. In addition, they can be kept for an extended period of time at 70°C or in liquid nitrogen.
Cell Wall Components and Antigenic Structure
Chlamydiae possess three types of major antigens: (a) antigens specific to the genus, (b) antigens specific to the species, and (c) antigens specific to the serotype.
Genus-specific antigen
- This antigen is heat-stable, complement-fixing, and species-specific.
- It resembles the LPS of Gram-negative bacilli.
- It exists in both EBs and RBs.
- Ether, chloroform, or methanol can be used to extract the antigen. CFT is used to detect the antigen.
Species-specific antigen
- This antigen is found on the surface of the envelope and is species-specific.
- This antigen is present in all Chlamydia strains.
Serotype-specific antigen
- This antigen is only present in a few chlamydiae species.
- They are located in the main outer membrane proteins (MOMPs) of Chlamydia species and are useful for intraspecies typing.
Typing of species
- Chlamydia species are divided into distinct serovars and serologic variations based on these antigens.
- C. trachomatis is comprised of three biovars: (a) trachoma biovar causing trachoma and inclusion conjunctivitis (TRIC), (b) lymphogranuloma venereum (LGV) biovar causing LGV, and (c) serovars causing mouse pneumonitis.
- Based on antigenic variations in the MOMPs, these biovars have been further categorised into 20 serotypes.
- The trachoma biovar is comprised of thirteen serotypes (A, B, Ba, C, D, Da, E, F, G, H, I, Ia, J, Ja and K). The LGV biovar comprises just five serotypes (L1, L2, L2a, L2b, and L3).
Pathogenesis and Immunity
C. trachomatis is an intracellular bacterium that causes disorders in a variety of human body systems.
Virulence factors
- The ability of C. trachomatis to replicate intracellularly within the infected cell is the major virulence mechanism.
- The bacteria inhibit the union of phagolysosomes and cellular liposomes, preventing the host cell from killing the bacteria intracellularly.
- Multiple C. trachomatis infections contribute to the pathophysiology of the infected eye in trachoma.
Pathogenesis of C. trachomatis infection
- C. trachomatis primarily causes disease by (a) destroying infected host cells directly during multiplication and (b) eliciting inflammatory responses in the host.
- C. trachomatis penetrates the host by microscopic abrasions or skin wounds.
- The bacteria react uniquely with the receptors found on nonciliated columnar, cuboidal, and transitional epithelial cells.
- The mucous membranes of the conjunctiva and genitourinary system, including the urethra, endocervix, endometrium, fallopian tube, and respiratory tract, include these epithelial cells.
- The LGV biovar multiplies in the lymphatic system’s mononuclear phagocytes. Typically, pathological lesions are seen in lymph nodes draining the initial site of infection.
- Granuloma is a pathologically typical lesion. Subsequently, the inflammatory process extends to neighbouring tissues, and lymph node rupture results in the creation of abscesses or sinus tracts.
- As seen in trachoma, infections with trachoma serovars are linked with a significant inflammatory response composed of neutrophils, lymphocytes, and plasma cells.
Host immunity
- C. trachomatis infections do not develop long-lasting immunity.
- In contrast, C. trachomatis reinfection often results in a robust inflammatory response and substantial tissue destruction.
- These responses are accountable for vision loss in people with chronic ocular infections, as well as sterility and sexual dysfunction in patients with genital infections.
Clinical Syndromes
C. trachomatis causes numerous illnesses. It is a significant global cause of vaginal and ocular infections. The LGV biovar of C. trachomatis produces lymphogranuloma venereum (LGV) and ocular LGV. Trachoma, adult inclusion conjunctivitis, newborn conjunctivitis, infant pneumonia, and urogenital infections are caused by C. trachomatis trachoma biovar.
Lymphogranuloma venereum
- C. tracheatitis LGV biovar (serotypes L1, L2, L2a, L2b, and L3) causes lymphogranuloma venereum.
