What is an autoclave?
An autoclave is a device that is used for sterilization / disinfection by means of steam under pressure, and it is found in hospitals, labs, industry.
Items are placed in a chamber where they are exposed to high-pressure saturated steam (commonly 121 °C for 15–20 minutes) so that microbes and spores are killed.
The sterilization is achieved by moist heat, which denatures proteins, and so cell membranes and enzymes are inactivated permanently.
In many labs, surgical suites and research facilities, this equipment is relied on daily, and labs use it for glassware, media, instruments.
The process is described simply: air is removed (by gravity or vacuum), steam is introduced, pressure is raised, temperature increases, lethality is achieved.
Bacillus subtilis spores and other resistant forms are targeted, though biological indicators (such as Geobacillus stearothermophilus) are recommended for validation.
Autoclaves are constructed from stainless steel, with a locking door, gauges for pressure/temperature, and valves (which must be maintained), and they are heavy-duty.
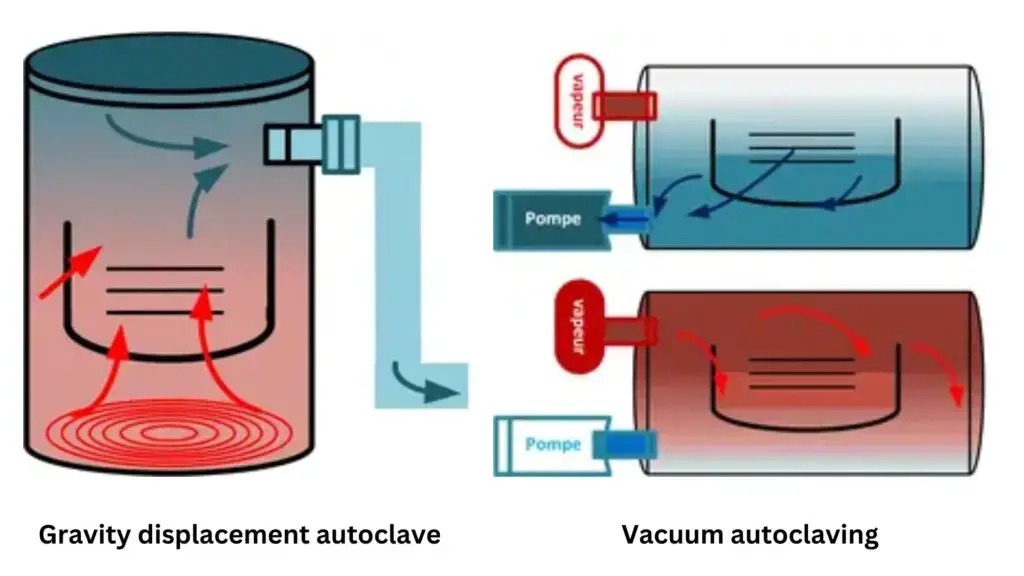
Gravity-displacement and pre-vacuum types are used — in the first, air is pushed out by steam, in the second, air is removed by vacuum making cycles faster, but more costly.
If air pockets are left, or pressure is not held, sterilization may fail, contamination risk is increased, and the load are compromised.
Tape indicators will change color when heat/steam exposure occurs, but biological spore tests are more dependable; chemical indicators are useful, yet they are not foolproof.
After the cycle, pressure is released slowly and items are cooled before removal, otherwise liquids boil over or glassware can crack.
Regular maintenance, cleaning and validation are required, because valves or deposits can cause inefficiency — neglect leads to safety/experimental errors.
It is considered greener than many chemical sterilants since water and heat are used, though energy use can be high depending on size / load.
In short, an autoclave is a vital, practical instrument for ensuring that instruments, media and waste are rendered free of viable microbes, maintaining aseptic conditions.
Definition of Autoclave
An autoclave is a machine that uses steam under pressure to sterilize materials by killing bacteria, viruses, and spores.
Autoclave Pressure and Temperature Chart
| STERILIZER | TEMPERATURE | PRESSURE | TIME |
|---|---|---|---|
| Steam autoclave | 121°C (250°F) | 15 psi | 15 min |
| Unwrapped items | 132°C (270°F) | 30 psi | 3 min |
| Lightly wrapped items | 132°C (270°F) | 30 psi | 8 min |
| Heavily wrapped items | 132°C (270°F) | 30 psi | 10 min |
| Dry heat wrapped | 170°C (340°F) | – | 60 min |
| 160°C (340°F) | – | 120 min | |
| 150°C (300°F) | – | 150 min | |
| 140°C (285°F) | – | 180 min | |
| 121°C (250°F) | – | 12 hrs | |
| Dry heat (rapid flow) unwrapped items | 190°C (375°F) | – | 6 min |
| Dry heat (rapid flow) packaged items | 190°C (375°F) | – | 12 min |
| Chemical vapor | 132°C (270°F) | 20-40 psi | 20 min |
| Ethylene oxide | Ambient | 8-10 hours |
Principle of an Autoclave – How an Autoclave Works?
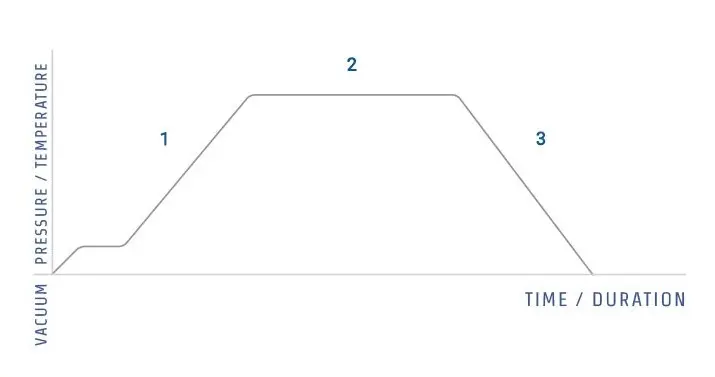
The principle of an autoclave is mainly based on the steam under pressure, by which the temperature of boiling water is increased beyond 100°C, usually about 121 °C or sometimes even higher (134°C).
In this system, sterilization is achieved by moist heat, because proteins of microorganisms are denatured / coagulated when they come in contact with saturated steam.
The heat transfer from the steam to the load happens very quickly, since the latent heat is released when steam condenses on the cooler surface, that’s the main killing step actually.
Inside the autoclave chamber, the air is first displaced (either by gravity or vacuum method), so that uniform heating is achieved in every part of the load.
The pressure itself doesn’t kill the microorganisms, it only helps to reach higher temperatures of the steam, which ensures that even resistant spores of Geobacillus stearothermophilus are destroyed.
Typically, sterilization cycle is maintained at 15 psi pressure and 121°C for around 15–20 min though sometimes more time is used for large volumes or dense materials.
The principle is that the higher pressure inside the chamber allows steam to remain liquid at a temperature above its normal boiling point, so it delivers much more energy per unit area compared to dry heat.
When the steam condenses, large amount of latent heat is released, and this heat rapidly raises the temperature of the load surface, leading to destruction of enzymes, proteins, and cell membranes.
