What is Bombardment (Biolistics) Method?
- The bombardment method, often called biolistics, is an advanced technique widely utilized for genetic transformation in plants and various organisms. It provides a distinct approach to introducing foreign DNA into target cells, which has significant implications for genetic engineering and biotechnology.
- Biolistics, derived from “biological ballistics,” employs high-velocity microprojectiles, such as gold or tungsten particles. These tiny particles serve as carriers for the DNA, which is coated onto their surfaces. When propelled at high speeds, these microprojectiles penetrate the cell walls and membranes of target tissues, delivering the genetic material directly into the cytoplasm or nucleus. This direct method of DNA delivery is particularly beneficial for transforming cells that are typically difficult to modify using conventional methods, such as Agrobacterium-mediated transformation.
- The mechanics of the biolistics process involve a gene gun or a particle inflator. In the gene gun method, a burst of gas propels the microprojectiles into the target tissue. Alternatively, in the particle inflator system, helium is used to accelerate the particles. Regardless of the method used, the primary goal remains the successful transfer of DNA into the cells. This technique is particularly advantageous because it can be applied to a wide range of tissues, including those that are recalcitrant to other transformation methods.
- Following the bombardment, the introduced DNA can integrate into the host genome through various mechanisms, allowing for stable expression of the new traits in subsequent generations. This aspect is crucial for developing genetically modified organisms (GMOs) with desirable characteristics, such as improved resistance to pests, enhanced nutritional content, or increased tolerance to environmental stressors.
- The biolistics method has undergone considerable refinement over the years. Researchers continually optimize parameters such as particle size, velocity, and target tissue type to enhance transformation efficiency. This iterative process has led to improved success rates and reduced cell damage, which are critical for maintaining the viability of the target tissues post-transformation.
- Moreover, biolistics is not limited to plant systems; it has been applied in various fields, including animal and microbial genetics. In animal research, for instance, biolistics can facilitate the study of gene function, gene therapy, and the development of transgenic animals. In microbiology, the method aids in the transformation of bacterial cells, enabling the exploration of gene function and regulation.
Principle of Bombardment (Biolistics) Method
- The principle of the bombardment (biolistics) method, commonly referred to as particle bombardment, revolves around the physical delivery of DNA into plant cells using high-velocity microprojectiles. This technique has gained traction in plant biotechnology due to its effectiveness and versatility.
- The core mechanism of biolistics is centered on the use of microprojectiles, which are typically constructed from tungsten or gold. These particles are meticulously coated with DNA, ensuring that the genetic material is effectively introduced into the plant cells. The biolistic device, commonly known as a gene gun, propels these microprojectiles at high speeds into the target cells or tissues.
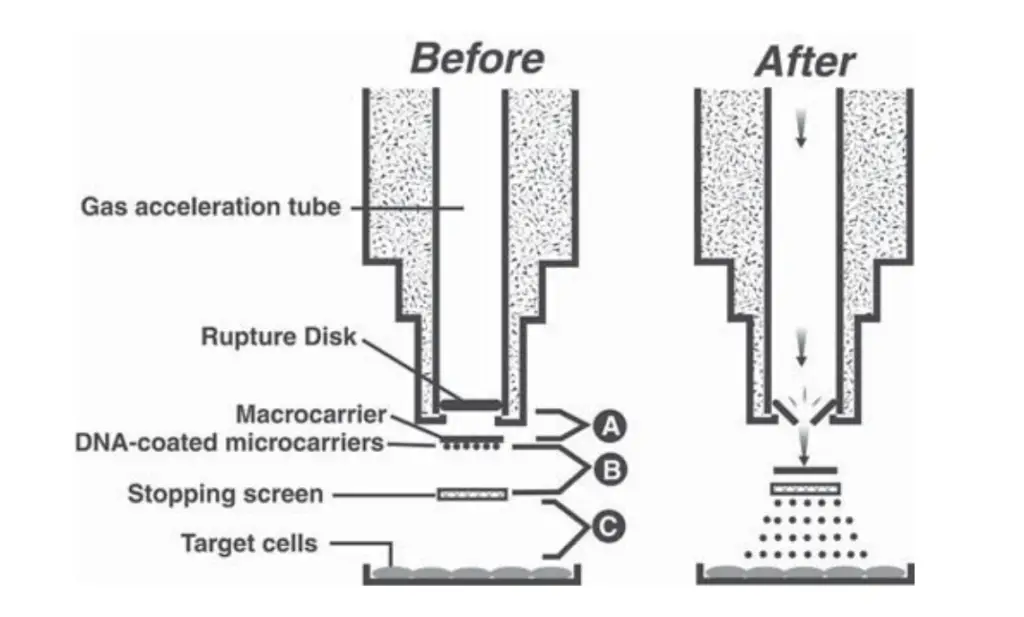
- The propulsion of the microprojectiles is achieved through the generation of high-pressure helium gas within the biolistic device. This gas accelerates a macrocarrier, which serves as a larger carrier for the microprojectiles, towards a stopping screen. Upon striking this screen, the macrocarrier comes to an abrupt halt, while the microprojectiles continue their trajectory at elevated velocities. This high-velocity impact is crucial for penetrating the rigid cell walls and membranes of the targeted plant cells, facilitating the introduction of the DNA into the cells.