- The disease is most usually caused by serotype L2. LGV is an STD that affects the cervix, urethra, salpinges, and epididymis.
- The incubation period ranges between 1 and 4 weeks. Additionally, fever, headache, and myalgia are related symptoms. The next stage of the disease is inflammation and swelling of the lymph nodes draining the main site of infection.
- Most frequently, regional lymph nodes, such as inguinal lymph nodes in males and intrapelvic and pararectal lymph nodes in females, are affected.
- With the establishment of draining fistulas, these lymph nodes become painful, swollen, unstable, and may eventually rupture. These lymph nodes are known as buboes.
- Fever, chills, anorexia, headache, and myalgia are the additional symptoms. Proctitis is a common symptom of LGV in women.
- This develops as a result of the lymphatic spread of bacteria from an infected cervix or vagina. Proctitis can also arise in men due to lymphatic spread from the urethra or after anal contact.
- In situations of untreated LGV, the infection may advance to a chronic ulcerative stage, resulting in the formation of ulcers, strictures, fistulas, or genital elephantiasis. In some instances, the infection may have resolved at this stage.
- The initial lesion that occurs on the external genitalia of patients with LGV is a tiny, painless papule or an inflammatory lesion.
- The most common infection sites are the penis in men, the fourchette in women, and the rectum in homosexuals. On sometimes, initial lesions may be seen in extragenital locations (e.g., fingers and palms).
- The absence of pain is the characteristic symptom that distinguishes ulcers from painful ulcers caused by herpes simplex virus infection and syphilis.
Ocular LGV
- Additionally, C. trachomatis LGV biovar causes ocular LGV.
- This causes Parinaud’s oculogenital conjunctivitis.
- It is characterised by inflammation of the conjunctiva together with lymphadenopathy of the periauricular, submandibular, and cervical lymph nodes.
Trachoma
- Trachoma is an eye infection caused by the C. trachomatis serotypes A, B, Ba, and C.
- This syndrome is characterised by follicular hypertrophy, papillary hyperplasia, pannus development, and cicatrization in the latter stages.
- Patients with follicular conjunctivitis first present with generalised inflammation affecting the entire conjunctiva.
- Subsequently, the problem advances with the creation of pannus, which suggests invasion of the corneal blood vessels and, ultimately, eyesight loss.
- Vision loss is the most significant and severe complication of trachoma.
Adult inclusion conjunctivitis
- Infection with C. trachomatis strains associated with genital infection is the cause of adult inclusion conjunctivitis (A, B, Ba, and D–K).
- This disease is more prevalent among sexually active individuals. The illness can also affect newborns.
- Important manifestations include a uniocular and less frequently a binocular red eye, ocular discharge, significant hyperemia, papillary hypertrophy, and a prominent follicular conjunctivitis.
- If left untreated, the illness advances to a chronic relapsing course, keratitis, and possibly iritis.
Neonatal conjunctivitis
- This is the infant version of conjunctivitis with inclusions. The syndrome manifests in infants who acquire the illness from a contaminated birth canal.
- Typically, infants born to expectant moms with chlamydial infections of the cervix are infected.
- The incubation period ranges between 5 and 12 days. The condition is characterised by swelling of the infant’s eyelid, hyperemia, and purulent discharge.
- Conjunctival scarring and corneal vascularization occur in long-lasting infections that are left untreated.
Infant pneumonia
- C. trachomatis causes newborn pneumonia in infants between 4 and 16 weeks of age.
- It is one of the leading causes of pneumonia in infants. Sixty percent of infants born to infected mothers are infected.
- Variable, but typically lasting two to three weeks after birth.
- Respiratory symptoms, such as rhinitis with cough and wheeze, characterise this illness. The child’s temperature is often normal during the sickness.
Urogenital infections
- Urogenital infection is C. trachomatis’ most common source of infection. About 80% of infected females and 50% of infected males exhibit no symptoms.
