| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Eurotiomycetes |
| Order: | Eurotiales |
| Family: | Trichocomaceae |
| Genus: | Aspergillus |
| Species: | A. fumigatus |
Aspergillus fumigatus
Aspergillus fumigatus can tolerates high temperatures and can thrives on decaying organic matter, Aspergillus fumigatus is widely distributed in soil, compost, indoor dust, and other environmental niches, where it is routinely detected by air sampling.
Aspergillus fumigatus is classified within the genus Aspergillus (phylum Ascomycota), and it is recognized as a small-spored, filamentous fungus.
When inhaled, the tiny conidia ( ~2–3 µm ) are deposited in the lower respiratory tract, and they are commonly phagocytosed by alveolar macrophages unless host defenses are weakened.
Although exposure is widespread, disease is typically restricted to people with weakened immunity or with structural lung disease, and invasive infection is often accompanied by angioinvasion and tissue necrosis.
Because of its thermotolerance, growth at elevated temperatures (up to about 50 °C ) is permitted, which is thought to contribute to environmental persistence and to its pathogenic potential.
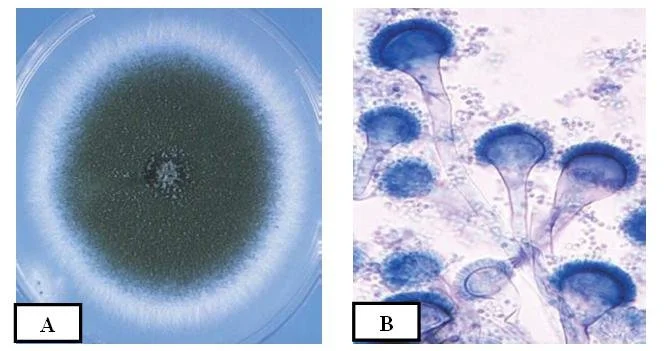
On culture media, rapid colony formation with blue-green, powdery conidial layers is usually produced, and microscopic features (septate hyphae, conidiophores with vesicles) are used for identification.
Large numbers of conidia are released.
If conidia are not efficiently cleared, germination is enabled and hyphal growth is set in motion, which can be followed by tissue invasion.
Invasive aspergillosis is most often attributed to Aspergillus fumigatus.
When hyphae invade blood vessels, thrombosis and infarction can be produced, and systemic dissemination may then occur.
Several virulence factors, including gliotoxin and other secondary metabolites, have been described and are thought to modulate immune responses, thereby aiding immune evasion.
Antifungal therapy is commonly administered with voriconazole, and alternatives such as amphotericin B are also employed in certain clinical scenarios.
Resistance mechanisms (notably point mutations in cyp51A, and efflux transporter up-regulation) are being reported with increasing frequency, and environmental azole exposure has been implicated.
Diagnostic confirmation is often pursued using imaging, culture, histopathology, galactomannan antigen testing, and PCR, but limitations are present with each method, so clinical correlation is required.
Hospital outbreaks or clusters have been associated with contaminated air systems or construction dust, and, in such settings, HEPA filtration / environmental controls are recommended.
Because spores are ubiquitous and difficult to remove entirely from indoor air, infection risk is mitigated by HEPA filtration, environmental controls, and prophylactic strategies in high-risk wards.
Researchers sequence genomes to track resistance, and clinicians adjust therapy based on susceptibility testing; yet gaps in surveillance and knowledge are still present.
In laboratory research, Aspergillus fumigatus is widely used as a model organism for studying fungal pathogenesis, spore germination, and host–pathogen interactions, and, at times, their genomes are sequenced for comparison.
It poses a special threat to transplant recipients and to people receiving prolonged corticosteroids or other immunosuppressive therapies.
Clinicians must balance antifungal efficacy with toxicity, and they must promote stewardship and environmental monitoring because resistance emerges and spread is facilitated by environmental reservoirs.
General Characteristics of Aspergillus spp
- Aspergillus spp. are widely distributed in soils, decaying plant material, indoor dust, and other Environmental niches, and they are commonly recovered by air-sampling.
- Colonies are usually fast-growing on common culture media, and characteristic textures and colours are produced (often woolly, powdery; blue-green to yellow-brown).
- Microscopic structures are characterized by septate hyphae, conidiophores bearing vesicles, and chains of conidia which are typically borne in large numbers, making dissemination facile.
- Because spores are small (often 2–5 µm) and lightweight, airborne dispersal is facilitated, and inhalation by humans/animals is therefore frequent.
- Many species are saprophytic, and environmental nutrient recycling (eg, decomposition of plant debris ) is assisted by their enzymatic systems.
- Thermotolerance is often displayed, and growth at elevated temperatures (some strains up to ~50 °C) is permitted, which is thought to correlate with environmental resilience and pathogenic potential.
- Asexual reproduction is predominantly observed, with conidia being produced in vast numbers; sexual stages are less commonly seen, but have been reported for some taxa.
- Cell walls are composed largely of glucans, chitin, and mannoproteins, and these components are implicated in host immune recognition as well as in drug target interactions.
- Many extracellular enzymes (proteases, lipases, cellulases) are secreted and are used for nutrient acquisition, and also, they may contribute to host tissue damage during infection.
- Secondary metabolites (mycotoxins) are produced by certain species, and mycotoxins such as aflatoxins, ochratoxins, and others have been associated with food contamination and toxigenic effects.
- In clinical contexts, colonization may be observed without disease, but invasive disease is usually restricted to persons with impaired immunity or with structural lung damage, and angioinvasion is a hallmark of severe infection.
- Host defenses are normally effective, and inhaled conidia are usually cleared by mucociliary action and by alveolar macrophages, yet when defense is compromised, germination is enabled and hyphal invasion may follow.
- Antifungal resistance has been reported, and mechanisms such as target-site mutations (eg, in cyp51 genes), efflux pump up-regulation, and biofilm-related tolerance are implicated.
- Environmental use of azole fungicides has been linked to cross-resistance in clinical isolates, and surveillance of resistance is therefore considered increasingly important.
- They produce abundant conidia that are easily aerosolized, and this trait increases exposure risk in both community and healthcare settings.
- Morphological identification is often employed, but molecular methods (PCR, sequencing of ITS / β-tubulin / calmodulin genes) are increasingly used for precise species-level identification, especially when treatment decisions are influenced.
- In the laboratory, growth conditions (temperature, media, CO₂ ), colony morphology, and microscopic features are recorded, and reference strains are used for comparison.
- Some species are exploited industrially for enzyme production or biotechnology applications, yet biosafety concerns and toxigenicity limit where and how they are applied.
- Air-handling systems, building works, and water-damaged materials are commonly implicated in indoor amplification, and HEPA filtration / environmental controls are recommended in high-risk wards.
- Allergic manifestations (eg, allergic bronchopulmonary aspergillosis, allergic fungal rhinosinusitis) are triggered in sensitized individuals, and diagnostic tests (skin testing, specific IgE ) are used in conjunction with clinical criteria.
- Genomes have been sequenced for multiple species, and comparative genomics is being used to map virulence factors, metabolic pathways, and resistance determinants.