- Once the microprojectiles breach the cell walls, the coated DNA is released into the cytoplasm of the target cells. For successful transformation, it is essential that the DNA reaches the nucleus, as this is where integration into the host genome can occur. This integration is a key objective of the biolistics method, as it allows for stable incorporation of foreign DNA into the plant’s chromosomal DNA. As a result, the introduced genes can be expressed in subsequent generations of the transformed plant, thereby achieving lasting genetic modifications.
- Besides stable integration, biolistics also enables transient gene expression. In this scenario, the introduced DNA is expressed for a limited duration without integrating into the genome. This feature is particularly beneficial for researchers studying gene function and expression patterns, as it allows for a more immediate observation of the effects of introduced genes.
- The versatility of the biolistics method is one of its most significant advantages. It can be applied to a wide array of plant species and tissues, including those that are challenging to transform via traditional methods such as Agrobacterium-mediated transformation. This flexibility makes biolistics a valuable tool in the realm of plant biotechnology, as it expands the possibilities for genetic engineering across diverse plant types.
- In summary, the principle of the bombardment method is fundamentally about the physical delivery of DNA-coated microprojectiles into plant cells. This technique not only facilitates the introduction of foreign genetic material but also supports both stable and transient expression of genes. Its effectiveness across various plant tissues and species underscores its importance in advancing research and applications in plant biotechnology.
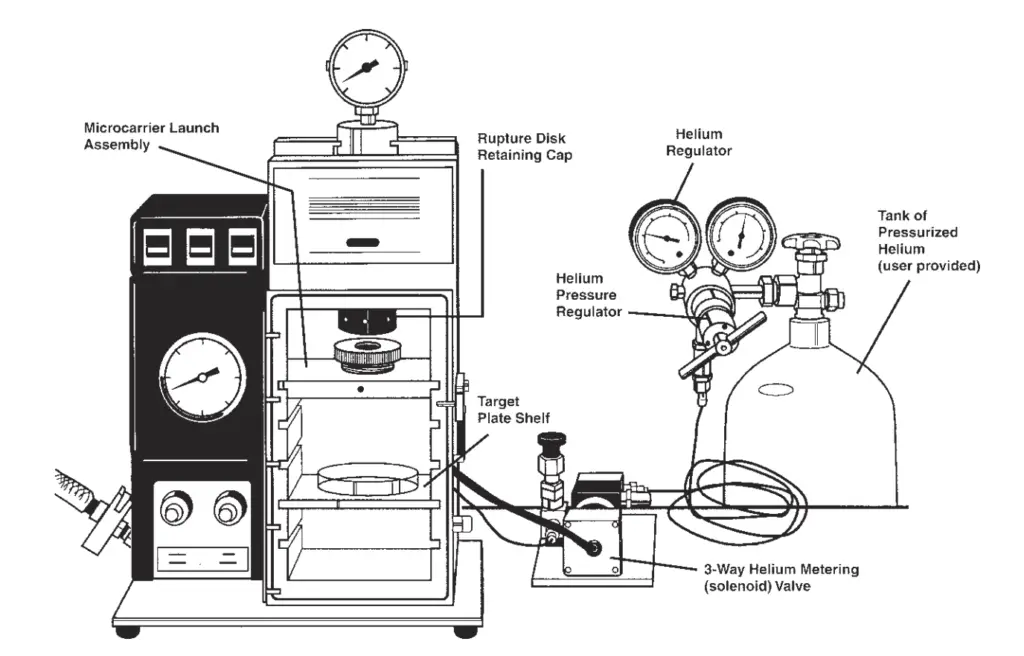
Materials Required for Bombardment (Biolistics) Method
- Reagent Quality: All reagents used in this process must be of tissue culture or molecular biology grade to ensure the highest level of purity and effectiveness.