After the sterilization cycle is complete, the pressure is released slowly, because sudden depressurization can cause liquids to boil over, or containers to break apart due to expansion.
In operation, it is made sure that all trapped air are removed, since air pockets act as insulators and prevent proper contact of steam with the material (which may cause incomplete sterilization).
The effectiveness of the autoclave depends on proper temperature–pressure–time relation, and if any one of them is not achieved properly, the sterilization process fails or becomes unreliable.
Such as, in laboratory practice, the tape indicators are used that change color when exposed to steam, though biological spore tests are more accurate for checking the sterilization efficiency.
After cooling, the load is removed carefully since condensed moisture on the surfaces may still be hot, and also sterility is maintained until used.
The working of autoclave, it can be simply stated, is a practical application of Boyle’s law and Charles’s law, where an increase in pressure raises the boiling point of water, producing higher-temperature steam.
So the autoclave works on the basic principle that — when steam under pressure contacts an object, heat energy is transferred, proteins are denatured, and all microorganisms including spores are killed, making the object sterile.
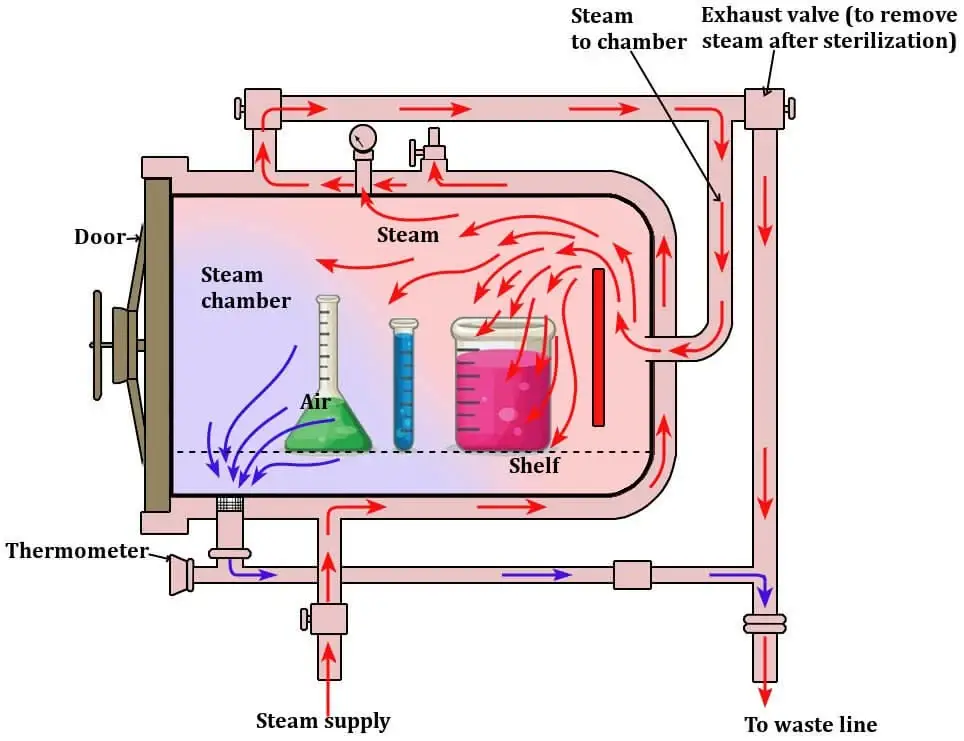
Autoclave Parts/ Components
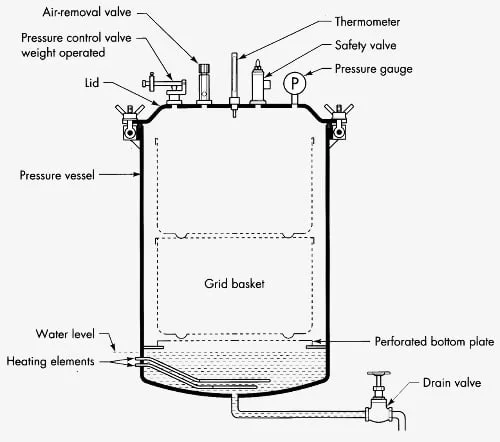
- Vessel / Chamber – is the main body which is made of stainless steel, and is built to resist high pressure and heat, it is where the load are placed and steam is held.
- Jacket – is provided around some chambers for even heating and to reduce condensation inside, and it helps temperature uniformity (used in larger autoclaves ).
- Door / Lid – is sealed with gaskets and a locking mechanism, the door is bolted or latched tightly, and sometimes doors are pneumatically operated which must be checked.
- Control System / Interface – is used to set and monitor temperature, time, pressure (121 °C / 15 psi etc) and the operator will be able to start cycles, though simple dials are still used in some models.
- Safety Valve / Pressure Relief Valve – is fitted as a fail-safe to open when pressure exceeds safe limits, this valve prevents overpressure accidents.
- Steam Generator / Heater – is used to produce steam when external steam is not supplied, and heating elements or boilers are installed for that purpose.
- Vacuum System – is found in pre-vacuum (and post-vacuum) types, air is removed by vacuum pumps so that steam penetration is improved, they are important for porous loads.
- Thermostatic Trap / Steam Trap – is installed to let condensate and air escape while keeping steam in, which ensures dry saturated steam is present in chamber.
- Gauges / Sensors – are provided (pressure gauges, thermocouples, sometimes humidity or differential sensors) and readings are taken to validate the cycle, though sensor drift can occur.
- Racks / Trays / Shelves – is used to support items and to allow steam circulation around the load, and spacing is required so steam can reach all surfaces.
- Seals / Gaskets / Door Sealing Mechanism – are critical components and they are replaced when wear is seen, poor sealing will let air in and sterilization will fail.
- Valves / Piping / Exhaust Valve – are fitted to regulate steam flow, to vent air or exhaust steam, and piping to drains or condensate tanks is included, check them regularly.
Approximate Conditions for Autoclave sterilization
| Organism | Vegetative Cells | Spores |
| Yeasts | 5 minutes at 50-60 degree centigrade. | 5 minutes at 70-80 degree centigrade. |
| Molds | 30 minutes at 62 degrees centigrade. | 30 minutes at 80 degrees centigrade. |
| Bacteria | 10 minutes at 60-70 degrees centigrade. | 2 to over 800 minutes at 100 degrees centigrade. 0.5 – 12 minutes at 121 degrees centigrade. |
| Viruses | 30 minutes at 60 degrees centigrade. |
Operating Procedures of Autoclave – How to Use an Autoclave?
Preparation – Instruments are cleaned and decontaminated, gross soil is removed and corrosion is looked for before packing.
Packaging – Items are wrapped in permeable wraps or pouches (sterilization pouches / wraps) that allow steam penetration and chemical indicators are included inside the packs.
Loading – Trays are arranged to allow steam circulation, heavy items are placed low and light items above, spacing is maintained so steam contact is ensured.