- Because they are ubiquitous, complete avoidance is impractical, but risk reduction is achieved by environmental controls, stewardship of antifungal use, and by protecting susceptible hosts (eg, neutropenic patients) with prophylactic measures.
Epidemiology
Globally, infections caused by Aspergillus spp. are recognized to cause a spectrum ranging from allergic disease to chronic and invasive forms, and many estimates are made to understand its burden.
The annual incidence of chronic pulmonary aspergillosis (CPA) is estimated at ~1,837,272 globally (with ~340,000 deaths) according to recent fungal burden studies.
In many regions, the occurrence of invasive aspergillosis (IA) is rising especially among immunocompromised patients; over 2,113,000 cases are estimated annually in certain high-risk settings.
In patients with asthma, allergic bronchopulmonary aspergillosis (ABPA) is estimated to affect about 2.5 % of adults with asthma, globally amounting to ~4.8 million people.
In individuals with cystic fibrosis (CF), ABPA prevalence is estimated between 1 % and 15 %, depending on setting and diagnostic criteria.
Among tuberculosis (TB) patients, CPA complicates a fraction; meta-analysis suggests that ~9 % of TB patients have CPA during treatment, rising to ~13 % after treatment.
The case-fatality rate of aspergillosis depends heavily on host factors, ranging between 20 % to 72 % in many reports.
Many Aspergillus infections are under-diagnosed, because diagnosis is difficult, and surveillance is inconsistent in many countries.
In ICUs, critically ill or ventilated patients are increasingly being recognized as having IA, especially if corticosteroids or broad-spectrum antibiotics have been used.
The distribution of Aspergillus species in human disease shows geographic variation: in Asia/Africa/ Middle East, A. flavus is often the second most common after A. fumigatus; in Europe, A. niger may be second.
Azole-resistant A. fumigatus (ARAf) isolates are being increasingly reported; in some hospital surveys, ~6.7 % of A. fumigatus isolates were azole-resistant.
In ICU patients, the rate of azole non-susceptible isolates may be higher (~9.7 %) especially in those with prior azole exposure.
The mortality of patients with azole-resistant infections is high; in one study the mortality rate among ARAf cases was ~50 %, compared to ~36.5 % in azole-susceptible cases.
Regional variation in incidence and species distribution is influenced by climate, patient population, agricultural fungicide use, and healthcare practices.
In COPD cohorts hospitalized, ~1.3 % of admissions in Spain developed IA, indicating that chronic lung disease contributes substantially to the burden.
Estimates indicate that in Europe, COPD admissions (~1,100,000/year) may contribute tens of thousands of IA cases annually
Among underlying risk groups, the highest risk is seen in neutropenia, hematologic malignancy, hematopoietic stem-cell transplantation, organ transplantation and prolonged immunosuppression.
It is observed that non-fungal risk conditions like liver cirrhosis, advanced COPD, or ICU stay also predispose to IA, though at lower rates.
The burden estimates are likely conservative, because many cases are missed, diagnostic tools are lacking in many regions, and many deaths are not attributed to fungal disease.
Surveillance gaps, absence of routine antifungal susceptibility testing, and variability in diagnostic capacity contribute to geographic heterogeneity in reported epidemiology.
Habitat of Aspergillus fumigatus
In many environments Aspergillus fumigatus is found as a saprotroph on decaying organic matter (leaf litter, compost heaps, wood chips), where nutrients are abundant and decomposition is ongoing.
The fungus is widely distributed in soil, especially in cultivated fields, garden soils, and rich humus layers, where moisture and organic substrates support growth.
In compost piles and self-heating organic substrates the thermotolerance of A. fumigatus is exploited, and high temperatures (e.g. 40–50 °C) are tolerated, allowing proliferation in such habitats.
In outdoor air A. fumigatus is ubiquitous: spores are released and dispersed by wind, thus the airborne habitat is a major reservoir.
Within built environments it is often detected on indoor surfaces, in dust, ventilation ducts, walls, and in homes’ air-conditioning / HVAC systems, especially where moisture is present.
In stored food, grains, hay, and agricultural products, growth is allowed when moisture and temperature permit; thus stored-product habitats are utilized.
Bird nests, droppings, and roosting surfaces are colonized, and feathers or debris in avian environments are used as substrate; thus avian-associated microhabitats are included.
Wet habitats (marshy lands, saline soils, mangrove swamps), sewage, sludge, and slime in industrial / waste-water treatment settings have been reported as occasional isolations, indicating broader ecological flexibility.
In aquatic / water-adjacent environments, the fungus is sometimes detected (in fresh water, coastal areas), though growth is more limited compared to terrestrial sites.
In general, oxygen-rich (aerobic) settings are preferred, and growth is favored on substrates exposed to open air or gas exchange rather than deep anaerobic zones.
Because spore dispersal is facilitated, many new habitats are colonized passively; conidia are carried by air, water, insects or dust, and thus remote sites may be seeded.
The species is cosmopolitan, and a broad geographic distribution is observed — temperate, tropical and subtropical zones are all inhabited, though local abundance may vary.
Habitat suitability is expected to shift under climate change, with northward / altitudinal expansions predicted for A. fumigatus as temperatures rise and habitat zones shift.
Morphology of Aspergillus fumigatus
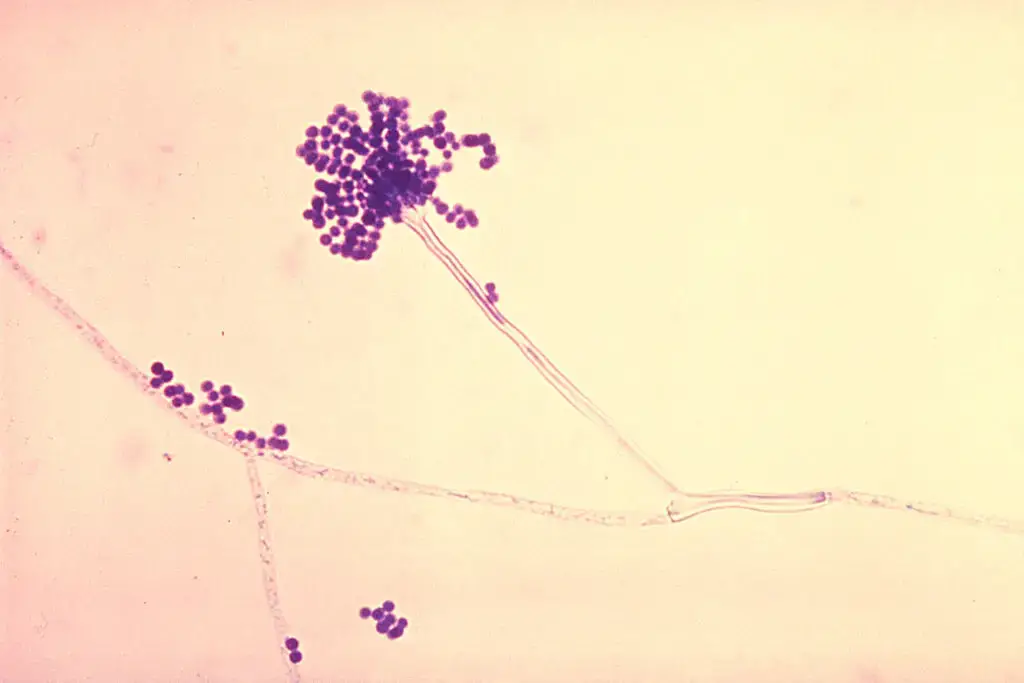
- In microscopic view, Aspergillus fumigatus is composed of septate, hyaline hyphae that branch dichotomously and invade tissues or substrates.