- Chlamydia is the most prevalent sexually transmitted disease in the world.
- The clinical symptoms in symptomatic patients are urethritis (nongonococcal urethritis), epididymitis, proctitis, and conjunctivitis in males, and mucopurulent cervicitis, endometritis, and salpingitis in females.
- Perinephritis, chronic pelvic discomfort, and pelvic inflammatory disease can occur from an ascending infection.
- Women infected with C. trachomatis, particularly serotype C, are about 6.5 times more likely to develop cervical cancer than those without infection.
Reiter’s syndrome: Reiter’s syndrome is a triad consisting of recurrent conjunctivitis, polyarthritis, and urethritis or cervicitis, and is usually associated with C. trachomatis-caused genital infection. This disorder affects both males and females. At the onset of arthritis, 50–60% of patients with Reiter’s syndrome had a vaginal infection with C. trachomatis. EBs have been discovered in the synovial fluid of patients with arthritis.
Geographical distribution of Chlamydia and Chlamydophila
- C. trachomatis-caused LGV is extremely widespread in Asia, Africa, and South America. The disease is sporadic in Europe, Australia, and the United States.
- In poor countries, 10% of genital ulcer illness is caused by LGV. Chlamydia gonorrhoeae and gonorrhoea caused by Neisseria gonorrhoeae frequently coexist.
- These two infections are the most common causes of epididymitis in adult males who engage in sexual activity.
- It is estimated that over 500 million people are affected with trachoma worldwide.
- The Middle East, Africa, the Far East, and India are endemic regions for the illness. 7–9 million persons suffer from blindness due to this illness.
- Trachoma is very frequent in these nations because to overpopulation, inadequate sanitation, and poor personal hygiene.
- All of these conditions facilitate infection transmission. In addition, C. trachomatis is the most common cause of newborn pneumonia around the world.
Habitat of Chlamydia and Chlamydophila
- C. trachomatis is a human-specific pathogen.
- It is present in the conjunctiva and genitourinary tract of affected individuals.
- C. trachomatis inhabits the human respiratory and gastrointestinal systems.
Reservoir, source, and transmission of infection
- Humans are the only natural host of Chlamydia trachomatis and are hence the only important reservoir of infection.
- Infected ocular secretions are the most common source of trachoma eye infection.
- Occasionally, respiratory secretions and human excrement might serve as an infection source.
- Eye-to-eye contact transmits trachoma via (a) droplets, (b) infected hands, and (c) contaminated clothing.
- All of these techniques facilitate the transmission of ocular discharges from infected children’s eyes to those of healthy youngsters.
- Trachoma can also be transferred through inhalation of respiratory droplets or consumption of infected food and water.
- The source of infection for adult inclusion conjunctivitis is genital secretions. Adult inclusion conjunctivitis is mostly transmitted through orogenital contact and self-inoculation.
- Although uncommon, eye-to-hand transmission of adult inclusion conjunctivitis has been observed. Babies born vaginally to C. trachomatis-infected mothers acquire conjunctivitis with inclusions.
- C. trachomatis eye infection affects roughly 25% of newborns whose moms have C. trachomatis in their vaginal canal.
- Additionally, infected vaginal discharge is the source of infection for neonatal pneumonia. 10–20% of infants get pneumonia during birth due to an infected birth canal. Chlamydial genital infections are typically transmitted by oral, anal, and vaginal contact.
Laboratory Diagnosis
There are numerous laboratory methods for diagnosing chlamydial infections, as described here. However, the sensitivity of these procedures is dependent on (a) the type of the disease, (b) the site of infection from which the material is collected, and (c) the population of patients who are investigated.
Specimens
- Specimens from the urethra, cervix, rectum, oropharynx, and conjunctiva are collected often.
- In addition to blood, respiratory secretions, sputum, lung, and other tissues, other specimens are collected and evaluated. Bubo pus is also effective for diagnosing LGV.