Indicators – Sterilization indicators (chemical strips, autoclave tape) are placed and Biological Indicators BI (eg. Bacillus spores) are used periodically for validation.
Cycle Selection – The cycle is selected according to load type (commonly 121°C /15 psi for 15–20 min or 134 °C for shorter cycles) and settings are set.
Water Level – The water reservoir is checked and filled with distilled or de-ionized water as required, debris is inspected in drain/reservoir.
Bowie-Dick / Vacuum Test – For vacuum autoclaves a Bowie-Dick test is run daily to confirm air-removal effectiveness.
Sealing – Sealed containers are not autoclaved, lids are loosened or removed and pressure-bearing vessels are vented or otherwise prepared before processing.
Operation Start – The chamber door is closed and locked, interlocks are verified, safety checks are done and then the cycle is started.
Monitoring – Temperature, pressure and time are monitored continuously and readouts are recorded, anomalies are noted and alarms are responded to.
Safety / PPE – Personal Protective Equipment (PPE) is worn by operators (goggles, heat-resistant gloves, lab coat) and emergency procedures are known.
Cooling and Depressurization – The chamber is allowed to cool and depressurize fully before opening, rapid opening is avoided to prevent steam-burns.
Unloading – Loads are removed carefully, items are dried or allowed to dry and then stored aseptically, hot surfaces are handled with caution.
Recordkeeping – Cycle parameters, operator ID, load contents and indicator results are logged (manual or electronic) and records are retained per protocol.
Biological Monitoring – Biological indicators are incubated, results are reviewed and any failure is investigated because failed loads are considered non-sterile.
Maintenance – Routine maintenance is scheduled and documented, gaskets and valves are checked and chamber cleaning/calibration is performed per manufacturer recommendations.
Troubleshooting – If cycle failures are observed the unit is taken out of service, a qualified technician is contacted, the autoclave is inspected and follow-up testing is done.
Avoid Overloading – Overfilling the chamber is avoided since steam penetration will be impaired and sterilization may fail.
Validation and Quality Control – Periodic validation (process challenge) is performed and logs are audited routinely, quality control samples are reviewed.
Emergency Procedures – In case of steam leaks or pressure anomalies the emergency stop is used and the area is evacuated if required, maintenance is notified and incidents are documented.
Special Loads – Liquids are processed in appropriate containers (vented, no sealed caps), sharp instruments are contained and porous loads are arranged to allow steam access.
Access and Signage – Access to the autoclave area is controlled and warning signs are posted, unauthorized use is restricted and training records are maintained.
Air removal form Autoclave
It is very essential to remove air from the autoclave for proper sterilization. Air trapped inside may make it difficult to attain sterility because air is not as effective in sterilization as steam. There are several methods for removing air in autoclaves:
- Gravity displacement was used in many older / simpler autoclaves and it works by steam entering chamber and pushing air downward out through drain
- Downward steam displacement is variant of gravity where steam enters top (or side) and air is forced out bottom
- Steam pulsing / steam flush is used: repeated pulses of steam are injected then vented to dilute and push out residual air
- Vacuum / pre-vacuum (dynamic air removal) is applied: chamber is evacuated (air sucked out) before steam admission
- Alternating vacuum & steam pulses (super-atmospheric / subatmospheric cycles) is used in some advanced autoclaves for tough loads
- Freesteaming is allowed (vent kept open) for a period so turbulent steam flushes remaining air
- Automatic air purge / venting during steam admission is used for simple loads (high mass, low surface area) where minimal air pockets exist
- Use of HEPA / biohazard filters on exhaust line is used when air removal might release contaminated gas into lab environment
- Post-vacuum drying assist is used: after sterilization, vacuum is applied to remove residual steam / air to make load drier
Compatible/incompatible materials for the autoclave – Which Materials You can Autoclave?
Autoclaves are useful machines for cleaning many materials. However, it is essential to know the materials that could be safely put in an autoclave and those that shouldn’t. Here are some examples of materials that may go into an autoclave and those that cannot go in one:
Autoclave Compatible Materials:
- Borosilicate glass (e.g. Pyrex type, Type I) is commonly accepted to be autoclaved without severe damage.
- Stainless steel (304, 316 and their low-carbon versions) is used routinely in autoclaveable instruments.
- Polypropylene (PP) plastics are often tolerated under steam and pressure conditions (used as secondary containers often).
- Polycarbonate (PC) is sometimes used, though it may weaken after many cycles.
- Polyetheretherketone (PEEK) medical-grade plastics are considered steam-resistant and suitable for many cycles.
- Polysulfone (PSU / PSF / PPSU) plastics are used in applications needing heat and steam resistance.
- Polytetrafluoroethylene (PTFE, e.g. Teflon) is accepted for parts that need chemical resistance plus autoclave stability.
- Silicone (liquid silicone rubber, silicone tubing etc.) is tolerated under steam/autoclave conditions.
- Textiles / natural fibers (e.g. cotton linens, hospital fabrics) are autoclaveable in many clinical / lab settings.
- Paper / filter paper / steam-penetrable wrapping materials (if placed in proper waste bags or with venting) are included in autoclaveable loads.
- Latex / Vinyl gloves may be autoclaved when placed inside biohazard waste bags (so that direct steam exposure is moderated).
- Media / aqueous solutions / culture stocks (not sealed) are autoclaveable material types (liquid loads) under correct cycle.
Autoclave Incompatible Materials
- Acids, bases & organic solvents are not to be autoclaved since they can react, corrode, or volatilize.
- Chlorides / sulphates / salts / seawater are disallowed because they can cause corrosion / deposit formation inside the chamber.
- Chlorine / hypochlorite / bleach are incompatible because toxic gases or corrosive byproducts may be produced.
- Non-stainless steel metals / unalloyed iron or ordinary steel are often incompatible because they corrode under steam / pressure.
- Plastics like polystyrene (PS) are incompatible (they melt or deform under autoclave conditions).
- Polyethylene (PE / LDPE / HDPE) is disallowed (low melting point) under steam sterilization.
- Polyurethane materials are not to be autoclaved as their properties degrade or they may be damaged.
- Polyvinyl chloride (PVC) and nylon / acrylic plastics are incompatible (can release harmful gases or melt).
- Liquids in sealed containers must not be autoclaved (they can explode due to internal pressure).
- Flammable / reactive / corrosive / toxic / radioactive materials are never acceptable for autoclaving.
- Paraffin-embedded tissue is incompatible (paraffin will melt, clog, contaminate).
- Material that would touch interior surfaces (melt / adhere) is disallowed because it may damage chamber.
- Pharmaceuticals / pills / cytotoxic drugs / carcinogens / mutagens are generally incompatible because of chemical hazard / unpredictable behavior.