- The conidiophores are smooth-walled, erect, and they bear a terminal swelling (vesicle) at apex, from which spore-forming structures emerge.
- On the vesicle, phialides (flask-shaped cells) are attached, usually in a uniseriate arrangement (i.e. phialides directly on vesicle, no metulae) in many strains.
- The vesicle diameter is generally in the range of ~20–30 µm, and phialides measure ~6–8 × 2–3 µm (length × width) in many isolates.
- Chains of conidia (asexual spores) are borne basipetally (i.e., newest at base, older toward tip) from phialides, forming long columns of spores.
- Conidia are small (about 2.5–3 µm diameter), echinulate (i.e. finely roughened/spiky) in many isolates, though smooth surface conidia have also been observed for pigment-deficient strains.
- Some strains produce white (pigmentless) conidia, due to loss or absence of spore pigmentation pathways.
- In a colony, hyphae are embedded in an extracellular matrix (ECM) of secreted materials, which helps structural integrity and perhaps resistance to stress.
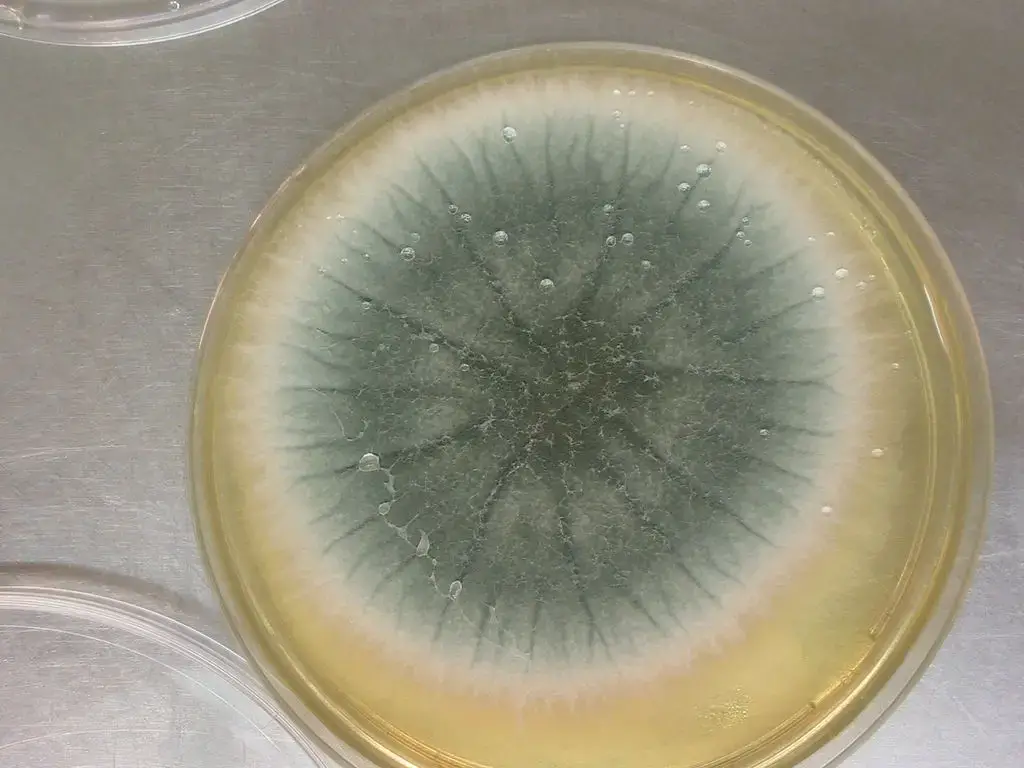
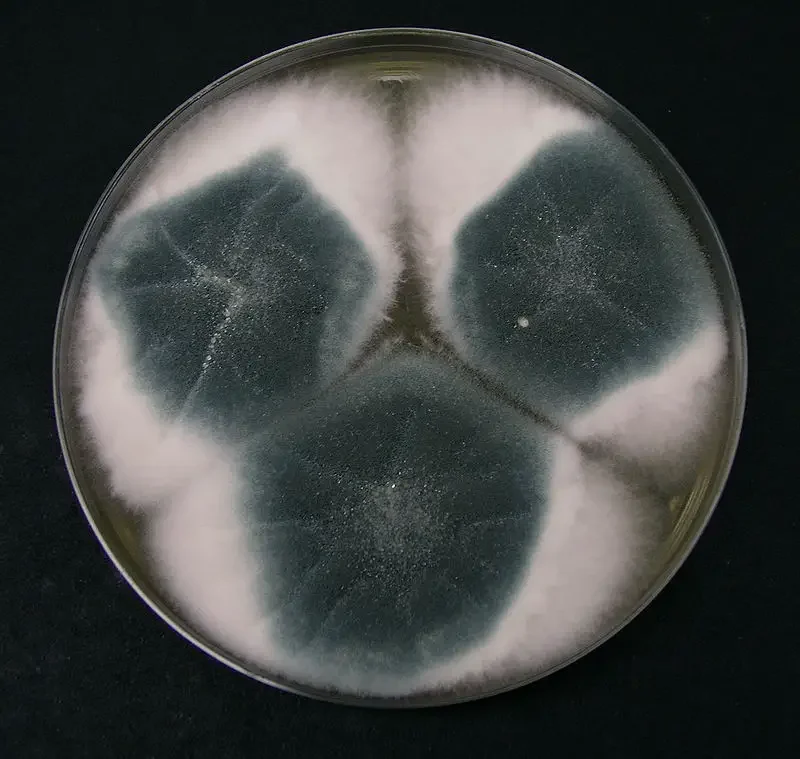
- When grown on agar media, colonies are often initially velvety / powdery, with surface colour turning grey-green or blue-green, and reverse (underside) coloration being pale or tan.
- The colony margin may appear radiating (with hyphal filaments extending outward), and sporulation is often denser in the central region.
- The morphological stages during germination proceed: resting conidia → swelling → germ tube emergence → hyphal elongation / branching → septation / mature hyphae.
- Multinucleate cells are present: each hyphal segment contains multiple nuclei (i.e. coenocytic in the sense of multiple nuclei, though septation is present).
- Cell wall is composed of layers rich in chitin, β-glucans, mannoproteins, and in spores, melanin or melanin-like pigments (DHN-melanin) may be present to protect against environmental stresses.
- Under extreme heat or stress, the conidia may exhibit resilience; viability under high temperature (e.g. up to 70 °C) has been documented, though growth is generally limited above ~50 °C.
- The morphology is somewhat conserved globally (i.e. low major variation in conidial / phialide dimensions) though minor strain-to-strain differences are noted in pigment, phialide length, spacing.