- Culture and Preparation of Plant Cells:
- Plant Material: Embryogenic Vitis vinifera L. ‘Chardonnay’ cell suspension cultures serve as the starting material for the transformation process.
- Medium for Cell Suspension Cultures: The growth medium consists of Murashige and Skoog (MS) basal medium enriched with macro- and microelements, vitamins, inositol, 18 g/L of maltose hydrate, 4.6 g/L of glycerol, and 5 μM β-napthoxyacetic acid (NOA). The pH is adjusted to 5.8 using KOH prior to autoclaving. A stock solution of NOA is prepared by dissolving 20.2 mg in 2 mL of 1 M KOH, adding 90 mL of Type I water, stirring, and then filter sterilizing.
- Flasks: Use 250-, 500-, and 1000-mL Erlenmeyer flasks, which should be capped with aluminum foil and autoclaved to maintain sterility.
- Filtration Tools: A double-screen mesh (1.1 mm² pore size) in a polypropylene funnel is required to filter the cell suspensions effectively.
- Pipets and Accessories: Disposable 10- and 25-mL plastic pipets, cotton-plugged and sterile, along with a compound microscope, glass slides, and cover slips for observational purposes.
- Stirring Equipment: A magnetic stir plate equipped with an autoclaved stir bar is essential for mixing the media.
- Centrifuge Tubes and Bottles: Graduated 12- or 15-mL conical centrifuge tubes and 100-mL media bottles with screw cap lids should also be autoclaved.
- Additional Equipment: 1-mL sterile polyethylene transfer pipets, a Büchner funnel (8 cm in diameter, autoclaved), a size arm flask (1 L, autoclaved), a vacuum source, and 7-cm diameter Whatman no. 2 filter papers, all autoclaved to maintain sterility.
- Bombardment Medium: The bombardment medium is composed of half-strength MS-HF (hormone-free) medium with osmotica, including 30 g/L of sucrose, 0.125 M mannitol, 0.125 M sorbitol, and 2.5 g/L of Phytagel. The pH is adjusted to 5.8 with KOH before autoclaving. The medium should be dispensed in 10-mL aliquots onto sterile, circular filter paper contained in Petri plates, which can be stored at room temperature for up to one month.
- Preparation of DNA-Coated Microcarriers:
- Macrocarriers and Holders: Macrocarriers and macrocarrier holders for the biolistic device (such as those from Bio-Rad) are fundamental to the process.
- Ethanol for Sterilization: Utilize both 70% and 95% ethanol for the sterilization of equipment.
- Sterilization Equipment: Glass beakers and Petri plates (both autoclaved) are required along with sterile Kimwipes or paper towels.
- Desiccation Setup: Place desiccant in glass Petri dishes with a stable, dust-free platform for loading DNA-coated particles onto macrocarriers, using Drierite brand desiccant.
- Gold Microcarriers: Gold particles (0.75 μm in diameter) are necessary for coating with DNA. These can be sterilized using isopropanol, while the coating process involves several specific reagents such as plasmid DNA in sterile TE buffer, 2.5 M CaCl₂, and 0.1 M spermidine, all of which must be filter-sterilized and stored appropriately.
- Bombardment Process:
- Biolistic Instrument: The Biolistic® PDS-1000/He Instrument (Bio-Rad) is crucial for the actual bombardment procedure.
- Helium Gas Supply: A high-pressure helium gas cylinder (2400–2600 psi) of grade 4.5 or 5.0 is required.
- Vacuum Pump: An oil-filled rotary vane vacuum pump with a pumping speed of 90–150 L/min is essential to create the necessary vacuum conditions.
- Additional Equipment: Rupture disks (sterilized with isopropanol), stopping screens (sterilized by autoclaving), safety glasses, a hair net, and latex gloves are also necessary for maintaining safety and sterility throughout the process.
- Storage for Bombarded Plates: An opaque plastic box sterilized with 70% ethanol is recommended for storing bombarded plates.
- Postbombardment Medium Reduction:
- Medium Composition: The medium used post-bombardment is 1/2 MS-HF without osmotica, including MS medium with half-strength macro- and microelements, vitamins, inositol, sucrose, and Phytagel. The pH is adjusted to 5.8 before autoclaving and dispensing.