Types of Autoclave
Autoclave can be different types based on their uses;
- Gravity displacement autoclave is used where steam enters chamber and pushes air downward out through vent (classic / simple type)
- Pre-vacuum (or vacuum-assisted) autoclave is used where air is sucked out before steam is introduced to improve penetration
- Steam pulsing / pulse type autoclave is used where alternating steam injection & exhaust is done to remove residual air
- Class N autoclave is considered basic type (gravity method) for simple solid loads not having internal channels
- Class S autoclave is intermediate class blending features of gravity + limited vacuum or steam contro
- Class B autoclave is advanced type where full vacuum + controlled steam cycles are used, capable of sterilizing porous, hollow, wrapped loads
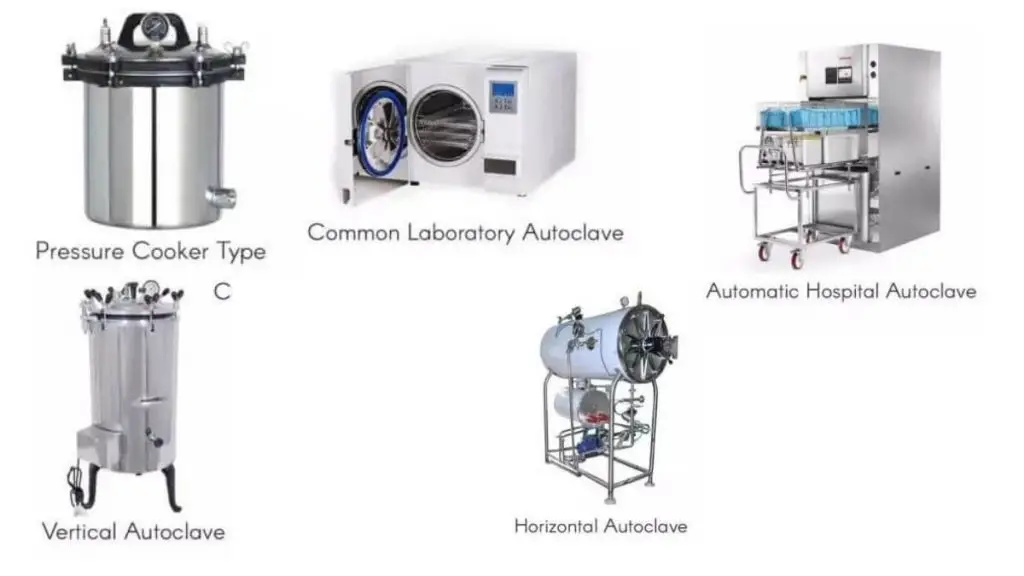
- Vertical autoclave is type having chamber oriented vertically, top lid opening (used in small labs)
- Horizontal autoclave is type with front-loading, horizontal chamber (used for larger loads)
- Pass-thru autoclave is used where one side opens to non-sterile zone and the other to sterile zone, to avoid cross contamination
- Waste / industrial autoclave is type used for sterilizing large volume waste / industrial items (batch or continuous)
Classes of autoclaves
Here is the different classes of autoclaves;
- Class N autoclave is defined as basic type autoclave, used for solid non-porous / “unwrapped” loads.
- Pros– Simplicity is afforded; cost is lower so small clinics / labs can use it.
- Cons– It cannot sterilize porous loads or hollow instruments; steam penetration is limited; cycle times may be longer.
- Class B autoclave is regarded as highest class with vacuum / pre-vacuum cycles allowing sterilization of porous, hollow, wrapped instruments.
- Pros -Very versatile — can sterilize porous, hollow, wrapped loads; efficient because vacuum ensures steam penetration; fastest effective sterilization.
- Cons– Higher cost (purchase & maintenance); more complex design and possibly more energy / parts required; users must maintain vacuum systems well or performance degrades.
- Class S autoclave is intermediate class placed between N and B, with certain selective capabilities (some hollow / some packaged loads), but limitations as defined by manufacturer.
- Pros– More flexible than N, some wrapped / limited hollow loads may be handled; better control in some models.
- Cons – It still lacks full vacuum capability so certain complex loads can’t be reliably sterilized; versatility is limited vs B.
What Is The Autoclave Cycle Time Frame?
The autoclave cycle time frame is commonly described as the total period from chamber pressurization to depressurization and drying.
It is most often divided into three stages – heating (come-up), holding (sterilization), and cooling/drying, and each stage are affected by load type, size, packaging, and autoclave design.
For routine lab glassware and wrapped items, 15–30 minutes at 121°C (250 °F) is frequently specified, but longer times are required for heavy loads or dense packs.
Liquids are treated differently and are usually given extended cycles because, when heated, they can boil-over if pressure is released too quickly, so slow exhaust is used.
The heating/come-up time is influenced by chamber volume and starting temperature, and may be 10–20 min for small units, longer for big ones; sometimes, pre-heating is used to speed it up.
The holding time at 121°C or at 134 °C (higher temp, shorter time) is the period during which microbial inactivation is intended to be achieved, and it is calculated based on the most heat-resistant target.
Pre-vacuum / vacuum-assisted cycles are usually faster because air is removed before steam is admitted, and for comparable loads they may finish in 30–40 min while gravity-displacement cycles can take 60–90 min or more.
Drying is often overlooked, yet it is included in total cycle time, and if drying is required the cycle will be extended by 10–30 minutes depending on load and packaging.
Biological indicators (such as Bacillus stearothermophilus) are recommended to be used to validate the time/temperature combination, because sterilization is not assumed, it must be demonstrated.
When liquids are sterilized, slower exhaust and longer cooling are mandated to prevent superheated boiling, this is why liquid cycles are longer, and users are warned to wait.
Cycle durations are often reported on the autoclave display and on record logs, but they are sometimes misinterpreted, operators must check what time is being shown (come-up vs. hold vs. total).
For critical instruments, shorter high-temp cycles (eg, 3–5 min at 134 °C) may be chosen, and for heat-sensitive items lower temp/longer time schedules are used instead.
Validation records, chemical indicators, and routine maintenance are to be kept, and failure to do so will render cycles unreliable, thus risk is increased.
Variability is expected between models, so manufacturers’ cycles are followed, they are not interchangeable without revalidation.
In practice, a typical total cycle time is reported as ~45–75 minutes for mixed loads (including drying), but this range is widened by load, chamber size, and cycle type.
Factors Affecting Sterilisation Effectiveness
Here are the list of different factors;
Temperature – Is the primary factor and it is usually specified (121°C, 134 °C) but variation is seen with steam quality and load, so effectiveness will be altered if the set-point is not reached or held long enough.
Pressure – Proper pressure (about 15 psi /103 kPa) is required to be maintained for saturated steam to contact surfaces, and if it fluctuates sterilization may be incomplete, the gauge sometimes are misread.
Exposure time – Longer holding periods are required for dense/large loads, short times are used for small/clean loads, and insufficient time will leave survivors (run-on, yes).
Steam Quality – Saturated, dry steam is expected, but when wet steam or non-condensable gases are present, heat transfer is reduced and pockets of air are formed which prevent sterilisation.
Air removal – Incomplete air removal was found to be critical because air acts as an insulator; pre-vacuum cycles are used to remove air, while gravity-displacement relies on steam displacing air and they sometimes fail.