Cultural Characteristics of Aspergillus fumigatus
- When cultured on solid media (eg, PDA, MEA, Czapek Dox), colonies of Aspergillus fumigatus are usually velvety to powdery, often with grey-green / blue-green pigmentation on the surface.
- On the reverse side of agar plates, coloration is often pale, ash-grey or olivaceous grey, with little or no exudate / soluble pigment being produced.
- The rate of growth is typically moderate to rapid, and a 5-day incubation at 37 °C is commonly used for observation of characteristic morphology.
- To promote sporulation, cultures are initially left unsealed (open to air) for first few days, then sealed after sporulation begins, so that conidia formation is optimized.
- In long-term storage, slants or plates are usually kept at ~4 °C and subcultured within ~6 months to maintain viability, as colony vigor declines over prolonged storage.
- In many isolates, pigmentation intensities, surface texture (velvety vs powdery), and sporulation density vary somewhat, and strain-to-strain variation is observed.
- Under various temperatures (e.g. 25 °C, 30 °C, 37 °C), colony morphology may differ slightly (e.g. density, pigment intensity), and some growth is still possible at ~25 °C (though less vigorous).
- In standard media the conidial heads are compact and columnar, and biseriate arrangement is often observed (i.e. metulae + phialides) in many strains.
- The substrate/mycelial mat beneath the sporulating layer often appears densely interwoven, and aerial mycelium may extend toward plate margins.
- Pigmentless or pale mutants (non-pigmented strains) may yield lighter coloured colonies (white / pale grey) in culture, lacking the usual bluish or greenish hue.
- Sporulation is abundant, and conidia are produced in massive numbers, which often gives a dusty / powdery appearance to mature colonies.
- Colony edges are often distinct, sometimes with radiating hyphae extending beyond core sporulation zone; margin irregularities may be present.
- Some soluble metabolic byproducts may diffuse into agar (weakly), but strong pigments or diffusible zones are generally absent or minimal.
- Culture media composition (carbon source, nitrogen source, pH) influences growth rate, sporulation, colony texture and pigmentation, though the “typical” colony form is robust across many common media.
- Because A. fumigatus tolerates relatively high temperature, incubation at 37 °C is often used to differentiate it from some other Aspergillus species whose growth is less robust at body temperature.
Culture media used for growth of Aspergillus fumigatus
Malt Extract Agar (MEA) is one of the most frequently recommended media for Aspergillus spp including A. fumigatus, because a rich supply of maltose / sugars promotes robust growth and sporulation.
Dichloran-Glycerol Agar (DG18) is also used, especially in environmental sampling, as it restricts spreading of fast-growing fungi and allows better isolation of Aspergillus colonies.
Czapek-Dox medium (CZA / Czapek medium) is often employed for culturing A. fumigatus, since defined salts and carbon source help in studying physiological traits.
Potato Dextrose Agar (PDA) is used in many labs for fungal growth including A. fumigatus, because it is versatile and supports general fungal propagation.
Sabouraud Dextrose Agar (SDA / Sabouraud agar) is employed frequently (often with antibiotics) to suppress bacterial contamination and permit fungal (incl A. fumigatus) growth.
Aspergillus Complete Medium (ACM) or “Aspergillus complete medium / agar” is used in long-term cultivation / storage of A. fumigatus in research settings.
Minimal media like Aspergillus Minimal Medium (AMM) are used in laboratory studies for conidial harvesting, growth quantification, and physiological experiments.
Selective Flamingo Medium has been developed to isolate A. fumigatus from environmental samples, by inhibiting competing fungi (especially Mucorales) and bacteria under specific incubation (e.g. 48 °C).
In antifungal resistance testing, RPMI 1640 agar (with 2% glucose) supplemented with antifungal agents is used as a screening medium for A. fumigatus isolates.
Broth media (e.g. Potato Dextrose Broth, Nutrient Broth, Czapek-Dox Broth, Malt Extract Broth) are used for liquid cultures, biomass production, enzyme studies, and growth kinetics of A. fumigatus.
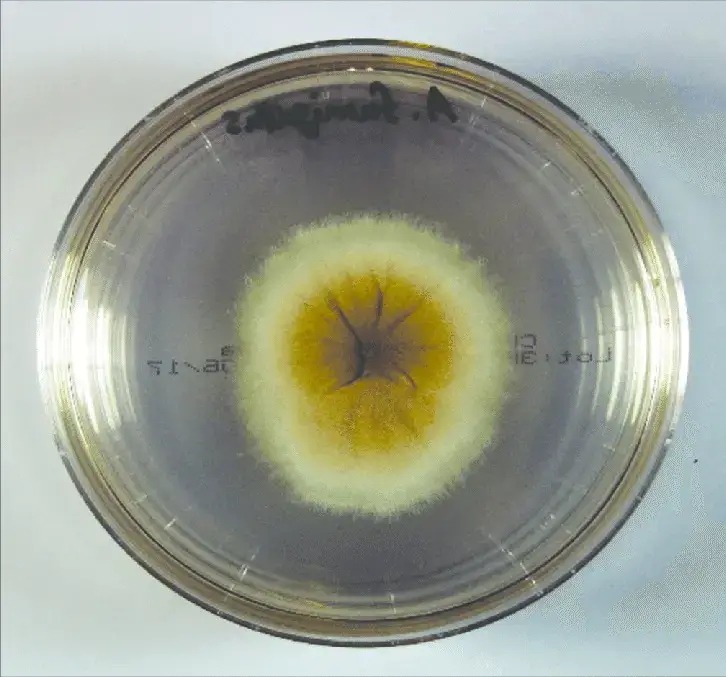
How to grow Aspergillus fumigatus in laboratory?
- A strain/isolate of Aspergillus fumigatus is first obtained (from a stock collection or clinical / environmental isolate) under biosafety conditions (BSL-2).
- The culture medium (e.g. Malt Extract Agar, MEA, or another suitable agar) is prepared, sterilized (autoclaved), cooled to ~45-50 °C, and poured into sterile Petri plates (∼25 mL for 9 cm plates) under aseptic conditions.
- From the stock, a small inoculum (mycelial plug or spore suspension) is aseptically transferred onto the agar plate, usually at the center.
- The inoculated plates are incubated inverted (agar side up) at ~37 °C (or sometimes 30 °C) for ~3–5 days (or as needed) under ambient air, avoiding sealing in the first days so that sporulation is promoted.
- After 3–5 days (or when growth is visible), sporulation is checked: conidia are produced abundantly on the colony surface, giving a powdery / greenish appearance.
- If needed, sealing of plates (e.g. with Parafilm) may be done after sporulation begins to prevent dehydration or contamination.
- A spore suspension is made by flooding the sporulated colony with sterile 0.05 % Tween 80 (or other surfactant solution), gently rubbing the surface to dislodge conidia, and collecting the suspension.
- The spore suspension is filtered (e.g. through sterile cotton / gauze) or allowed to settle, then adjusted (by dilution) to a desired spore concentration (e.g. 1 × 10⁶ to 1 × 10⁷ conidia/mL).
