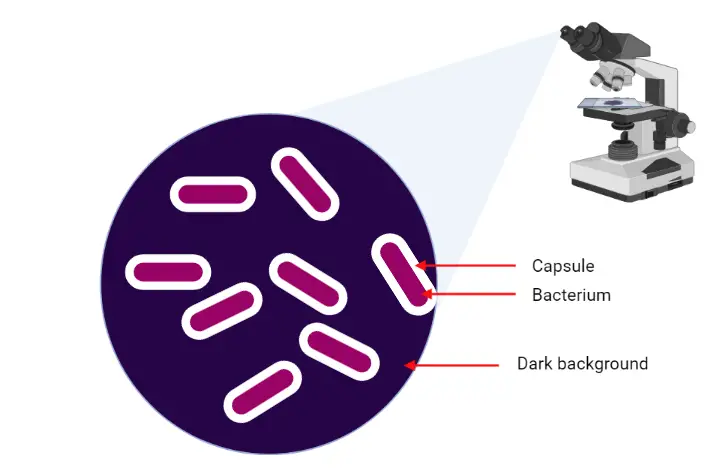
Capsule staining is the specialized staining method that is used to demonstrate the capsule present around certain bacterial cells. It is the outer gelatinous layer secreted by the cell which surrounds and adheres to the cell wall.
It is not common to all organisms and the cells having heavy capsules are usually virulent because the capsule protects the bacteria from the phagocytic activity of host cells. It is mainly composed of complex polysaccharides like levans, dextrans and celluloses.
Capsule staining is difficult because the capsular material is water-soluble and it may be removed if the smear is vigorously washed, and heating the smear may cause shrinkage of the cell which forms a clear zone that can be mistaken as capsule.
It is the process in which the capsular material is differentiated from the cell body by using a negative staining technique. In this method the background is stained by an acidic dye like India ink or Nigrosin and the cell is stained by a basic dye like Crystal violet. The capsule is non-ionic and does not take up the stain, so it appears as a clear halo around the stained cell.
This method is referred to as demonstrating the glycocalyx layer or capsule which is important as a virulence factor in many pathogens. In this staining the smear is not heat fixed, and water is not used for washing because capsule is highly water-soluble and gets easily distorted by heating or washing. In some methods like Anthony’s stain, 20% copper sulfate is used which acts as a gentle decolorizer and also counterstains the background lightly while keeping the capsule unstained.
Purpose of Capsule Staining
The main purpose of capsule staining is to visualize the capsule of a cell.
Principle of Capsule Staining
The principle of capsule staining is based on the special nature of the capsular material which is highly hydrated, water-soluble and mostly non-ionic in character. It is the outer polymeric layer that does not take up the usual acidic or basic stains because the capsule does not have sufficient electrical charge to bind these dyes. It is difficult to stain directly so the technique depends on creating contrast between the stained background, the stained cell and the unstained capsule. This is referred to as a type of negative staining where an acidic dye like India ink or Nigrosin stains the background, and a basic dye such as Crystal violet stains the bacterial cell.
The capsule remains clear because it is not penetrated by either dye, so it appears as a colorless halo surrounding the cell. It is important that the smear is not heat fixed because heating causes shrinking or dissolving of the capsule which gives a false appearance. Water is also avoided as it removes the water-soluble capsular material very easily. In some modified methods like Anthony’s stain, 20% copper sulfate is used which acts as a gentle rinsing agent that removes excess primary stain without disturbing the capsule. It also lightly stains the background while keeping the capsule as an unstained zone between the background and the cell.
Principle of Anthony’s Capsule Staining Method
The principle of Anthony’s capsule staining is based on the fact that the capsule is non-ionic in nature and does not take up most basic dyes. It is the process where the cell is stained but the capsule remains clear because the stain cannot bind to the polysaccharide layer. In this method crystal violet is used as the primary stain and copper sulfate is used as the decolorizer.
The crystal violet stains the bacterial cell and the protein background from milk or serum. The capsule does not react with the stain and stays as an unstained halo. When copper sulfate is applied, it removes the excess crystal violet from the smear. Some of the stain may be replaced by the copper sulfate in the capsule area giving a faint blue appearance. Water is not used here because it may dissolve the capsule.