Load size & arrangement – Overpacked trays or touching instruments are prevented from being contacted by steam properly, spacing is needed, and when items are nested sterilization will be patchy.
Material type – Porous items (textiles, gauze) are required to be given longer cycles than solids, and heat transfer differences are to be considered, metals behave differently.
Packaging material – Wrapping that allows steam penetration (eg, medical wraps) is preferred, while some plastics or films will block steam and/or melt, causing failure.
Moisture content – Excess water is sometimes present and will extend drying time and may cause recontamination, too little moisture in some cases reduces heat conduction, odd but true.
Steam penetration – All surfaces are to be contacted by steam for kill to occur, but long lumens, sealed joints or closed containers will block penetration and they remain contaminated.
Autoclave maintenance – Leaking seals, blocked drains, uncalibrated sensors are ignored sometimes, small faults are allowed to persist and cycle conditions will be altered, so effectiveness is reduced.
Operator practices – Wrong cycle selection, premature door opening, improper loading, or misuse are often blamed when sterilization is failed; training is required, but it is not always done.
Validation indicators – Biological indicators (such as Bacillus stearothermophilus) are recommended to be used for routine validation, chemical indicators are used for process checks, and failed indicators show that the process was ineffective.
Cooling / drying – Rapid depressurization or insufficient drying is implicated in condensation and contamination, drying phases are often extended to preserve sterility after cycle end.
Water / steam purity – Impurities, scale, and non-condensable gases reduce steam effectiveness and can cause spot corrosion on instruments, maintenance of water quality is recommended.
Mode of Action of Autoclave – How does the autoclave destroy bacteria?
The mode of action of an autoclave is primarily moist heat-mediated protein denaturation and hydrolytic damage, and it is delivered by saturated steam under pressure.
Cellular enzymes and structural proteins are irreversibly denatured / coagulated by heat and moisture, causing metabolic failure and loss of function.
Membrane disruption is caused by lipid melting and structural collapse, permeability is increased and leakage of cytoplasmic contents is produced.
Nucleic acids (DNA, RNA) are damaged by heat-induced strand breaks and depurination, reducing repair and replication capacity, so recovery is prevented.
Spores are inactivated when core rehydration is allowed and core proteins are denatured, thus the heat resistance is overcome (spore coat changes, core water uptake).
Pressure (eg, 15 psi) is applied so higher temperatures (121 °C and sometimes 134°C) are achieved and sustained, which shortens the time needed for kill.
Heat transfer is enhanced because steam condensation on cooler surfaces releases latent heat, therefore energy is efficiently delivered to microbial targets.
Steam penetration into porous loads and lumens is required, otherwise trapped air pockets insulate and sterilisation will be incomplete.
Moist heat is more effective than dry heat since hydrolytic reactions are accelerated and proteins are more readily disrupted, so lower temps / shorter times are needed.
Steam penetrates the load and transfers heat quickly, and operators see rapid come-up times on many modern pre-vacuum cycles.
Thermal inactivation kinetics are described by D-values (decimal reduction time) and z-values (temp change for tenfold D-value shift), which are used to predict log reductions, but they are often misunderstood.
Validation is commonly done with biological indicators such as Bacillus stearothermophilus spores, these indicators shows whether the combined heat/pressure/moisture action was adequate.
When rapid depressurization or premature venting is done, survival were sometimes observed (strange effect), because cooling and pressure change can prevent complete denaturation.
Overall cell death is produced by the combined effects: protein denaturation, membrane damage, nucleic acid breakage, and enzyme inactivation — all driven by steam/heat/pressure.
Precautions
Personal protective equipment – Gloves, goggles, and a lab coat should be worn, and eye/skin protection is insisted because splashes or steam burns are possible.
Loading practices – Trays are to be loaded with space between items so steam circulation is allowed, overpacking must be avoided, nested instruments will not be sterilized properly.
Correct cycle selection – The appropriate temperature/time cycle (eg 121°C or 134 °C) must be chosen for the load, wrong cycle selection are a common error and causes failure.
Packaging checks – Packaging materials must be inspected so steam penetration is permitted and weak wraps are not used, some plastics (eg, certain films) will melt or block steam.
Water level / quality – Distilled or demineralized water is recommended for steam generation, scale and impurities are to be prevented as they reduce efficiency and damage the unit.
Door operation – Doors are to be opened slowly after depressurization, rapid or premature opening is dangerous and will cause scalding, and steam jets can burn.
Indicator use – Biological indicators (such as Bacillus stearothermophilus) and chemical indicators are to be used routinely to validate cycles, failed indicators must be acted upon.
Maintenance schedule – Routine checks (gaskets, valves, drains, sensors) are to be performed and records are to be kept, when maintenance is neglected the cycles are rendered unreliable.
Handling of liquids – Liquid loads are to be processed with slow exhaust / long cooling phases to avoid boiling-over, bottles must not be sealed tightly and should be vented( loosely ) to prevent explosion.
Instrument prep – Instruments are to be cleaned of gross debris before autoclaving since organic matter protects microbes and reduces sterilization effectiveness.
Training of personnel – Operators must be trained and competency verified, mistakes like wrong loading or wrong cycle selection are commonly done by untrained staff.
Record keeping – Cycle parameters and indicator results are to be logged, absence of logs makes validation impossible and audit will fail.
Emergency procedures – In case of malfunction or steam leak, the autoclave is to be isolated and service called, do not attempt makeshift repairs, safety comes first.
Cooling / drying – Adequate drying time is to be ensured before removing packages, wet packs are easily recontaminated and they should not be put into sterile storage when damp.
Sterilization control / Quality Control of Autoclave / Validation of Autoclav
There are a variety of methods available to make sure that autoclaves ensures the goal of sterility. The efficiency of sterilization process that is performed by the autoclave can be monitored through:
Biological Indicators – are used to directly validate sterilization, typically Bacillus stearothermophilus (BI) spores are placed in a representative/ challenge pack and then incubated after the cycle, if growth is observed the cycle is failed.
The biological indicator (BI) method is mainly used to ensure that the sterilization process in autoclave is effectively killing all microorganisms especially the resistant spores.
In this method, spore strips or ampoules containing highly resistant bacterial spores such as Geobacillus stearothermophilus are used.
These spores are chosen because they can survive at high temperature (121°C / 132°C), so if they are destroyed, all other microbes will be too.
Before the cycle, BI is placed at several points in the load (especially in hardest-to-sterilize areas), sometimes also near drain or center of chamber.
The autoclave is then operated through its regular sterilization cycle—usually at 121°C for 15–20 minutes or sometimes longer depending on load size.
After completion of cycle, the biological indicators are carefully removed (sometimes allowed to cool before handling) to avoid burns or contamination.
Each indicator is then aseptically transferred to growth medium or incubator (usually 55–60°C for 24–48 hrs) depending on BI type.
A control (unexposed BI) is also incubated to confirm viability of spores used in the test.