- Aliquots (e.g. 1 µL or 10 µL) of the spore suspension are inoculated (spot-inoculated) onto fresh agar plates (or streaked) for colony isolation / experiments.
- Plates are incubated under the same conditions (inverted, at chosen temperature) and monitored daily for growth, morphology, sporulation, contamination.
- For long-term storage of culture stocks, subcultures may be made from single spores or mycelial plugs every few months; stock cultures are stored at 4 °C (on slants or plates) or frozen in glycerol (10 %) at –80 °C.
- Throughout, sterile technique, containment, and handling precautions are maintained (e.g. use of biological safety cabinet, mask, minimizing aerosolization) because A. fumigatus is a pathogen risk.
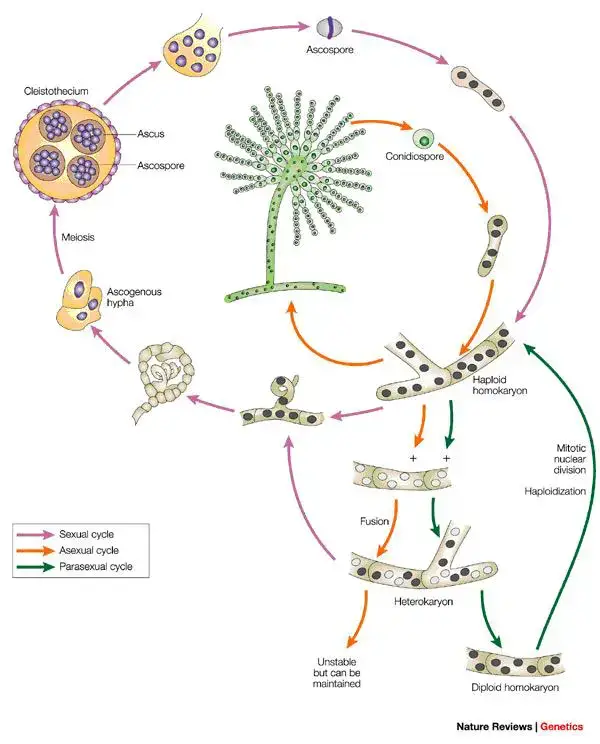
Life Cycle of Aspergillus fumigatus
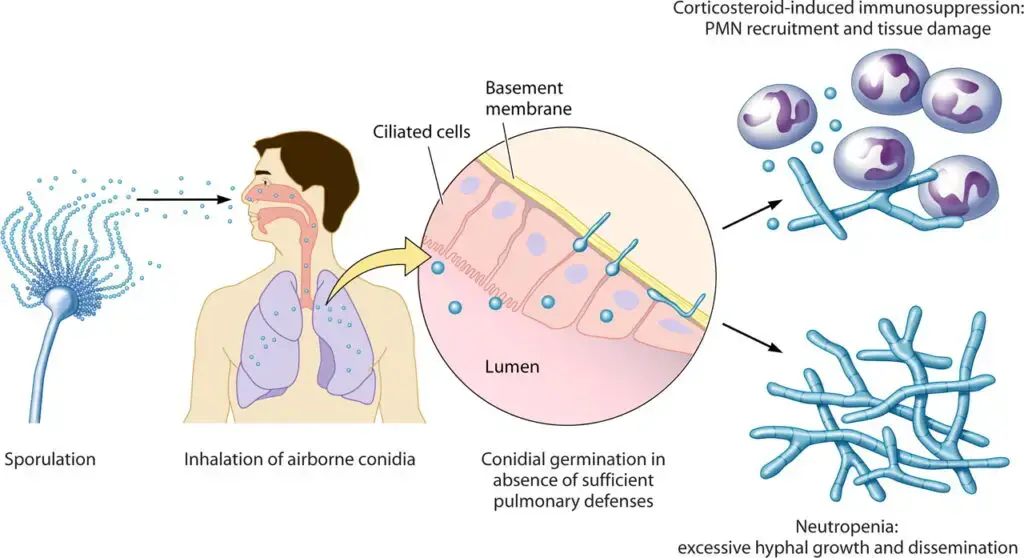
- In nature, Aspergillus fumigatus is primarily propagated by asexual reproduction, and conidia are borne on specialized conidiophores and are released into air for wide dispersal.
- Conidia are produced in vast numbers.
- The conidia, being small ( ≈2.5–3 µm ) and hydrophobic, are rendered resistant to desiccation and environmental stress, and dormancy is usually maintained until favorable conditions occur.
- Upon contact with suitable substrate, germination is initiated, swelling is observed, a germ-tube is formed, and subsequent hyphal elongation is then established.
- Hyphae are produced and branching is initiated, so that a mycelium (network of hyphal filaments) is formed on the substrate.
- From aerial mycelium, conidiophores are differentiated, terminal vesicles are produced, phialides are arranged on vesicles, and chains of conidia are subsequently generated.
- Spores germinate when host defenses are weak.
- Released conidia are dispersed by wind, dust, water-droplets/ insects and human activity, which permits colonization of distant substrates and of indoor environments.
- Sexual reproduction is rarely observed in nature, but under special/laboratory conditions complementary mating types may meet and cleistothecia (sexual fruiting bodies) can be formed, inside which ascospores are produced.
- Within cleistothecia, karyogamy and meiosis are completed, and resultant ascospores are released to germinate into new haploid mycelia.
- In humans, inhalation of conidia is the principal route of exposure.
- In the respiratory tract, conidia are normally phagocytosed by alveolar macrophages and mucociliary clearance is relied upon to prevent germination, unless immunity is compromised or corticosteroids are present.
- When host defenses are impaired, germination is enabled, hyphal growth is promoted, and tissue invasion / angioinvasion may be effected leading to thrombosis and infarction.
- Secondary metabolites (eg, gliotoxin) are produced and are implicated in modulation / suppression of host immune responses.
- Genetic variation is generated mainly by mutation during asexual propagation, and occasional sexual recombination may contribute to diversity and to emergence of novel traits (eg, antifungal resistance).
- The cycle is therefore dominated by asexual propagation in the environment, yet the capacity for sexual reproduction is retained and may be important for long-term evolution.
Pathogenesis of Aspergillus fumigatus
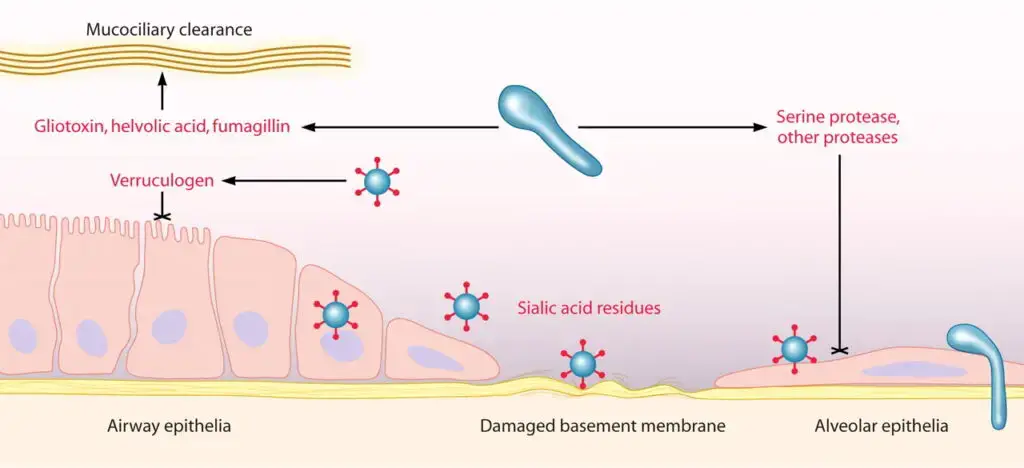
Infection is typically initiated when conidia are inhaled (deposited in airways / alveoli) and evade mucociliary clearance.