- Analysis of Gene Expression:
- GUS Staining Solution: The preparation of GUS histochemical staining solution involves mixing components such as type I water, EDTA, sodium phosphate, potassium ferrocyanide, and Triton X-100, followed by the addition of 5-bromo 3-chloro 3-indolyl β-D-glucuronic acid (X-Gluc).
- Analysis Equipment: This includes sterile forceps, sterile Petri plates, an incubator set at 37°C, a stereomicroscope, a plastic sheet with an imprinted grid for measurement, and a cell counter.
- Embryo Selection, Germination, and Regeneration:
- Selective Medium: Kanamycin monosulfate (Km) stock at 25 mg/mL is prepared and added to a selective medium containing 1/2 MS-HF with sucrose, activated charcoal, Bacto-agar, and Km.
- Germination and Growth Media: Embryo germination medium and plant growth medium (woody plant medium) are prepared to facilitate the growth of the transformed cells.
Protocol for Bombardment (Biolistics) Method
The bombardment method, also known as biolistics, is a sophisticated technique employed for introducing foreign DNA into plant cells. This protocol outlines the essential steps for preparing plant cells and DNA-coated microcarriers, executing the bombardment, and analyzing the results. Rigorous attention to detail and sterile conditions are paramount throughout the process to ensure successful transformation.
- Preparation Timeline and Sterile Environment
Preparation for bombardment should commence six days in advance. All procedures must occur within a laminar flow hood to mitigate the risk of microbial contamination. - Culture and Preparation of Plant Cells
- Maintenance of Cells:
- Embryogenic suspension cells must be cultivated in GM+NOA medium within 250- or 500-mL Erlenmeyer flasks, at a speed of 120 rpm, kept in the dark at 23 ± 1°C.
- Each week, refresh the medium by removing and replacing half of the spent medium using a sterile plastic 10- or 25-mL pipet.
- Filter cells through a sterile screen mesh using a funnel to eliminate larger clumps as necessary.
- Pre-Bombardment Cell Check:
- Utilize cells for bombardment four days after subculture. Prior to use, check for microbial contamination by placing a sample on a glass slide under a compound microscope.
- Cell Preparation for Bombardment:
- Transfer all cells required for bombardment through a sterile screen mesh into a 1-L sterile Erlenmeyer flask. Add a sterile stir bar and mix on a magnetic stir plate at a low speed.
- To standardize cell density, take a 10-mL sample of the suspension, allow it to settle for 15 minutes (or 30 minutes for very fine suspensions), and adjust the density to 0.2 mL of settled cell volume per 10-mL sample by modifying the volume of GM+NOA medium.
- Application of Cells on Filter Paper:
- Place a sterile Whatman no. 2 filter paper in a Büchner funnel atop a 1-L side-arm flask. Moisten the filter with 1 mL of GM+NOA medium using a sterile transfer pipet.
- While stirring the cell culture, collect 5 mL of cells and spread them onto the filter. Apply slight vacuum to remove excess liquid and evenly distribute the cells.
- Transfer of Cells to Bombardment Medium:
- Use sterile forceps to transfer the filter paper with attached cells to the bombardment medium.
- Maintenance of Cells:
- Preparation of DNA-Coated Microcarriers
- Sterilization of Macrocarriers and Holders:
- Submerge macrocarrier holders in a glass beaker and macrocarriers in a Petri dish filled with 70% ethanol for 15 minutes.
- Afterward, remove them using sterile forceps and allow them to dry in a laminar flow hood.
- Sterilization of Gold Particles:
- Weigh 30 mg of gold particles, heat them at 180°C for 12 hours, and subsequently mix with 0.5 mL of isopropanol.
- Perform multiple washing steps with sterile water to remove any remaining contaminants, resulting in a suspension in 0.5 mL of 50% (v/v) glycerol/type I water.
- Coating Gold Particles with DNA:
- Vortex-mix gold particles, then sequentially add 5 μL of plasmid DNA, 50 μL of 2.5 M CaCl2, and 20 μL of 0.1 M spermidine, vortexing gently between additions.
- Incubate the mixture on a continuous vortex mixer, pellet the microcarriers, and resuspend them appropriately for bombardment.
- Sterilization of Macrocarriers and Holders:
- Bombardment Execution
- Preparation of Equipment:
- Familiarize with the bombardment apparatus and adhere to safety protocols, including the use of safety glasses and gloves.
- Set the PDS-1000/He parameters to 1300 psi helium and ensure the chamber is sterilized with 70% ethanol.
- Loading and Activation:
- Position the rupture disk within the gas acceleration tube and load the macrocarrier holder containing microcarriers.