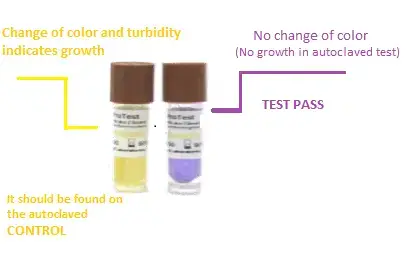
After incubation, the tubes/vials are examined visually. Presence of turbidity / color change / growth indicates survival of spores which means sterilization failed.
If no growth is seen (medium remains clear or color unchanged), it shows all spores were destroyed and sterilization was effective.
Sometimes BI contains color-changing media (e.g. purple to yellow), making detection simpler.
Interpretation must be compared with physical parameters (time, temperature, pressure) and chemical indicators for confirmation.
The results are documented in validation report with details like cycle parameters, BI locations, incubation data, and final results.
Failure of BI means the autoclave process must be reviewed, recalibrated or revalidated before further use.
Regular validation using BI is required as per GMP/ISO guidelines to assure consistent sterilization performance of autoclave units.
Chemical Indicators – are placed inside and outside packs to show exposure to steam/time/temperature, Class 5 integrators (chemical integrators) are often used because they correlate better with BI but they are not a substitute.
The chemical indicator (CI) method is mainly applied to check that the required conditions of sterilization like temperature, time and steam exposure were achieved inside the autoclave chamber.
In this process, chemical indicator tapes/strips/paper cards are used which contain heat or steam sensitive chemicals that undergo color or physical change when exposed to sterilization parameters.
These indicators are classified in various Classes (1–6) as per ISO 11140–1 standard, depending upon how many parameters they monitor.
The indicators are usually placed both outside (for exposure indication) and inside (for internal condition verification) of each pack or load before the sterilization run.
The autoclave is then operated through normal sterilization cycle like 121°C / 134°C for 15–30 mins (as per load requirement).
During the process, the chemical ink or reagent inside the indicator reacts with heat and moisture causing a visible change of color or pattern.
After completion of cycle, the indicator tapes/strips are examined carefully for uniform color change which shows that proper sterilization parameters were achieved.
If the expected color does not appear, it means incomplete exposure or failure in steam penetration.
Some indicators may show a transition like light pink to dark brown or blue to black, depending upon the manufacturer specification.
The results from CI are compared with the autoclave’s physical data (like temperature chart and pressure record) for confirmation of accuracy.
Although chemical indicators do not confirm microbial kill, they are used for routine monitoring because of their quick and easy interpretation.
The performance of CI should be verified periodically, and defective or expired ones must not be used since chemical sensitivity decreases with time.
Validation record includes – indicator type/class, batch no, position in load, cycle data, and observation of color change.
In case of inconsistent result, the cycle should be repeated and autoclave parameters rechecked before releasing the sterilized material.
Hence, chemical indicators act as visual evidence that the sterilization cycle conditions have been met inside the autoclave system.
Bowie-Dick test – is run daily on pre-vacuum autoclaves to detect incomplete air removal, operators run it at start-up and failures prompt corrective action, they are simple but sensitive to loading/conditions.
The Bowie–Dick test is mainly performed to check the efficiency of air removal and steam penetration in pre-vacuum type autoclaves.
A Bowie–Dick test pack is used which contains chemical indicator sheet placed between layers of porous material (like cotton or paper).
The test pack may be commercially prepared or sometimes made in-house with similar standard conditions.
Before starting, the autoclave chamber should be empty and at room temperature to ensure accurate test result.
The test pack is placed horizontally on the bottom shelf of the chamber directly above the drain area (since it’s the most challenging spot for air removal).
The sterilizer door is closed and a standard Bowie–Dick cycle is selected which generally runs at 134°C for 3.5–4.0 minutes with full vacuum phase.
During the cycle, air is evacuated and replaced by saturated steam; the chemical indicator sheet inside the pack responds to the penetration of steam and heat.
After cycle completion, the test pack is removed carefully and allowed to cool slightly before opening to observe the result.
The indicator sheet is then checked visually – a uniform color change (usually blue/black or pink to brown) over the entire sheet indicates that the steam has penetrated properly and air was completely removed.
A non-uniform or patchy color change means incomplete air removal or vacuum leak inside the chamber.
Such as, lighter areas or streaks of original color often suggest trapped air pockets or cold spots during the process.
In case of failed test, the autoclave must not be used for sterilization until the cause (like vacuum pump malfunction, gasket leakage) is identified and corrected.
The test result should be recorded with details like cycle temp, time, pressure, test date, operator name and result interpretation.
Bowie–Dick test is usually performed daily before first sterilization load to verify that air removal system of autoclave is functioning correctly.
Hence, by this test proper functioning of the pre-vacuum phase is confirmed, ensuring complete steam penetration for effective sterilization performance.
Physical Monitoring – time, temperature and pressure are recorded by gauges, thermocouples and data-loggers and these readouts are reviewed against set-points for each cycle.
Process Challenge Device (PCD) – are used to simulate worst-case loads (lumens, wrapped trays) and chemical/biological indicators are placed inside to assess steam penetration, performance is judged by indicator outcome.
Vacuum / leak test – is performed to verify chamber integrity and the efficiency of air removal, when leaks are detected cycles are invalidated and repairs are required.
Calibration & Maintenance – Sensors, valves, gaskets and gauges are to be calibrated and serviced on schedule, neglect of this leads to unreliable validation and false confidence.
Temperature mapping – is conducted during qualification using multiple probes to identify cold spots in the chamber, mapping is repeated after major service or relocation of the unit.
Sterility testing (culture) – samples from processed loads are incubated in growth media to check for survivors, it is slower than indicators yet provides direct evidence when needed (used for critical/ high-risk items).
Indicator tape / External Indicators – are relied upon for routine visual confirmation of exposure, they change appearance but they do not prove sterility, they are to be used with internal indicators.
Record keeping – Cycle parameters, BI/CI results, maintenance and calibration logs are to be retained and reviewed routinely, missing or inconsistent records are red flags at audit.
Operator competency checks – Staff are to be trained and competency-assessed for loading, cycle selection, interpretation of indicators and emergency responses, training gaps are to be corrected promptly.
Acceptance criteria & SOPs – Clear acceptance limits (eg, BI negative, integrator change, temp within ±2 °C) are to be defined in SOPs and the documented procedures are to be followed without exception.
Corrective action / Revalidation – Failed indicators or test anomalies require loads to be quarantined, investigation performed, repairs done and revalidation (including BI and mapping as appropriate) is to be completed before return to service.
Uses of Autoclave
Autoclaves are flexible and used in many ways. Common autoclave uses:
- The autoclave is mainly used for sterilization of materials by using steam under pressure to destroy all types of microorganisms including spores.
- It is widely applied in microbiology laboratories for sterilizing culture media, glassware (like pipettes, flasks, petri dishes), and other lab instruments before or after experiments.
- In medical and hospital areas, it’s used for sterilizing surgical instruments, dressings, syringes, and other patient care materials that must be free from microbes.
- It’s also used for decontamination of biohazardous waste, such as contaminated agar plates, used pipette tips, and other infectious laboratory disposables before disposal.