Conidia are phagocytosed by alveolar macrophages and killed within phagolysosomes in immunocompetent hosts, but occasionally survival is permitted, especially if host defense is impaired.
When conidia survive, germination is enabled, germ tubes and hyphae are formed, and tissue invasion is initiated.
Hyphal growth is promoted, and invasive penetration into pulmonary epithelium and endothelial layers is effected (angioinvasion).
During angioinvasion, damage to vascular endothelium may occur, thrombosis and infarction may be induced, and dissemination to other organs is possible in severe cases.
Several virulence factors are produced (or expressed) which contribute to pathogenesis; for example gliotoxin, proteases, hydrophobin RodA, and enzymes for nutrient acquisition.
The RodA protein coats conidia, masking immune recognition (PAMPs), and thus impeding innate immune activation.
Oxidative stress defenses (e.g. catalases, superoxide dismutases, antioxidants, production of mannitol) are employed by the fungus to resist reactive oxygen species produced by host phagocytes.
Nutrient acquisition systems (notably siderophore-mediated iron uptake, nitrogen assimilation, proteolytic degradation of host proteins) are upregulated to support fungal growth in host tissues.
Secondary metabolites such as gliotoxin exert immunosuppressive / cytotoxic effects on immune cells (macrophages, neutrophils), inhibit NF-κB signaling, impede leukocyte functions, and promote apoptotic pathways in host cells.
Signaling pathways in the fungus (e.g. regulation of stress response, hypoxia adaptation, cell wall synthesis regulation) are activated to adapt to host microenvironments (low oxygen, nutrient limitation).
Inflammation is induced in host tissue, and cytokine/chemokine responses are mounted; the host inflammatory damage may exacerbate tissue necrosis.
The balance between fungal virulence and host immunity determines whether disease (invasive, chronic, or allergic forms) will be manifested or controlled.
Clinical manifestation of Aspergillus fumigatus infection
In many cases, a hypersensitivity / allergic response is manifested (especially in asthmatics or cystic-fibrosis patients).
- Wheezing is often produced.
- Cough (sometimes with brownish mucus plugs or flecks) may be expectorated.
- Shortness of breath is commonly observed.
- Occasionally low grade fever, malaise or general weakness may be present.
- Over time, bronchiectasis (especially central bronchiectasis) can be produced in the lungs.
In the “fungus ball” / aspergilloma form, the infection may remain localized (often in preexisting lung cavities).
- Many patients are asymptomatic, or only mild cough is manifested.
- Hemoptysis (coughing up blood) is a frequent manifestation (which may range from mild to severe).
- Shortness of breath and fatigue may occur, as the fungus ball grows or irritates surrounding tissue.
In chronic pulmonary aspergillosis (CPA) forms (often in patients with underlying lung disease), a more indolent course is produced.
- Persistent cough (often non-resolving) is presented.
- Weight loss and anorexia are frequently manifested.
- Fatigue / malaise is often complained of.
- Hemoptysis (sometimes intermittent) may be present.
- Night sweats, low grade fever over weeks to months can occur.
Invasive aspergillosis (especially invasive pulmonary aspergillosis) is the most severe manifestation and is usually produced in immunocompromised hosts.
- Fever that is persistent (often not responsive to broad antibiotics) is produced.
- Cough (often nonproductive initially) and dyspnea (difficulty breathing) are manifested.
- Pleuritic chest pain (pain worsened by deep breathing) is often complained of.
- Hemoptysis (coughing blood) may be observed as the infection invades blood vessels (angioinvasion).
- Rapid progression of respiratory failure may develop in severe cases.
- Dissemination to other organs (brain, kidneys, heart) may occur in severely immunosuppressed patients.
When the sinuses / rhinocerebral forms are involved, particular local symptoms are manifested.
- Nasal congestion, nasal pain or sinus pain are produced.
- Bloody (hemorrhagic) nasal discharge or bloody mucus in nasal cavities is possible.
- Facial swelling (on one side) and headache may be manifested.
- Orbital symptoms (drooping eyelids, proptosis) or involvement of adjacent structures may occur.
Other localized / less common sites may be involved, producing more focal manifestations.
- Skin or soft tissue may be involved (especially in immunocompromised) — localized cellulitis, ulceration may be manifested (though rare).
- Ear canal (otomycosis) or external auditory symptoms may appear (itching, discharge) when ear is colonized.
- Sinus fungal disease may cause chronic sinusitis-like symptoms: headaches, facial pressure, nasal discharge.
Complications and further consequences are often manifested when disease is unrecognized / untreated.
- Fibrosis, scarring and structural lung damage may be developed (especially in allergic / chronic forms).
- Massive hemoptysis (life threatening bleeding) can complicate aspergilloma or invasive disease.
- Respiratory failure, multi‐organ failure and death may occur in invasive/disseminated disease.
Laboratory diagnosis Methods of Aspergillus fumigatus
Direct microscopy is often done first on clinical specimens (e.g. sputum, BAL, tissue).
- A KOH (potassium hydroxide) preparation may be used to clear debris and hyphae can be visualized.
- Use of optical brighteners / fluorescent stains (eg calcofluor white) is sometimes employed to enhance detection of fungal elements.
- The presence of septate, acute-angle branching hyphae is suggestive (but not definitive) of Aspergillus genus.
- Because sporulation is rarely seen in vivo, specific identification to A. fumigatus is seldom achieved by microscopy alone.
Culture is performed on respiratory, tissue or sterile fluid specimens.
- Samples are inoculated on appropriate fungal media (e.g. Sabouraud agar) and incubated (usually at 37 °C).
- Growth is typically observed within 1 to 3 days (sometimes slower) if viable fungus is present.
- Once growth is obtained, macroscopic and microscopic morphology (e.g. conidiophore structure) may be used to suggest A. fumigatus.
- However, culture is insensitive (many false negatives) and contamination / colonization must be distinguished.
Histopathology / tissue examination is used when biopsy / surgical specimens are available.
- The tissue is processed and stained (eg Gomori methenamine silver, PAS) to visualize hyphae invading tissue.
- Evidence of tissue invasion (angioinvasion, necrosis) strengthens the diagnosis of invasive disease.
- The combination of morphology + location (sterile tissue) is used to distinguish colonization vs true infection.
Antigen detection / serologic assays are non-culture based methods.
- The galactomannan (GM) assay is used in serum or BAL fluid; a polysaccharide from the Aspergillus cell wall is detected.
- In BAL fluid, GM has moderately good sensitivity & specificity.