- Insert the target cells into the chamber, close the door, and activate the bombardment unit.
- Bombardment Procedure:
- After achieving the specified vacuum, trigger the firing mechanism to burst the rupture disk. Following bombardment, vent the chamber and remove the bombarded cells.
- Preparation of Equipment:
- Post-Bombardment Treatments
- Incubation:
- Incubate the bombarded and control plates in the dark at 23 ± 1°C for two days to promote cell recovery and DNA integration.
- Medium Osmotic Potential Reduction:
- Transfer the cells to Petri plates with 1/2 MS-HF medium without osmotica to begin reducing osmotic potential. This should be performed approximately 16 hours after bombardment.
- Incubation:
- Analysis of GUS Expression
- Assessment Timeline:
- Perform a transient GUS expression assay 48 hours after bombardment, comparing results with negative controls.
- Use sterile forceps to transfer filter papers with cells to fresh plates, applying 600 μL of X-gluc solution. Incubate at 37°C overnight, noting that transformed cells will exhibit blue coloration.
- Long-Term Monitoring:
- Conduct follow-up analyses over a 3–6 month period to evaluate rates of long-term GUS expression, indicative of stable transformation.
- Assessment Timeline:
- Embryo Selection, Germination, and Regeneration
- Selection Medium Transition:
- Two days post-bombardment, transfer cells to selective medium containing 10 mg/L of Km for embryo induction. This process requires periodic transfers to fresh selective medium with increasing concentrations of Km.
- Germination and Growth:
- Once putative Km-resistant embryos are identified, transfer them directly to germination medium, adjusting light and temperature conditions to promote development.
- Continuously transfer embryos to fresh medium and ultimately to plant growth medium for root elongation and shoot formation.
- Selection Medium Transition:
Particle bombardment transformation, also known as biolistics, is a sophisticated method employed in genetic engineering to introduce foreign DNA into target cells. This procedure involves a sequence of meticulously orchestrated steps that ensure the successful incorporation of genetic material into the cells. The following is a flow chart of the essential steps involved in this process, providing clarity and structured guidance for students and educators.
- Preparation Phase (Week Prior to Bombardment)
- –6 Days: Sterilization of Supplies
- Sterilize all necessary supplies, including Whatman filter papers, Sharkskin filter papers, funnels, flasks, and water, to ensure a contamination-free environment for cell culture and transformation.
- –5 Days: Media Preparation
- Prepare the required media for the transformation procedure, including:
- GM+NOA suspension culture medium for maintaining cells.
- 1/2 MS-HF bombardment medium with osmotica to support cell health during bombardment.
- 1/2 MS-HF medium without osmotica for post-bombardment recovery.
- 1/2 MS-HF selective medium to facilitate the selection of transformed cells.
- Prepare the required media for the transformation procedure, including:
- –4 Days: Subculture Embryogenic Cell Suspensions
- Subculture or refresh the embryogenic cell suspensions to ensure they are in optimal condition for bombardment.
- –6 Days: Sterilization of Supplies
- Bombardment Week (Week of Bombardment)
- –1 Day: Setup and Preparations
- Configure the gene gun parameters, ensuring correct distances as described in the accompanying figure.
- Weigh the gold particles (microcarriers) and place them in an oven overnight for sterilization.
- Sterilize macrocarriers, holders, and stopping screens to maintain a sterile environment.
- Assemble the macrocarriers into holders, readying them for the bombardment process.
- Key Day (Bombardment Day)
- Examine the embryogenic cell suspension for microbial contamination using a microscope.
- Prepare cells on filter paper for the bombardment.
- Sterilize microcarriers to eliminate any potential contaminants.
- Coat microcarriers with DNA, ensuring that the genetic material is effectively bound to the particles for successful transfer.
- Proceed with the bombardment of cells, introducing the DNA-coated microcarriers into the target cell population.
- Incubate the bombarded cells in the dark at 23 ± 1°C to facilitate initial recovery and integration of the foreign DNA.
- –1 Day: Setup and Preparations
- Post-Bombardment Phase (Following Bombardment)
- +1 Day: Initial Medium Transfer
- Transfer the cells to a medium without osmotica, performing the first transfer approximately 16 hours post-bombardment.
- Conduct a second transfer approximately 24 hours after bombardment to further support cell recovery.
- +2 Days: Transfer to Selective Medium
- Transfer the cells to selective medium to initiate selection of transformed cells.