Microscopy
- The laboratory diagnosis of chlamydial infection is based on the demonstration of chlamydial inclusion bodies in diverse clinical specimens.
- These inclusion bodies are visible in specimens stained with Giemsa, Castaneda, Machiavello, or Gimenez.
- C. trachomatis infections of the conjunctiva, urethra, and cervix are identified by the presence of reniform inclusion bodies surrounding the nucleus in stained smears of the conjunctiva, urethra, and cervix.
Iodine staining of conjunctival scrapings is a straightforward and expedient approach for diagnosing TRIC. Iodine stains the glycogen matrix of C. trachomatis inclusion bodies. However, iodine staining has a low sensitivity and is only positive at specific stages of inclusion body growth in infected cells. This is more beneficial for demonstrating chlamydial inclusion bodies in clinical specimen-inoculated cell cultures.
Culture
- C. trachomatis infection can be diagnosed more precisely by isolating C. trachomatis in cell cultures. To isolate Chlamydia, clinical specimens are injected into several cell lines.
- The sensitivity of cell cultures for C. trachomatis isolation is boosted by (a) pretreatment with cycloheximide (i.e., a metabolic inhibitor, which suppresses the metabolism of host cells) and (b) the use of irradiation cell lines (treated McCoy cells are most commonly used cell lines for isolation of C. trachomatis).
- Infection with C. trachomatis in cell culture is evidenced by the formation of intracellular inclusion bodies.
- These are detected using iodine stains or antibodies conjugated with fluorescence. The cultivation procedures are challenging and costly.
- These are the favoured techniques for isolating C. trachomatis from rectal specimens, while non-culturing techniques are typically negative. The culture demonstrates a sensitivity between 50 and 90% and a specificity of 99.9%.
Antigen detection
- By using direct fluorescent antibody (DFA) labelling and enzyme immunoassay, Chlamydial antigen can be detected in clinical specimens (EIA).
- Antibodies produced against the Chlamydia MOMP or the cell wall LPS are utilised to detect antigens in clinical specimens using these two techniques.
- Both DFA staining and EIA have a sensitivity of roughly 80% and a specificity of 95%. However, both procedures are labor-intensive and call for trained workers.
Serodiagnosis
- Serodiagnosis is based on the identification of anti-C. trachomatis antibodies in serum. Patients with LGV have a very high blood antibody level.
- The diagnosis of LGV is aided by antibody-based diagnostics. To identify particular antibodies in sera, CFTs, microimmunofluorescence (MIF), and ELISA are utilised:
- CFT employs a genus-specific LPS antigen for antibody detection. Antibody titers of 1:256 or higher in a single serum or a fourfold increase in antibody titers in matched sera are highly indicative of LGV.
- The MIF test is a particular test that utilises antigens that are species- and serovar-specific, such as Chlamydia MOMPs. The MIF test verifies that a vehicle is an LGV.
- ELISA is a genus-specific test that, like CFT, employs LPS antigen.
- C. trachomatis-caused urogenital infections are not reliably diagnosed by serological testing based on antibodies.
- Since antibodies are present in circulation for an extended length of time, antibody titers cannot distinguish between recent and historical infections.
- To diagnose Chlamydia pneumonia in newborns, ELISA testing for IgM antibody detection is quite beneficial.
Frei’s skin test
- The Frei’s test is an intradermal skin test that is used to diagnose LGV. As antigen, a heat-inactivated C. trachomatis LGV serovar produced in the yolk sac of an embryonated egg is employed, and 0.1 mL of antigen is injected intradermally in the forearm, along with a control antigen made from an uninfected yolk sac.
- Frei’s skin test is a delayed hypersensitivity reaction in which a positive reaction is indicated by the formation of a 7 mm in diameter inflammatory macule on the test arm after two days.