- In serum, sensitivity is lower (especially in non-neutropenic or patients on antifungals) and false negatives are common.
- The (1,3)-β-D-glucan (BDG) assay (a fungal cell wall component) may be used in serum; it is non-specific (detects many fungi) but can serve as screening tool.
- Combined use of GM + BDG (especially from BAL) may raise overall diagnostic accuracy.
Molecular methods (DNA / PCR / sequencing) are increasingly applied.
- Real-time PCR specific for A. fumigatus (or genus Aspergillus) is done on clinical specimens (tissue, BAL, serum) to detect fungal DNA.
- Internal Transcribed Spacer (ITS) regions are often targeted to differentiate A. fumigatus from other fungi.
- Multiplex PCR and panfungal PCR approaches may be used; results must be interpreted in clinical + radiologic context.
- Next-generation sequencing (mNGS) is emerging (especially for difficult / atypical cases), though its fungal detection sensitivity is variable
Serology / antibody / immunologic tests (especially for allergic / chronic forms).
- Measurement of Aspergillus-specific IgG / precipitins may support diagnosis in chronic pulmonary aspergillosis (CPA) or allergic bronchopulmonary aspergillosis (ABPA).
- Total IgE and specific IgE to A. fumigatus are measured in allergic forms (ABPA).
- A positive precipitin reaction against A. fumigatus is often found in ABPA (though cross-reactivity or background positivity may occur).
Species / isolate identification and susceptibility testing (when culture yields isolate).
- Once a fungal culture is recovered, further identification (e.g. morphological keys, MALDI-TOF, sequencing) is done to confirm A. fumigatus.
- Antifungal susceptibility testing (AFST) is performed (e.g. for azole resistance) to guide therapy.
- Molecular detection of resistance markers (e.g. mutations in CYP51A) is sometimes applied.
Other / experimental or adjunctive methods may be used in research or in specialized centers.
- Detection of fungal volatile organic compounds (VOC) in breath is being explored.
- Advanced spectroscopy (e.g. Raman, tip-enhanced Raman) and imaging-mass techniques are under study.
- Combined algorithmic approaches (e.g. integrating imaging, biomarkers, PCR) are frequently recommended.
Treatment of Aspergillus fumigatus
- The first-line therapy for invasive aspergillosis is usually voriconazole (a mold-active triazole) because it has been shown to be superior to amphotericin B in trials.
- Use of liposomal amphotericin B (L-AmB) or other lipid formulations of amphotericin B is reserved as an alternative or in case of intolerance/contraindication to voriconazole.
- Isavuconazole is increasingly considered as another alternative first or salvage agent.
- Echinocandins (e.g., caspofungin, micafungin) are generally not recommended as monotherapy for primary treatment but can be used in salvage or in combination in selected patients.
- In salvage therapy (when first-line fails or resistance/toxicity is encountered), switching the class of antifungal is recommended (e.g., from voriconazole to amphotericin or isavuconazole or posaconazole), or combining agents may be attempted.
- Antifungal therapy duration is variable but typically 6-12 weeks or more, depending on the site of disease, immunosuppression, and radiologic/clinical response.
- Therapeutic drug monitoring (TDM) of azoles (voriconazole, posaconazole, and itraconazole) should be performed to ensure adequate levels and avoid toxicity.
- Surgical intervention is considered in localized disease readily accessible (e.g., fungal sinusitis, localized cutaneous/soft tissue disease, removal of aspergilloma) to debride necrotic tissue or to reduce burden.
- In allergic aspergillosis (e.g., ABPA), antifungals such as itraconazole are used adjunctively; corticosteroids are often used to reduce inflammation.
- In chronic pulmonary aspergillosis or aspergilloma, surgery may be the preferred option for symptomatic cases, though antifungals may have limited efficacy as sole therapy.
- Reduction or reversal of immunosuppression (if possible) is an important adjunct to therapy so that host defenses are improved.
- Combination antifungal therapy (e.g., voriconazole + echinocandin) may be considered in selected high-risk patients, though evidence is weak/mixed.
- In patients who require ongoing immunosuppression after successful therapy, secondary prophylaxis with mold-active azoles may be used to prevent relapse.
- Supportive care (e.g., management of renal function, liver monitoring, and electrolyte balance) must be done while therapy is ongoing, because antifungals carry risks of toxicity.
Prevention and Control of Aspergillus fumigatus
- Exposure to Aspergillus spores is attempted to be minimized in high-risk persons (immunocompromised, neutropenic) by avoiding dusty/moldy environments.
- Use of personal protective equipment (PPE), such as N95 respirators, is recommended when exposure to dust or demolition/construction sites cannot be avoided.
- In hospital settings, environmental control measures are instituted (e.g., HEPA filtration, air handling, ventilation, and positive pressure rooms) to reduce airborne spore load.
- During construction/renovation/demolition work, barriers (plastic sheeting, sealed zones), dust suppression (water sprays), negative pressure in work zones, and restricted access are enforced to prevent spore dissemination into clinical areas.
- In critical wards, protected environments (also called “mold-protected rooms”) with HEPA-filtered positive-pressure ventilation are used for high-risk patients.
- Air sampling/environmental surveillance is periodically conducted to monitor spore counts in key hospital zones (ICU, transplant wards) and to detect contamination early.
- Maintenance of HVAC (heating, ventilation, air-conditioning) / duct systems, filters, and water systems is done regularly to prevent fungal growth and spread.
- Cleaning, disinfection, and decontamination of surfaces, equipment, and environments (especially after construction) are carried out, with protocols for spore removal from dust and debris.
- Hospital policies are made (and enforced) to restrict the introduction of plants, potted soil, cut flowers, and construction materials in patient care areas.
- Risk assessments (infection control risk assessments, ICRA) are performed prior to building works to tailor protective measures and scheduling (e.g., works done when fewer vulnerable patients are present).
- Antifungal prophylaxis (e.g., mold-active azoles) may be used in selected high-risk patients (e.g., hematologic malignancy, HSCT) to reduce the probability of invasive aspergillosis, though it is treated as an adjunct, not a substitute, for environmental controls.
- In community/home settings, simple personal measures (avoid gardening, avoid piles of leaves/compost, wear a mask when gardening, and clean wounds promptly) are adopted to reduce spore exposure.
- Training/education of staff, construction crews, housekeeping, and clinical personnel is emphasized so that awareness of risk and proper procedures is maintained.
- During outbreaks, epidemiologic investigation, strain typing, and source tracing are used to identify environmental source(s) and control spread.
- Policies of continuous review/audit of control measures are maintained (if spore levels increase, action is taken) so that prevention is dynamic, not static.