Thus, the principle is that the cell and surrounding material are stained by crystal violet, while the capsule is seen as a clear or slightly bluish halo due to its inability to combine with the dye. This contrast helps in demonstrating the presence of the capsule.
Principle of Negative Staining Method (India Ink/Nigrosin Method)
The principle of the negative staining method is based on the use of acidic dyes which cannot penetrate the bacterial cell or the capsule. It is the process where the background is stained dark while the cell and the capsule remain unstained. India ink, Nigrosin or Congo red are acidic in nature and they carry a negative charge. The bacterial cell surface also carries a negative charge, so the stain is repelled.
Because the stain does not enter the cell, the cell appears as a clear or lightly coloured structure. The capsule, being non-ionic and water soluble, also does not take the stain. As a result, the capsule is seen as a clear halo between the dark background and the stained cell when a counterstain like crystal violet is added.
This principle allows high contrast because the background becomes dark and the capsule is kept intact without heat fixing. It helps in demonstrating encapsulated organisms clearly.
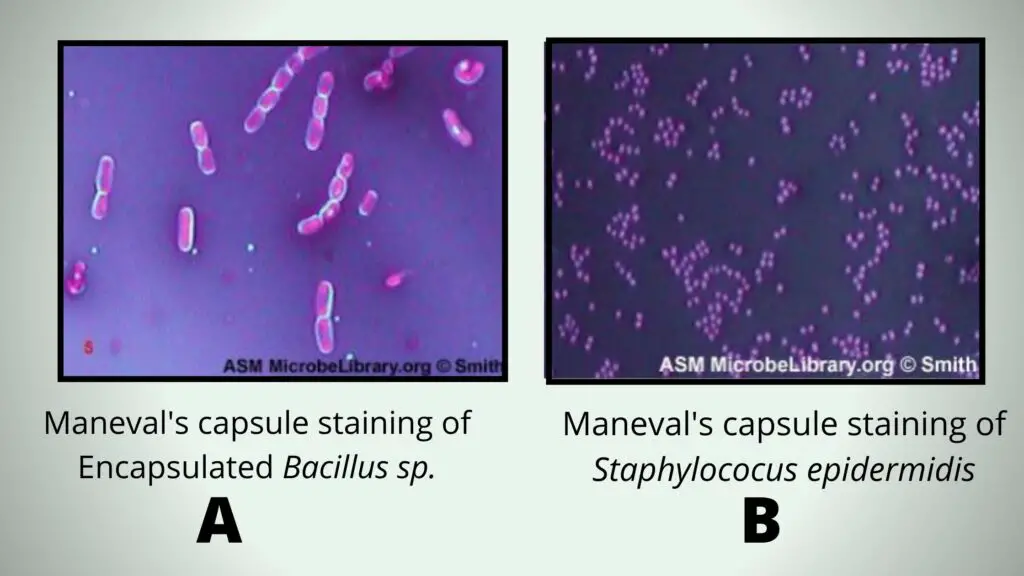
Principle of Maneval’s Capsule Staining Method
The principle of Maneval’s capsule staining is based on using two stains that give contrast between the background, the cell, and the capsule. It is the process where Congo red is used first to colour the background, while the capsule and the cell remain unstained at this stage. Congo red is an acidic dye and it does not bind to the negatively charged bacterial surface.
After this, Maneval solution is applied. It contains acid fuchsin, ferric chloride, acetic acid and phenol. The acetic acid lowers the pH and changes the colour of Congo red to dark blue. At the same time the acid fuchsin stains the bacterial cell bright red or pink. The capsule is non-ionic and does not combine with these dyes, so it remains as a clear halo around the stained cell.
Thus the principle is that the background becomes dark blue, the cell becomes red due to the basic dye, and the capsule appears as a colourless zone because it does not react with either stain. This contrast helps in clear demonstration of the capsule.
Requirement
Capsule staining needs some basic materials and the reagents depending on the method used. It is the process where the capsule is demonstrated without getting destroyed because heating is avoided. The materials are arranged before starting the staining to keep the smear preparation continuous.
Some of the main materials required for capsule staining are–
- Microscopic slides
- Inoculating loop
- Microscope with 100x objective lens (oil immersion)
- Immersion oil
- Gas burner (but heat fixing is not done in capsule staining)
- Test bacteria culture grown in milk broth or litmus milk broth
- Staining tray
- Staining rack
- Slide holder
- Disposable gloves
- Absorbent paper for air drying
- Serum protein (used when organism is not grown in milk broth and it helps in giving a protein background)
Staining Reagents (Method-wise)
I. Anthony’s Capsule Stain Reagents
These are used for demonstrating capsule with a simple staining technique.