- Perform an analysis of the reporter gene, such as the GUS assay, to evaluate transient expression of the introduced gene.
- +3 Days: Evaluation of GUS Expression
- Examine the plates for GUS-positive blue spots, indicating successful transformation and expression of the reporter gene on the filter paper.
- +1 Day: Initial Medium Transfer
- Weeks Following Bombardment (Post-Bombardment Weeks)
- +30 Days: Fresh Selective Medium Transfer
- Transfer the cells to fresh selective medium and conduct a reporter gene assay to monitor transient expression levels.
- +60 Days: Embryo Development Check
- Inspect the plates for the development of embryos and transfer the confirmed embryos to germination medium.
- Move any remaining cells to fresh selective medium and perform a reporter gene assay for long-term expression analysis.
- +90 Days: Final Check and Transfer
- Conduct checks similar to those performed at +60 days, ensuring that the germinated embryos are properly transferred to plant growth medium for further development.
- +30 Days: Fresh Selective Medium Transfer
Important Notes
Below are important notes regarding this method that elucidate its nuances and best practices, aiding both students and educators in understanding its complexities.
- Embryogenic Cell Cultures as Ideal Targets
- Embryogenic cell cultures are preferred for biolistic transformation due to their uniformity and high regeneration capacity. Proembryogenic cells, which are finely divided, spread easily on filter papers, enhancing their suitability for bombardment. Additionally, small cell clusters facilitate the selection of transformants, minimizing the occurrence of non-transformed cells escaping selection.
- Role of Osmotica in Bombardment Medium
- Supplementing the bombardment medium with osmotica, such as mannitol or sorbitol, has been shown to improve stable transformation rates across various suspension-cultured cells. While the advantages are evident for suspension cultures, the impact of osmotica is less pronounced when utilizing intact tissues, such as leaves or whole embryos. It is posited that the osmotica help reduce cellular damage by inducing plasmolysis, which prevents protoplasm leakage from bombarded cells.
- Selection of Particle Types and Sizes
- Bio-Rad provides gold particles in various sizes, with 0.6 μm and 1 μm being most effective for plant cell transformation. Tungsten particles are also an option for many plant species and are cost-effective. However, they may present issues, such as heterogeneous size and potential DNA degradation, which could negatively affect transformation efficiency. Therefore, users are encouraged to review Bio-Rad bulletins for detailed information on particle types and sizes.
- Considerations for DNA Purity and Preparation
- The purity of DNA is crucial; it should be devoid of RNA or protein to prevent microprojectiles from clumping. Techniques such as cesium chloride gradient centrifugation or using a plasmid purification kit (e.g., Qiagen) are recommended for DNA purification. Furthermore, it is essential to maintain fresh spermidine stocks, as they can degrade even when frozen, which may dramatically reduce transformation efficiency.
- Rupture Disks and Pressure Considerations
- Rupture disks are available in a range of bursting pressures, from 450 to 2200 psi, with 1100 psi being the most common for plant tissues. Higher pressure disks can impart greater velocity to carriers but may also result in increased tissue damage. Therefore, the choice of rupture disk should align with the robustness of the target tissue.
- Dispensing Techniques for Culture Medium
- When dispensing 10 mL of medium onto plates, it is advisable to either swirl the plates to ensure uniform coverage or pipette additional medium and then remove excess until only 10 mL remains. This technique helps achieve an even distribution of the medium over the cells.
- Laboratory Setup and Efficiency
- Effective design of bombardment experiments is essential to ensure comfort and efficiency for operators. In practice, a maximum of 50 to 60 plates can be bombarded in a single day by two individuals working collaboratively. One technician prepares the target cells while the other prepares the DNA-coated microcarriers, facilitating a smooth workflow.
- Cell Culture Management and Contamination Control
- As cell populations increase in flasks, they should be divided into multiple flasks to maintain optimal growth conditions. There is no strict formula for this division; technicians should develop an eye for maintaining a desirable population density indicated by a creamy white or light yellow coloration and small cell clusters. Moreover, contamination control is paramount. Weekly subcultures should include streaking samples onto bacterial and plant growth media to monitor for contamination, with further examination of cells using microscopy to detect any microbial presence.
- Handling of Microcarriers and Technique Consistency
- The bore of a 5-mL pipet can be too small, leading to blockages from cell clumps. Therefore, practitioners should strive to minimize cell loss and ensure uniform spreading across filter papers, which may require practice. Macrocarriers and holders may be assembled and sterilized together; however, it is advisable to use alcohol sterilization to avoid shrinkage issues that can occur with autoclaving.