- Within pharmaceutical industries, autoclaves are used to sterilize raw materials, final products, and components like rubber stoppers or glass vials which are sensitive to microbial contamination.
- In research and educational institutions, autoclaves help in sterilizing culture media and reagents used for biological and chemical studies.
- Some industries use them for processing food / beverage containers and packaging materials to ensure extended shelf life and safety.
- In tissue culture work, sterilization of nutrient media, instruments, and explants is done by autoclave to maintain aseptic condition.
- It’s also applied in veterinary laboratories for sterilizing surgical tools and contaminated animal waste material.
- The autoclave is even used in tattoo parlors and dental clinics, where tools come in direct contact with body fluids and must be sterilized between clients.
- For waste management, infectious materials are sterilized before being discarded to prevent spread of pathogens in environment.
- Sometimes used for sterilization testing, where validation of process efficiency is performed through biological/chemical indicators.
- Autoclaving ensures safety and hygiene by completely killing bacteria, viruses, fungi, and bacterial spores through moist heat at 121°C – 134°C under pressure.
Advantages of Autoclave
- The autoclave is widely used and is considered to be highly effective for sterilization, because moist heat under pressure is employed to kill microbes including spores.
- Complete sterilization is usually achieved, and therefore confidence in sterilized items is provided by the method.
- In many labs, the process is preferred since temperature and pressure are precisely controlled, and consistency is maintained.
- Shorter cycle times are offered by autoclaves when compared to dry heat, and so throughput is improved, this is practical for routine work.
- The procedure is non-toxic, no harmful chemicals are required, and hence environmental exposure is minimized.
- Operating cost is kept relatively low because only water and electricity are used for steam generation, making it economical.
- Various materials such as glassware, metal instruments, rubber and textiles are sterilized without major damage, though delicate items are handled cautiously.
- Uniform heat penetration is achieved by steam, so every surface is contacted; pockets of air are reduced, and sterilization is more reliable.
- No chemical residue is left behind after the cycle, which makes it safer for medical/pharmaceutical use.
- The process is validated/monitored by biological/chemical indicators, and records are maintained for quality control.
- Both solids and liquids are able to be sterilized, which increases the autoclave’s versatility for lab, clinical and industrial tasks.
- As a closed system the user exposure to contaminated material is reduced, and safety is thereby improved.
- Autoclaves are energy efficient compared with some alternatives, and thus they are favored in many settings.
- They is regarded as dependable and simple to operate, with automatic controls that assist even less experienced staff.
- Because broad applicability is shown across medical, research, pharmaceutical and food sectors, the autoclave remains a standard method for achieving reliable, economical, and effective sterilization.
Limitations of Autoclave
- The autoclave cannot be used for heat-sensitive or moisture-sensitive materials since high temperature and steam may damage them.
- Some plastic materials are deformed or melted during the sterilization cycle because of high heat (121°C–134°C).
- Powders, oils, and fats cannot be sterilized effectively, as steam cannot penetrate them completely.
- The process is unsuitable for volatile or flammable substances, because such materials may react or explode when exposed to heat and pressure.
- In some cases, metal instruments may become corroded after repeated autoclaving due to moisture and oxidation effects.
- Improper loading of chamber can result in incomplete sterilization since steam may not reach all parts of the load uniformly.
- The drying phase for porous loads (like cloth or dressing materials) may take longer time and sometimes incomplete drying causes recontamination.
- Autoclaving can’t be used for electronic devices or delicate equipment, as steam exposure can permanently damage internal parts.
- The method requires energy and water, hence not ideal for places with limited utility availability.
- Sometimes, the maintenance of pressure and temperature gauges is critical; if calibration is off, sterilization fails without immediate indication.
- It’s also limited by its capacity, only a certain amount of material can be sterilized at a time depending on chamber size.
- Handling of hot instruments or load after cycle completion may cause burns or injuries if safety precautions are not followed.
- For certain materials, chemical residue or rust formation may occur after frequent exposure to moist heat.
- The autoclave requires regular validation and monitoring to ensure its consistent performance, otherwise false sterilization assurance may happen.
- So, though highly effective for many items, the autoclave is restricted only to those materials that can withstand both moisture and high temperature-pressure conditions.
Examples of Autoclave
Tuttnauer autoclave
Tuttnauer is a manufacturer of autoclaves and other sterilization equipment. Tuttnauer autoclaves are used in a variety of settings, including hospitals, dental offices, laboratories, and research facilities, to sterilize a wide range of materials.
Tuttnauer autoclaves use steam under pressure to sterilize materials, and the temperature of the steam can reach 121-134°C (250-273°F). This high temperature is necessary to kill all types of microorganisms, including spores of thermophilic bacteria, which are resistant to lower temperatures.
Tuttnauer autoclaves are available in a range of sizes and models to suit the needs of different users. Some models are designed for use in small laboratories or dental offices, while others are larger and suitable for use in hospitals or research facilities.
Tuttnauer autoclaves are known for their reliability and durability, and the company offers a range of maintenance and repair services to ensure that their autoclaves are operating at optimal performance.
Midmark autoclave
Midmark is a manufacturer of medical and dental equipment, including autoclaves. Midmark autoclaves are used to sterilize a wide range of materials, including medical instruments, laboratory glassware, and textiles.
Midmark autoclaves use steam under pressure to sterilize materials, and the temperature of the steam can reach 121-134°C (250-273°F). This high temperature is necessary to kill all types of microorganisms, including spores of thermophilic bacteria, which are resistant to lower temperatures.
Midmark autoclaves are available in a range of sizes and models to suit the needs of different users. Some models are designed for use in small laboratories or dental offices, while others are larger and suitable for use in hospitals or research facilities.
Midmark autoclaves are known for their reliability and durability, and the company offers a range of maintenance and repair services to ensure that their autoclaves are operating at optimal performance.
Quiz Practice on Autoclave
What is the primary purpose of an autoclave in healthcare and laboratory settings?
a. Sterilizing surgical instruments
b. Refrigerating samples
c. Heating food
d. Cleaning glassware
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: a [/expand]
Which component of an autoclave is responsible for indicating the pressure inside the chamber during sterilization?
a. Pressure Gauge
b. Water Level Indicator
c. Whistle
d. Control Panel
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: a [/expand]
What is the significance of a safety valve in an autoclave?
a. To control the temperature
b. To prevent contamination
c. To release excess pressure
d. To regulate the timer
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c [/expand]
In an autoclave, which component is responsible for maintaining the appropriate water level?
a. Pressure Gauge
b. Water Level Indicator
c. Whistle
d. Safety Valve
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b [/expand]
What is the primary function of a vacuum generator in certain autoclaves?
a. To generate steam
b. To extract air and create a vacuum
c. To cool the chamber
d. To regulate pressure
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: b [/expand]
Which component of an autoclave helps to prevent damage to drainage pipes by cooling the effluent?
a. Pressure Gauge
b. Vacuum Generator
c. Wastewater Cooler
d. Electrical Heater
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c [/expand]
What is the primary role of the control panel in an autoclave?
a. To regulate pressure
b. To indicate the water level
c. To control electricity supply
d. To release excess pressure
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: a [/expand]
Which component is responsible for creating a hermetic seal on the autoclave to ensure proper sterilization?
a. Water Level Indicator
b. Safety Valve
c. Lid/Door
d. Pressure Gauge
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c [/expand]
What is the primary function of the electrical heater in an autoclave?
a. To indicate temperature
b. To create a vacuum
c. To generate steam by heating water
d. To regulate pressure
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: c [/expand]
In some types of autoclaves, what does the whistle do after the sterilization process is complete?
a. Regulates pressure
b. Releases excess steam
c. Sounds an alarm
d. Signals that sterilization is finished
[expand title=”Show answer” swaptitle=”Hide answer”] Answer: d [/expand]
FAQ on Autoclave
1. What are autoclave bags made of?
Autoclave bags made of two-millimeter-thick Polypropylene (PP).