- The nodule achieves its maximum size in four to five days. Two to six weeks after infection, the skin test becomes positive and remains thus for several years. However, skin tests are rarely utilised for diagnosis today.
Molecular Diagnosis
DNA probes and PCR are utilised to do a molecular diagnostic of C. trachomatis infection (polymerase chain reaction). Currently available DNA probes detect the presence of a 16S rRNA species-specific sequence. This technique is not particularly sensitive. PCR is used to diagnose infections with a sensitivity of 80–90% and a specificity of 99.9%, respectively. The approach is particularly useful for assessing urine samples, but less sensitive for detecting germs in vaginal swabs collected from infected women.
Treatment
- Azithromycin is the antibiotic of choice for treating chlamydial infections of the vaginal tract. This antibiotic has the benefit of being administered in a single dose, is well tolerated, and has few contraindications.
- Generally, tetracyclines are suggested for at least three weeks for the treatment of individuals with LGV.
- Children under 9 years of age, pregnant women, and patients unable to tolerate tetracyclines are treated with a macrolide such as erythromycin or azithromycin together with sulfisoxazole.
- For the treatment of vaginal and ocular infections, a 7-day course of doxycycline or fluoroquinolone (e.g., ofloxacin) is also beneficial.
- Erythromycin administered for 10–14 days is an effective treatment for infant conjunctivitis and infant pneumonia.
- For the treatment of neonatal ophthalmia, erythromycin can be taken orally and topically.
- Several weeks of local application and oral medication of erythromycin and tetracycline are beneficial in treating trachoma.
Prevention and Control
- Although it is difficult to prevent C. trachomatis infection, the morbidity (such as the blindness associated with trachoma) can be avoided by treating the disease immediately and preventing re-exposure to the bacterium.
- Chlamydial genital infections are avoided by utilising safe sexual practises and by treating symptomatic individuals and their partners as soon as possible.
Chlamydophila
- C. pneumoniae was originally identified from the conjunctiva of a Taiwanese infant in 1965, followed by the throat of a pharyngitis patient in 1983.
- Initially, it was believed that these organisms were C. psittaci strains TWAR (from Taiwan acute respiratory) because they produced inclusion bodies in cell cultures that resembled those produced by C. psittaci.
- The isolate from Taiwan was classified as TW-183, and the isolate from the pharynx was designated as AR-9.
- Initially, it was assumed that these two strains were connected to psittaci strains, but it was later determined that they were distinct.
- Previously, these two species were categorised as Chlamydia pneumoniae, however they have since been reclassified and put in the new genus Chlamydophila.
Chlamydophila pneumoniae
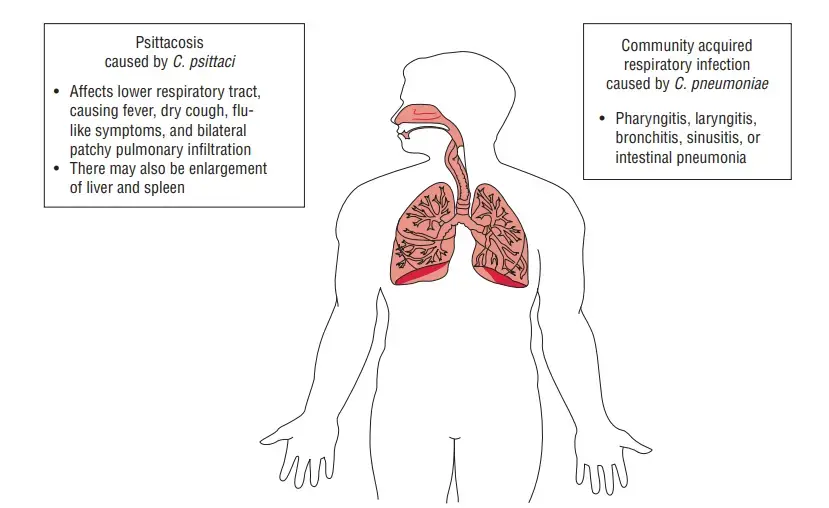
- After Streptococcus pneumoniae and Haemophilus influenzae, C. pneumoniae is the third most prevalent cause of pneumonia.