- Crystal violet 1% solution (primary stain)
- 20% Copper sulfate solution (decolorizer and also counterstain)
II. Maneval’s Capsule Stain Reagents
In this method capsule appears colourless against a stained background.
- Congo red (1% aqueous solution) for negative staining
- Maneval solution (cell stain) which contains–
- Acid fuchsin (0.05 g)
- Ferric chloride (3.0 g)
- Acetic acid (5 ml)
- Phenol liquified (3.9 ml)
- Distilled water (95 ml)
III. Negative Staining (India Ink or Nigrosin Method) Reagents
This process occurs when the background is stained whereas the capsule remains clear.
- India ink
- Nigrosin (10 g in 100 ml distilled water)
- Congo red (may also be used as negative stain)
- Crystal violet 1% solution (counterstain)
- Methylene blue or Eosin (as alternative counterstains)
IV. Hiss’s Stain Reagents
These are used for another capsule demonstration technique.
- 1% Crystal violet solution
- 20% Copper sulfate solution
- Normal saline (0.85 g/dl sodium chloride) mixed with organism suspension to enhance capsule visibility
Procedure of Capsule Staining
The procedure of capsule staining is done without heat fixing because the capsule is water soluble. It is the process where the smear is dried only in air and the stains are applied carefully so that the capsule is kept intact. These steps are followed in different methods.
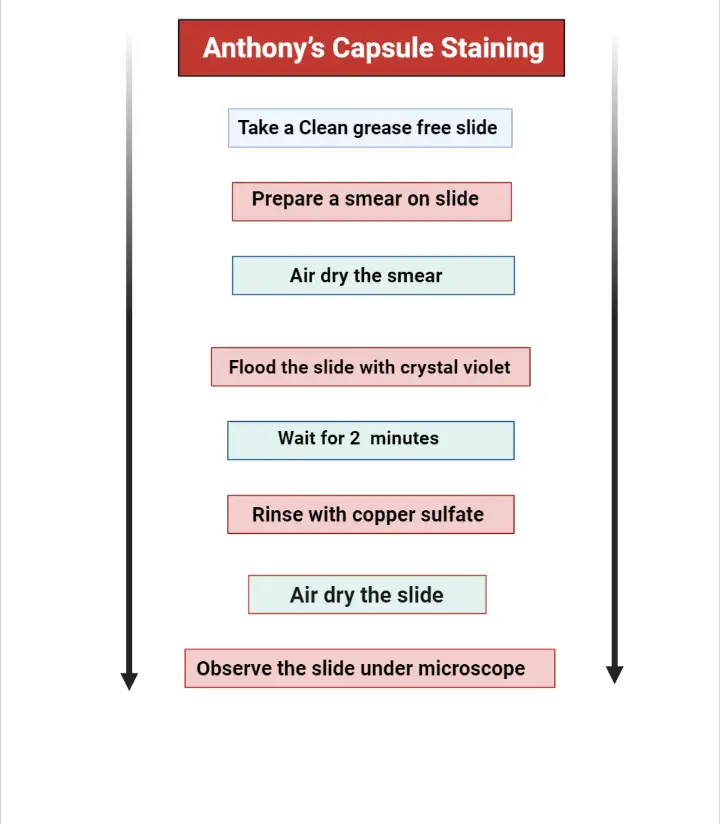
I. Anthony’s Capsule Stain Procedure
In this method crystal violet is used as the primary stain and copper sulfate works as the decolorizer. The smear is dried without heating.
- In the first step the skim milk broth culture is mixed gently and 2–3 loops of the culture is placed on the slide. Serum protein is added when the organism is not grown in milk broth.
- The sample is spread with the loop so that it covers nearly one inch of the slide.
- The smear is allowed to air dry completely. It is not heated because heating will damage the capsule.
- The dried smear is covered with 1% crystal violet for about two minutes.
- Excess stain is removed with 20% copper sulfate solution. The copper sulfate acts as decolorizer and also as light counterstain and water is not used here.
- The slide is shaken lightly to remove the remaining solution and then allowed to air dry. Blotting is avoided because the cells are not heat fixed.