Aspergillus fumigatus Images
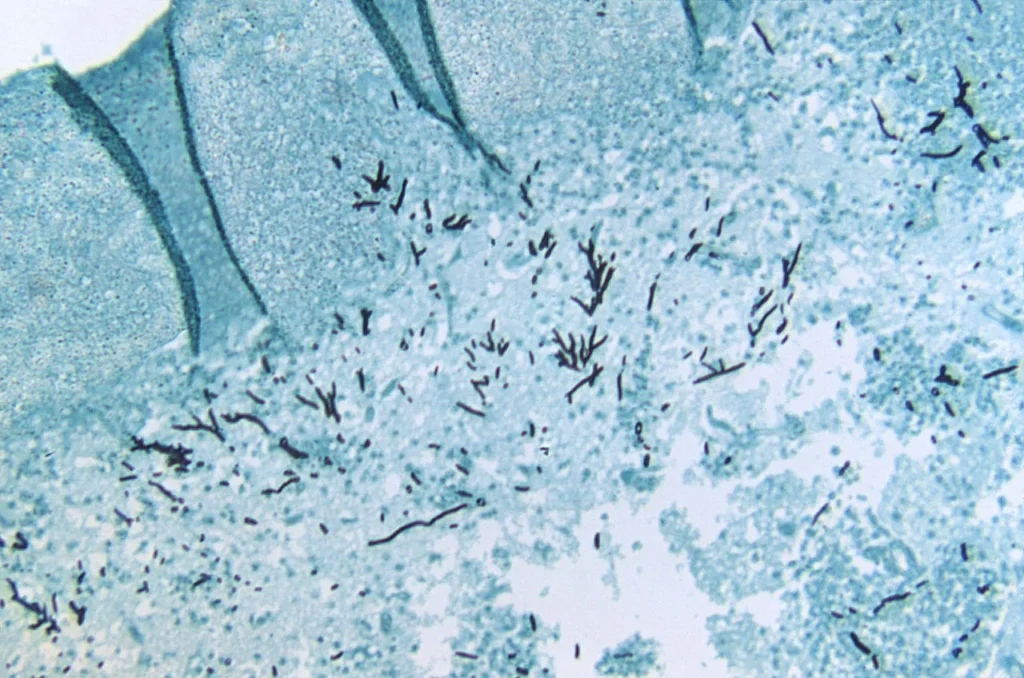
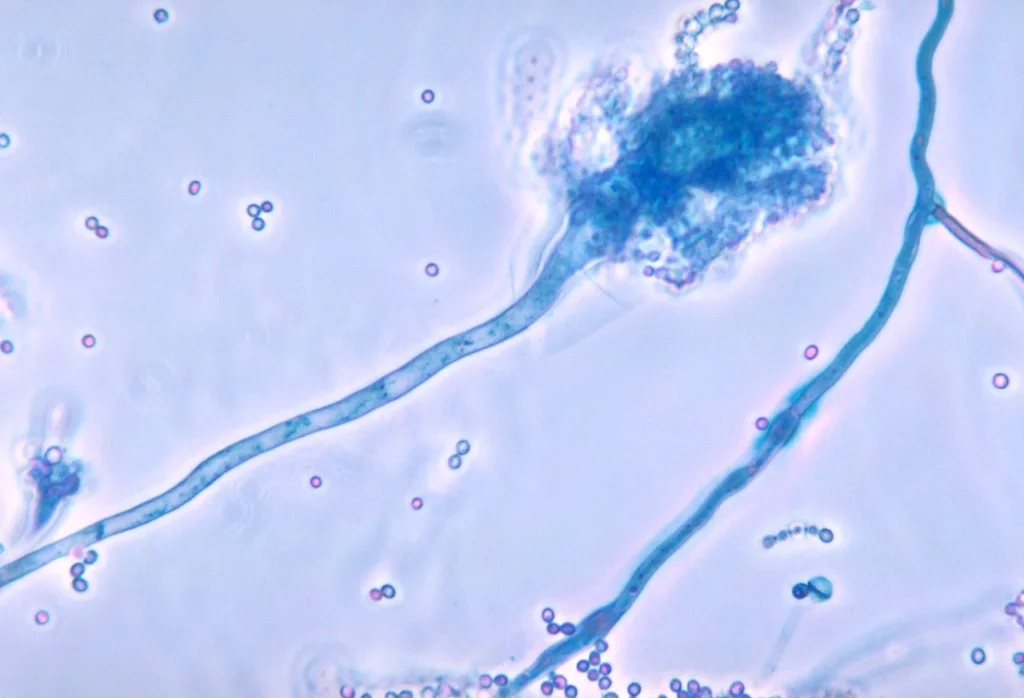
FAQ
What is Aspergillus fumigatus?
Aspergillus fumigatus is a fungus that is commonly found in the environment, especially in soil, compost, and decaying vegetation. It can cause a wide range of infections, especially in immunocompromised individuals.
How does Aspergillus fumigatus cause disease?
Aspergillus fumigatus can cause disease by invading the respiratory tract, blood vessels, and other tissues. It can also produce toxins that can damage cells and tissues.
What are the symptoms of Aspergillus fumigatus infection?
The symptoms of Aspergillus fumigatus infection depend on the site of infection, but can include fever, cough, chest pain, shortness of breath, and fatigue.
Who is at risk of Aspergillus fumigatus infection?
Individuals with weakened immune systems, such as those undergoing chemotherapy or stem cell transplantation, are at increased risk of Aspergillus fumigatus infection. Other risk factors include chronic lung disease and exposure to environmental sources of the fungus.
How is Aspergillus fumigatus infection diagnosed?
Aspergillus fumigatus infection is diagnosed through a combination of clinical signs and symptoms, radiographic findings, and laboratory tests such as culture, serology, and molecular assays.
What is the treatment for Aspergillus fumigatus infection?
The treatment for Aspergillus fumigatus infection involves antifungal medications, surgery, immunotherapy, supportive care, and prevention strategies. The choice of treatment depends on the severity of the infection and the patient’s underlying medical conditions.
Can Aspergillus fumigatus infection be prevented?
Yes, Aspergillus fumigatus infection can be prevented through environmental control, infection control measures, prophylactic antifungal therapy, vaccination (when available), and education.
Is Aspergillus fumigatus infection contagious?
No, Aspergillus fumigatus infection is not contagious and cannot be spread from person to person.
How common is Aspergillus fumigatus infection?
Aspergillus fumigatus infection is relatively uncommon in healthy individuals, but can be a significant problem in immunocompromised patients. It is estimated that up to 10% of patients with hematologic malignancies or stem cell transplantation develop invasive aspergillosis.
What is the prognosis for Aspergillus fumigatus infection?
The prognosis for Aspergillus fumigatus infection depends on the severity of the infection, the patient’s underlying medical conditions, and the timeliness and effectiveness of treatment. In general, the mortality rate for invasive aspergillosis is high, especially in immunocompromised patients.
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999 Apr;12(2):310-50. doi: 10.1128/CMR.12.2.310. PMID: 10194462; PMCID: PMC88920.
- Bandres MV, Modi P, Sharma S. Aspergillus Fumigatus. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482464/
- Latgé, J.-P., & Chamilos, G. (2019). Aspergillus fumigatus and Aspergillosis in 2019. Clinical Microbiology Reviews, 33(1). doi:10.1128/cmr.00140-18
- Latgé, J.-P. (1999). Aspergillus fumigatus and Aspergillosis. Clinical Microbiology Reviews, 12(2), 310–350. doi:10.1128/cmr.12.2.310
- https://microbewiki.kenyon.edu/index.php/Aspergillus_fumigatus_and_Aspergillosis
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.