- It is a major cause of respiratory illness in older children and adults around the world. C. pneumoniae causes infections that are typically asymptomatic.
- Pharyngitis, sinusitis, bronchitis, and pneumonia are the most prevalent symptoms of acute respiratory tract infection in symptomatic cases.
- C. pneumoniae is also a human-only infection and lacks an animal reservoir. Infections are spread from person to person via respiratory secretions, with no bird or animal host.
- Approximately 2–3 million cases of C. pneumoniae occur annually, with the majority of cases occurring in adults.
- By demonstrating C. pneumoniae antigen in specimens using enzyme immunoassay or DFA test, an infection is diagnosed.
- Culture-based diagnosis is not performed because C. pneumoniae grows poorly in cell cultures. Utilizing CFT, ELISA, or MIF, serodiagnosis is conducted.
- Antibodies have been detected in the sera of over fifty percent of the population. PCR can also be used to detect bacterial genome in a sample.
- For 10–14 days, tetracycline, doxycycline, erythromycin, and azithromycin are administered to treat C. pneumoniae infection. Infections caused by C. pneumoniae are difficult to control.
Chlamydophila psittaci
- C. psittaci is the pathogen responsible for psittacosis, a transmissible disease of parrots and psittacine birds.
- Psittacosis derives from the fact that the disease was initially observed in parrots, hence its name (Greek word psittakos means parrot).
- Ornithosis was the name given to a disease of nonpsittacine birds with comparable symptoms (Greek word ornithos means bird). Both disorders are now known as psittacosis.
- Infections in birds may manifest with or without symptoms. Infection symptoms may include respiratory infection, diarrhoea, and malnutrition.
- The bacteria are present in the blood, tissue, and feathers of sick birds, as well as the faeces, nasal discharges, aerosols, etc. C. psittaci is transmissible from birds to humans, as well as to sheep, cows, and goats.
- The virus is primarily transmitted to humans by psittacine birds, such as parrots, parakeets, cockatiels, and macaws.
- They become infected by inhaling the dried faeces, urine, or respiratory secretions of infected birds.
- Veterinarians, poultry workers, pet shop employees, pigeon farmers, and others are at a greater risk for this illness due to their occupations.
- Human-to-human transfer is uncommon. Ingestion of poultry does not induce infection either.
- Infections in laboratories are well known. The clinical condition caused by C. psittaci in humans ranges from a mild influenza-like sickness to a deadly pneumonia. The incubation period ranges between 5 and 14 days.
- In the initial phase of the illness, headache, high fever, chills, malaise, and myalgia are the predominant symptoms.
- Respiratory manifestations include nonproductive cough, rales, and consolidation. Untreated instances may proceed to encephalitis, endocarditis, pericarditis, and even mortality if they are severe.
- The laboratory diagnosis of the disease is primarily based on serological tests. C. psittaci infection is suggested by a fourfold increase in antibody titer between acute and convalescent serum, as measured by the CFT. The MIF test is a specialised diagnostic procedure used to validate the diagnosis.
Levinthal-Cole-Lille (LCL) inclusion bodies
- These are observed in the brain, yolk sac, cell cultures, and infected cells, such as alveolar macrophages, from patients infected with C. psittaci.
- These inclusion bodies are not iodine-stained and are more diffuse and irregular in appearance.
- Sulfadiazine and cycloserine had no effect on them. C. psittaci can be cultivated in cell cultures, such as L cells, after 5–10 days of incubation, however it is not commonly employed.
- C. psittaci infections may be treated with tetracycline, doxycycline, erythromycin, and azithromycin. Infection is prevented by preventing its spread among birds.