- The smear is observed under oil immersion lens. The cell and the milk background appear purple while the capsule is seen as a clear or faint halo.
II. Negative Staining Method (India Ink/Nigrosin Method) Procedure
This process occurs when the background is stained dark and the capsule remains clear.
- A small drop of negative stain (India ink, Nigrosin, Congo red) is placed on a clean slide.
- One loopful of bacterial culture is added and mixed into the dye.
- Using another slide, the mixture is dragged to form a thin film just like a blood smear.
- The film is left for air drying for few minutes. It is not heat fixed.
- The smear is then covered with crystal violet (1%) for one minute. This stains the cell but not the capsule.
- The slide is tilted to drain the stain slowly. Water is not used for rinsing.
- The slide is examined under oil immersion. The capsule appears as a clear halo around the violet stained cell on a dark background.
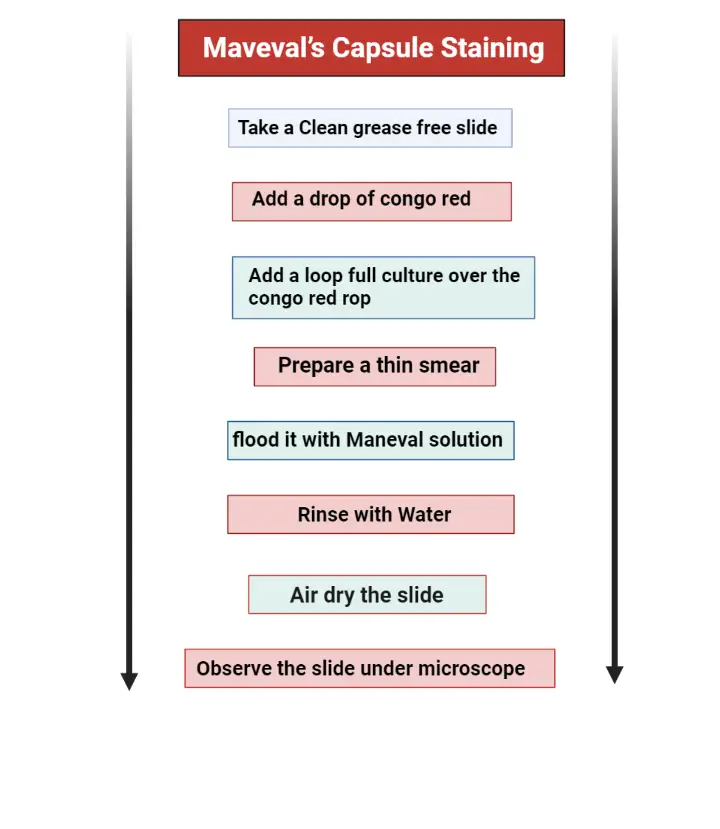
III. Maneval’s Capsule Staining Method Procedure
This method uses Congo red for background and Maneval solution for staining the cell.
- A few drops of Congo red (1% solution) is placed on the slide, without adding water.
- A small amount of culture is mixed in the Congo red.
- The smear is allowed to air dry. Heat fixing is not done here.
- Maneval solution is added gently over the smear and left for 5 minutes.
- The excess stain is drained carefully. Water is used for rinsing but in a very gentle manner by allowing water to flow slowly over the slide in the staining tray.
- The slide is placed on absorbent paper and left for air drying. It is not blotted.
- The smear is observed under oil immersion. The cell appears bright red or pink, the background becomes dark blue and the capsule is seen as a clear halo.
Result of capsule staining
The result of capsule staining is seen under the microscope after the smear is dried and stained without heat. It is the process where the cell, the capsule and the background can be differentiated clearly. The capsule appears as a clear area since it cannot bind the stain.
I. General Appearance
In capsule staining the appearance is based on triple contrast. These are–
- The capsule is seen as a clear or colourless halo around the cell.
- The bacterial cell is stained either violet, purple, or red depending on the dye used.
- The background is stained darker so that the capsule becomes visible.
It is the clear halo that indicates capsule formation. When there is no capsule, this clear halo is not observed.
II. Results of Specific Staining Methods
Negative Staining Method (India Ink/Nigrosin)
The cells are stained violet with crystal violet. The capsule appears as a clear halo. The background becomes dark or black due to India ink or Nigrosin. The halo is clearly seen between the violet cell and the black background.