- Importance of Consistent Techniques
- Consistent techniques in preparing microprojectiles and loading the biolistic apparatus are crucial, as variations in methodology can lead to substantial differences in transformation efficiency. Finger vortex-mixing prior to aliquoting microcarriers and immediate placement of macrocarriers in a desiccator post-loading can help mitigate clumping due to humidity exposure.
- Gene Gun Settings and Experimental Conditions
- Proper gene gun settings are critical for successful transformation. Settings should be checked prior to each bombardment, and adjustments made based on the type of tissue being transformed. Higher particle velocities can be achieved through increased helium pressure and shorter distances; however, caution must be exercised since these adjustments may also increase shockwave impact, potentially reducing stable transformation rates.
- Medium Preparation and Storage
- Maintaining appropriate vacuum conditions is important, as excessive vacuum may lead to boiling of the medium. Using slightly lower vacuum levels or increasing the gelling agent concentration in the medium can help prevent medium loss during the process. Additionally, the green fluorescent protein (gfp) gene can be used as a nondestructive reporter gene, offering flexibility in monitoring transformation.
- Optimization for Specific Cultivars
- The procedures and growth media outlined here have been specifically tailored for certain grapevine cultivars such as ‘Chardonnay,’ ‘Merlot,’ and ‘Pinot Noir.’ It is imperative for researchers to optimize media and selective agents based on the specific genotypes and cell culture lines they are working with.
- Embryo Incubation Techniques
- Lastly, embryos can be incubated either at 4°C in the dark for a chilling treatment followed by standard conditions or directly at room temperature with low light intensity. Both methods have shown similar outcomes in terms of embryo development, thus offering flexibility in incubation strategies.
Advantages of Bombardment (Biolistics) Method
The bombardment (biolistics) method, a key technique in plant biotechnology, offers numerous advantages that make it a preferred choice for researchers and practitioners. Here are the primary benefits of this method:
- Wide Applicability: Biolistics can be utilized across a diverse range of plant species and tissues, including those that are traditionally difficult to transform, such as certain woody plants and cereal crops. This broad applicability enhances its utility in various agricultural and research settings.
- High Transformation Efficiency: The method allows for efficient delivery of DNA into cells, resulting in higher transformation rates compared to some other techniques, particularly in recalcitrant species that may not respond well to Agrobacterium-mediated transformation.
- Stable and Transient Expression: Biolistics facilitates both stable integration of foreign DNA into the plant genome and transient expression of genes. This dual capability allows researchers to study gene function and expression patterns over different time frames, providing flexibility in experimental design.
- Reduced Contamination Risk: Since the biolistics method does not rely on biological vectors like bacteria, there is a lower risk of contamination with pathogens that could occur in other transformation methods, particularly those involving live organisms.
- Control Over Delivery Parameters: Researchers can optimize various parameters such as particle size, speed, and distance, tailoring the bombardment conditions to enhance transformation efficiency based on the specific requirements of the plant tissue being used.
- Direct Gene Delivery: The physical delivery of DNA directly into plant cells bypasses the need for cellular uptake mechanisms, which can be inefficient. This direct approach can improve the likelihood of successful transformation.
- Versatility in DNA Constructs: The method accommodates a wide variety of DNA constructs, including plasmids, linearized DNA, and even RNA molecules, allowing for diverse genetic modifications tailored to specific research goals or agricultural needs.
- Suitable for Complex Genomes: Biolistics is particularly advantageous for plants with complex genomes where traditional transformation methods may struggle, thereby facilitating the genetic engineering of more sophisticated traits.
- Rapid Results: The relatively straightforward and rapid execution of biolistics can yield results in a shorter time frame compared to other methods, making it valuable for time-sensitive research and applications.
Limitation of Bombardment (Biolistics) Method
While the bombardment (biolistics) method presents several advantages in plant biotechnology, it also has limitations that researchers should consider. Here are the primary drawbacks of this technique:
- Tissue Damage: The high-velocity impact of microprojectiles can cause mechanical damage to plant tissues, leading to cell death or reduced viability. This issue can be particularly pronounced in delicate or sensitive tissues.
- Variable Transformation Efficiency: The efficiency of transformation can vary significantly depending on the plant species, tissue type, and specific experimental conditions. Not all plant cells respond equally to bombardment, which can lead to inconsistent results.