2. Why are autoclave indicators used?
Autoclave indicators are used to make sure that articles have been sterilized. Autoclave tape, sensitivity marks on bags or wraps, and indicator capsules.
3. What are autoclave bags used for?
Autoclave bags are used in high heat sterilization applications in order to prevent low temp plastics inside the bag from sticking to the walls of the sterilizer
4. Can autoclave kill endospores?
Yes, By increasing the pressure, the autoclave reaches a boiling point of 100°C or higher (121°C) and kills endospores.
5. Can autoclave kill prions?
Yes, by exposing them to effective sterilisation temperatures for around 14 minutes longer than the standard 134°C cycle.
6. How autoclave kill microorganisms?
Autoclaves kill microorganisms by degrading nucleic acids and denaturing enzymes and other essential proteins.
how does an autoclave work
To sterilise items, autoclaves employ tremendous heat in the form of pressurised steam. An autoclave, like a pressure cooker, uses a locked door to produce a sealed chamber. The air within the chamber is then replenished with pressured steam until the goods within the chamber are adequately disinfected.
How does autoclaving kill bacteria?
Using steam heat, autoclaves elevate temperatures to the point where proteins within the cell walls of a microbe begin to denature and coagulate, resulting in the bacterium’s death and sterilisation.
Why is autoclaving items better for sterilization purposes than boiling them?
Autoclaves are more effective at sterilization than boiling because they use higher temperatures and pressures. Autoclaves use steam under pressure to sterilize materials, and the temperature of the steam can reach 121-134°C (250-273°F). This high temperature is necessary to kill all types of microorganisms, including spores of thermophilic bacteria, which are resistant to lower temperatures.
In contrast, boiling water only reaches a maximum temperature of 100°C (212°F), which is not sufficient to kill all types of microorganisms. Boiling is effective at killing most bacteria and viruses, but it is not effective at killing spores and certain types of fungi.
Additionally, autoclaves use a combination of heat and pressure to sterilize materials, which can be more effective at killing microorganisms than heat alone. The pressure inside an autoclave can reach 15 pounds per square inch (psi), which helps to kill microorganisms that may be resistant to high temperatures.
Overall, autoclaves are a more effective method of sterilization than boiling because they use higher temperatures and pressures to kill a wider range of microorganisms.
How long does it take an autoclave to sterilize goods?
The time it takes for an autoclave to sterilize goods depends on several factors, including the size and type of material being sterilized, the type of autoclave being used, and the sterilization cycle being used.
In general, autoclaves use one of two types of sterilization cycles: a gravity cycle or a pre-vacuum cycle. The gravity cycle is typically faster than the pre-vacuum cycle, as it does not require the removal of air from the autoclave chamber before sterilization. However, the pre-vacuum cycle is generally more effective at sterilization because it removes air from the chamber, which allows steam to penetrate materials more effectively.
The size and type of material being sterilized also affect the sterilization time. Larger items or items with complex shapes may take longer to sterilize because they have more surface area that needs to be exposed to steam. Similarly, materials with a high moisture content, such as liquids or wet fabrics, may take longer to sterilize than dry materials.
In general, sterilization times for an autoclave range from 30 minutes to several hours, depending on the factors mentioned above. It is important to carefully follow the manufacturer’s instructions and guidelines for the specific autoclave being used to ensure that the materials are adequately sterilized.
What temperature(s) can an autoclave reach?
Autoclaves use steam under pressure to sterilize materials, and the temperature of the steam can reach 121-134°C (250-273°F). This high temperature is necessary to kill all types of microorganisms, including spores of thermophilic bacteria, which are resistant to lower temperatures.
The temperature inside an autoclave is controlled by the pressure of the steam, with higher pressures resulting in higher temperatures. The pressure inside an autoclave can reach 15 pounds per square inch (psi), which helps to kill microorganisms that may be resistant to high temperatures.
It is important to note that the temperature inside an autoclave may not be uniform throughout the chamber. The temperature may be higher near the steam source and lower in other areas of the chamber. As a result, it is important to carefully follow the manufacturer’s instructions and guidelines for the specific autoclave being used to ensure that all materials are adequately sterilized.
How long do items stay sterile after autoclaving?
Autoclaved items will remain sterile as long as they are kept in a sterile environment and are not contaminated by microorganisms.
After autoclaving, it is important to handle the items carefully to avoid contamination. This may involve wearing sterile gloves and using sterile techniques to transfer the items to a sterile container or storage area.
The length of time that autoclaved items will remain sterile will also depend on the type of material being sterilized and the storage conditions. Some materials, such as metal instruments, may remain sterile for an extended period of time if they are stored in a dry, sterile environment. Other materials, such as biological cultures or tissue samples, may be more susceptible to contamination and may need to be used or stored under more stringent conditions.
Overall, it is important to carefully consider the type of material being sterilized and the storage conditions to ensure that autoclaved items remain sterile for as long as needed.
- (ANSI) American National Standards Institute Inc./(AAMI) Association for the Advancement of Medical Instrumentation
- https://www.britannica.com/technology/autoclave
- https://university.steris.com/course/understanding-steam-sterilization/
- https://www.cdc.gov/hicpac/Disinfection_Sterilization/13_0Sterilization.html
- https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/steam.html
- https://blink.ucsd.edu/safety/research-lab/biosafety/autoclave/index.html
- http://www.theratronics.ca/PDFs/Autoclave_Temperature_and_Time_Pressure_Chart.pdf
- https://tuttnauer.com/blog/autoclave
- https://en.wikipedia.org/wiki/Autoclave
- https://consteril.com/how-does-a-laboratory-autoclave-work/
- https://www.labkafe.com/blog/autoclave-definition-uses-working-principle-and-types-labkafe/
- https://www.fcbios.com.my/blogs/news-insight/autoclave-machine-principle-how-to-use-and-maintenance
- https://www.mlsu.ac.in/econtents/2214_Unit%201_Autoclave.pdf
- https://www.mesaustralia.com.au/blogs/news/autoclaves-principles-uses-types-procedures
Helpful Note, Thank You