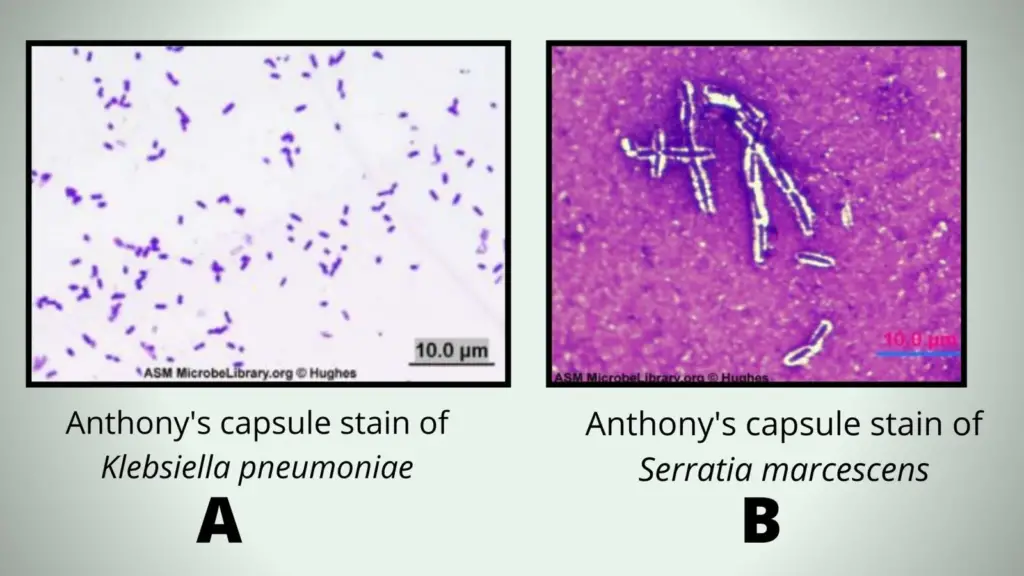
Anthony’s Capsule Stain
The cell is purple or violet because of crystal violet. The capsule becomes faint blue or transparent because copper sulfate acts as mild counterstain. The background remains purplish or light violet. A faint blue halo is seen around the purple cell.
Maneval’s Capsule Stain
The cell becomes bright red-pink due to acid fuchsin. The capsule is clear and unstained. The background appears dark blue because Congo red changes colour in acidic condition. A clear halo is seen between the red cell and dark blue surrounding.
Hiss’s Stain
The cell colour is dark violet. The capsule appears pale blue or light violet. The background becomes brighter. A pale blue halo can be seen around the cell.
III. Interpretation and Significance
A positive result is shown when a clear halo is seen around the stained cell. It is the indication that the organism is encapsulated. Capsules protect the cells from phagocytosis and increase virulence. This is important for distinguishing pathogenic strains.
Some of the organisms showing positive capsule staining are Klebsiella pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, Pseudomonas aeruginosa and Cryptococcus neoformans.
Uses of capsule staining
- It is used to visualize the capsule, since the capsule is not stained by ordinary stains and it appears as a clear halo around the cell.
- It is used to differentiate encapsulated and non-encapsulated cells.
- It is a part of specialized identification where the presence of polysaccharide layer (glycocalyx) is confirmed.
- It is used in detecting encapsulated fungal pathogens like Cryptococcus neoformans by India ink method.
- It is used to assess virulence factor because capsule formation is associated with virulent strains.
- It helps in determining the degree of pathogenicity of an isolate.
- It is used to confirm antiphagocytic property of pathogens since the capsule resist phagocytosis.
- It is helpful in diagnosing invasive diseases caused by encapsulated organisms like Streptococcus pneumoniae, Klebsiella pneumoniae and Neisseria meningitidis.
- It forms the basis for serological typing of capsular antigen, for example in Quellung reaction.
- It is used in vaccine development since capsular polysaccharides act as antigenic components of conjugate vaccines.
- It was important in early scientific discovery for identifying virulent “S” forms in classical transformation experiments.
- It helps in studying adherence and biofilm formation, because capsules assist in attachment to host tissues or other cells.
Advantages of capsule staining
- It is the most reliable method to visualize the capsule since the capsule does not take up ordinary stains and appears as a clear halo.