- Regeneration Challenges: After transformation, the regenerated plants must be capable of developing from the transformed cells. Some species may have low regeneration potential, complicating the recovery of viable plants.
- Gene Silencing: There is a potential for gene silencing, where the introduced genes may be downregulated or silenced by the plant’s regulatory mechanisms. This can limit the expression of desired traits in transformed plants.
- Integration Challenges: The integration of foreign DNA into the plant genome may not be stable, resulting in a loss of transgenes over successive generations. This instability can hinder the establishment of transgenic lines with consistent traits.
- Limited Precision: The random integration of DNA into the genome can lead to positional effects, where the expression of the inserted gene is influenced by nearby genomic elements. This randomness can result in unintended phenotypic consequences.
- Cost of Equipment: The biolistic devices, such as gene guns, can be relatively expensive to purchase and maintain, which may limit access for some research institutions or laboratories.
- Operator Skill Required: Successful execution of the bombardment technique requires a certain level of skill and experience from the operator. Variations in technique can lead to significant differences in transformation efficiency.
- Short-Term Expression Limitations: While biolistics can facilitate transient expression, the duration of this expression is often limited. This may not be suitable for experiments requiring long-term gene expression studies.
- Potential for DNA Damage: The physical force exerted during bombardment may cause DNA fragmentation or damage, impacting the functionality of the introduced genetic material.
Uses of Bombardment (Biolistics) Method
The bombardment (biolistics) method, also known as particle bombardment, is a versatile technique widely used in plant biotechnology for various applications. Here are some key uses of this method:
- Genetic Transformation: The primary use of biolistics is to introduce foreign DNA into plant cells, allowing for the creation of genetically modified organisms (GMOs). This can include inserting genes that confer desirable traits, such as pest resistance, herbicide tolerance, or improved nutritional content.
- Transgenic Plant Development: The method is instrumental in developing transgenic plants, which are essential for agricultural improvement. These plants can exhibit traits that enhance yield, disease resistance, or environmental tolerance, contributing to food security.
- Functional Genomics: Bombardment is used in functional genomics to study gene function and regulation. By introducing specific genes or gene constructs, researchers can analyze the effects of these genes on plant growth, development, and stress responses.
- Protein Production: Biolistics can be employed for the transient expression of recombinant proteins in plant cells. This approach allows for the rapid production of proteins, including enzymes, antibodies, and vaccines, without the need for stable transformation.
- Metabolic Engineering: The technique is applied in metabolic engineering to modify metabolic pathways within plants. By introducing genes that encode enzymes involved in specific biosynthetic pathways, researchers can enhance the production of valuable compounds, such as secondary metabolites.
- Research on Gene Expression: Biolistics facilitates the study of gene expression patterns in various plant tissues. By using reporter genes, such as green fluorescent protein (GFP) or β-glucuronidase (GUS), researchers can visualize and quantify gene expression in response to different environmental conditions.
- Crop Improvement: The method is valuable in improving crop varieties, especially those that are recalcitrant to traditional transformation techniques. It allows for the introduction of traits that can enhance crop performance in specific environments or under particular stresses.
- Development of Biopharmaceuticals: Biolistics can be utilized to produce biopharmaceuticals in plants, leveraging the ability of transgenic plants to synthesize complex molecules, such as therapeutic proteins and vaccines.
- Studying Plant-Pathogen Interactions: Researchers use biolistics to investigate the interactions between plants and pathogens. By introducing genes that confer resistance or susceptibility, scientists can better understand plant immune responses.
- Improving Transformation Protocols: The method is also used to optimize transformation protocols for various plant species. By experimenting with different microprojectile sizes, DNA concentrations, and bombardment parameters, researchers can enhance transformation efficiency and stability.
- Kikkert JR, Vidal JR, Reisch BI. Stable transformation of plant cells by particle bombardment/biolistics. Methods Mol Biol. 2005;286:61-78. doi: 10.1385/1-59259-827-7:061. PMID: 15310913.
- Prasad, Bishun & Kumari, Diksha & Sahni, Sangita & Ranjan, Tushar. (2022). TRANSGENIC PLANTS: METHODS AND CURRENT INNOVATIONS.
- Clough, S. J., & Bent, A. F. (1998). Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. The Plant Journal, 16(6), 735–743. doi:10.1046/j.1365-313x.1998.00343.x
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/biolistics
- https://www.sciencedirect.com/topics/immunology-and-microbiology/biolistic-transformation
- https://forages.oregonstate.edu/tallfescuemonograph/transgenesis/methods/biolistic