- It helps to differentiate encapsulated and non-encapsulated strains of bacteria.
- It is used for identifying important pathogens like Streptococcus pneumoniae, Klebsiella pneumoniae, Neisseria meningitidis, Haemophilus influenzae and Cryptococcus neoformans.
- It gives rapid preliminary diagnosis because confirmation of capsule indicates possible pathogenic nature.
- It is helpful to assess virulence since capsule formation is an important virulence factor.
- It helps to predict the severity of disease as encapsulated organisms are often associated with severe invasive infections.
- It confirms the antiphagocytic property of pathogens because capsule resist engulfment by immune cells.
- It can guide treatment because encapsulated pathogens require effective opsonization and proper immune response for clearance.
- It avoids heat fixation, which is important since heat can shrink or distort the capsule.
- It uses non-aqueous rinsing solutions (like copper sulfate) to prevent dissolution of the capsule during staining.
- It supports vaccine development because capsular polysaccharides are major antigenic components for conjugate vaccines.
- It is useful before serological typing methods such as Quellung reaction where capsular swelling is observed.
- It has historical importance as it helped to demonstrate the virulent “S” type in classical transformation experiments.
Limitations of capsule staining
- There is a high risk of capsule distortion because heat fixation is not allowed and heat can shrink or dissolve the capsule.
- Water cannot be used for rinsing since the capsule is water-soluble and may get washed away easily.
- The cells are not fixed to the slide, so slight pressure during blotting can remove the cells or damage the capsule.
- Smear preparation is difficult because vigorous shaking or rough handling can detach the capsule or slime layer.
- The capsule does not stain directly, so it is seen only as an unstained halo which makes the interpretation indirect.
- Negative stains like India ink may form artifacts due to colloidal particles showing Brownian movement.
- The stain often requires proper adjustment because too dense background can hide the cells, and thinned stain may reduce contrast.
- Sometimes the rinsing agent like copper sulfate gives faint blue colouring of the capsule which may reduce clarity.
- It is difficult to distinguish loose slime layer from true capsule since slime may not properly exclude the stain.
- Capsules that look similar in shape may have different protective properties which cannot be distinguished microscopically.
- Capsule size depends on culture conditions, age of culture, and medium, so a negative result may come due to poor growth conditions, not due to absence of capsule.
- Araujo, G. de S., Fonseca, F. L., Pontes, B., Torres, A., Cordero, R. J. B., Zancopé-Oliveira, R. M., et al. (2012). Capsules from Pathogenic and Non-Pathogenic Cryptococcus spp. Manifest Significant Differences in Structure and Ability to Protect against Phagocytic Cells. PLoS ONE, 7(1), e29561.
- Capsule staining – (Microbiology) – Vocab, Definition, Explanations. (2025). Fiveable Inc.
- Capsule Staining- Principle, Reagents, Procedure and Result. (n.d.). [Source not explicitly identified, often a website or blog].
- Encapsulated Bacteria. (2020, January 11). Medbullets Step 1. Medbullets Team.
- Expert Report: Capsule Staining – Principles, Techniques, and Clinical Pathology of the Bacterial Glycocalyx. (n.d.).
- Gao, S., Jin, W., et al. (2024). Bacterial capsules: Occurrence, mechanism, and function. [Unknown Journal], 10(21).
- Hartline, R. (2023, February 17). 1.13: Capsule Stain. Microbiology Laboratory Manual (Hartline). Biology LibreTexts.
- Hinsu, M. (n.d.). Capsule staining [Presentation slides]. Slideshare.
- Hughes, R. B., & Smith, A. C. (2007). Capsule Stain Protocols. American Society for Microbiology.
- Liu, X., Xu, Q., et al. (2025). Capsular polysaccharide enables Klebsiella pneumoniae to evade phagocytosis by blocking host-bacteria interactions. mBio, 16(3), e0383824.
- Sandeep Sir Medico. (n.d.). HISS’S STAIN.
- STAINS & STAINING. (n.d.). [Source not explicitly identified, possibly internal notes or slides].
- Uribe-Querol, E., & Rosales, C. (2017). Control of Phagocytosis by Microbial Pathogens. Frontiers in Immunology, 8, 1368.
- Wikipedia contributors. (n.d.). Bacterial capsule. Wikipedia.
- Zaragoza, O., et al. (2009). The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol, 68, 133–216.