- Aspergillus clavatus is a species of fungus in the genus Aspergillus.
- It was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.
- The name “Aspergillus” itself was used by Micheli (1729) for moulds with a brush-like spore structure.
- In taxonomy it is placed under family Aspergillaceae, order Eurotiales.
- During the 20th century, A. clavatus was grouped in a section called Clavati (or “clavatus group”) by Thom & Church (1926).
- Later, additional species (such as A. rhizopodus, A. longivesica, A. clavatonanicus) were added in that Section Clavati / Clavati section.
- In 1979 Samson concluded that Aspergillus pallidus is a white variant (synonym) of A. clavatus, based on identical DNA sequence data.
- A sexual stage (teleomorph) was described recently (2018) under the genus Neocarpenteles but under the “one fungus–one name” convention, the A. clavatus name was retained.
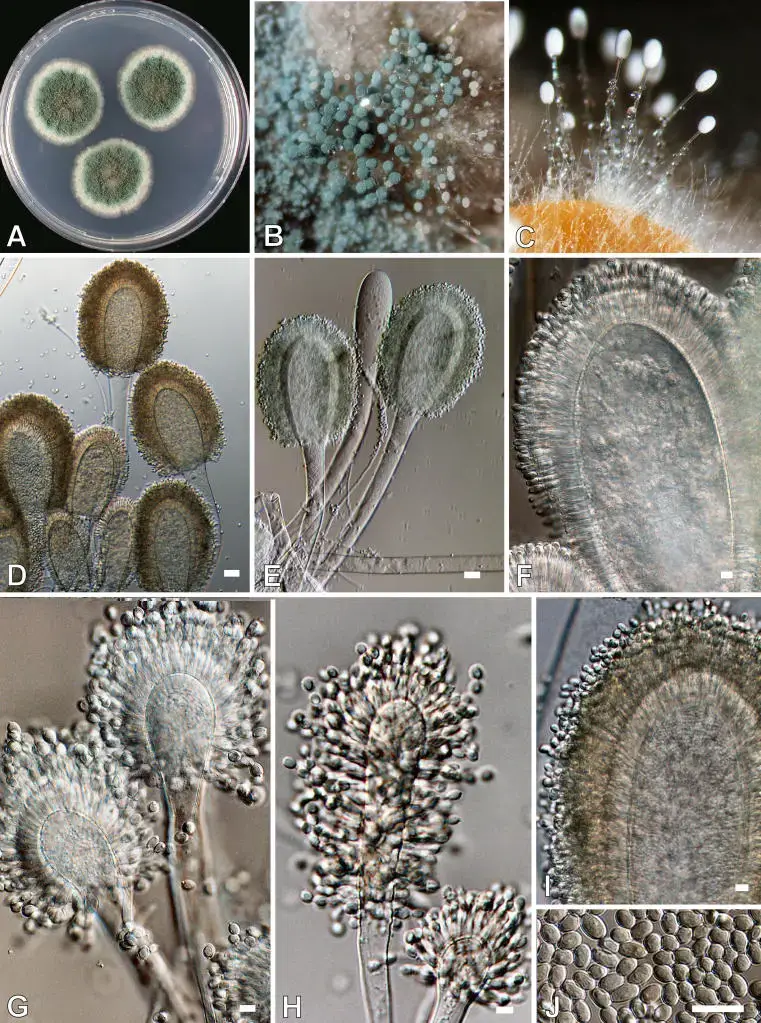
- Morphologically, the fungus is characterized by club-shaped (clavate) vesicles, uniseriate phialides over the entire surface, and blue-green uniseriate conidia.
- Conidia size is about 3–4.5 × 2.5–4.5 µm.
- Growth in culture: on Czapek’s agar, it can reach 3.0–3.5 cm in 10 days at 24–26 °C.
- Historically A. clavatus has been studied not only for its morphology but for its production of secondary metabolites / toxins (such as patulin).
- In 1942, Wiesner noted that A. clavatus produced an antibiotic (initially called clavatin, later clavacin), which was linked to patulin.
- In 1943, A. clavatus strains were reported capable of sterilizing liquid media infected with Staphylococcus aureus (i.e. antibacterial effects).
- Geographically, A. clavatus is cosmopolitan. It has been found in soil, dung, decomposing material across tropical, subtropical, Mediterranean and temperate regions.
- It is often seen as a spoilage fungus on cereals, stored grains (especially when moisture is present) and in alkaline environments (it is alkali-tolerant).
- In malting of barley, A. clavatus may grow and sometimes reach undesirable levels if conditions (temperature, humidity) are favorable.
- Pathogenicity: it is not a frequent aggressive pathogen, but is allergenic (causes hypersensitivity pneumonitis, e.g. malt-worker’s lung).
- Some cases of pulmonary infections / aspergillosis have been associated with A. clavatus, especially in immunocompromised persons.
- In animals, neurotoxicosis (in sheep) has been reported.
- Modern genomics: ~80 % of genes are shared with A. fumigatus among identified genes, but only ~30 % of secondary metabolite (SM) genes are conserved.
- A strain ATCC 1007 (= A. clavatus) is held in culture collections and is used for antibiotic / metabolite research.
- The past & present studies of A. clavatus reflect both its role as a mold of interest (toxins, antibiotics) and as a mild opportunistic organism.
Habitat of Aspergillus clavatus
- Aspergillus clavatus is usually found in soil (especially cultivated soils) and animal manure / dung.
- It is also isolated from rotting organic waste / decomposing material (plant debris, compost) in many habitats.
- In stored cereals / grains (rice, corn, millet etc) under humid / moist conditions it is seen as a spoilage mold.
- It has been detected in alkaline substrates because it is alkali-tolerant, allowing growth in high pH soils or decayed material.
- The geographic range is cosmopolitan: tropical, subtropical, Mediterranean, temperate zones are inhabited by it.
- It has been found in low frequencies in soils of India, Bangladesh, Sri Lanka, also in USA, Greece, Egypt, etc.
- It can colonize rhizosphere soils (soil close to roots) of plants like banana, ground-nut, wheat.
- It was recovered even from rock / cave substrates and deep stratigraphic cores (down to ~1200 m in Japan).
- Within indoor or built environments it is less common, but it may appear in moist / wet surfaces, damp walls, or humid storage areas.
- Due to its xerophilic (low-water requirement) tendencies it can occur on relatively dry substrates, especially where others fail.
- It is also isolated from insects (dead adult bees, honeycombs), bird droppings / feathers, showing it can inhabit / survive on biotic surfaces.
- Growth is favored in moderate temperature (~25 °C) but growth has been reported from min ~5 °C to max ~42 °C in culture, so its habitat range is broad in thermal tolerance.
- In agricultural soils it is often associated with crops like barley, potatoes, sugar cane, cotton, legumes.
- It is stimulated by nitrogen / NPK fertilizers (in some reports) in soils / compost, thereby becoming more abundant under enriched nutrient conditions.
- Its presence in a habitat is influenced by moisture, pH, nutrient content, substrate type (organic matter), temperature, and competition from other microbes.
- It may persist in habitats even when conditions become less favorable (e.g. dry periods) because of spore resilience / dormancy.
Morphology of Aspergillus clavatus
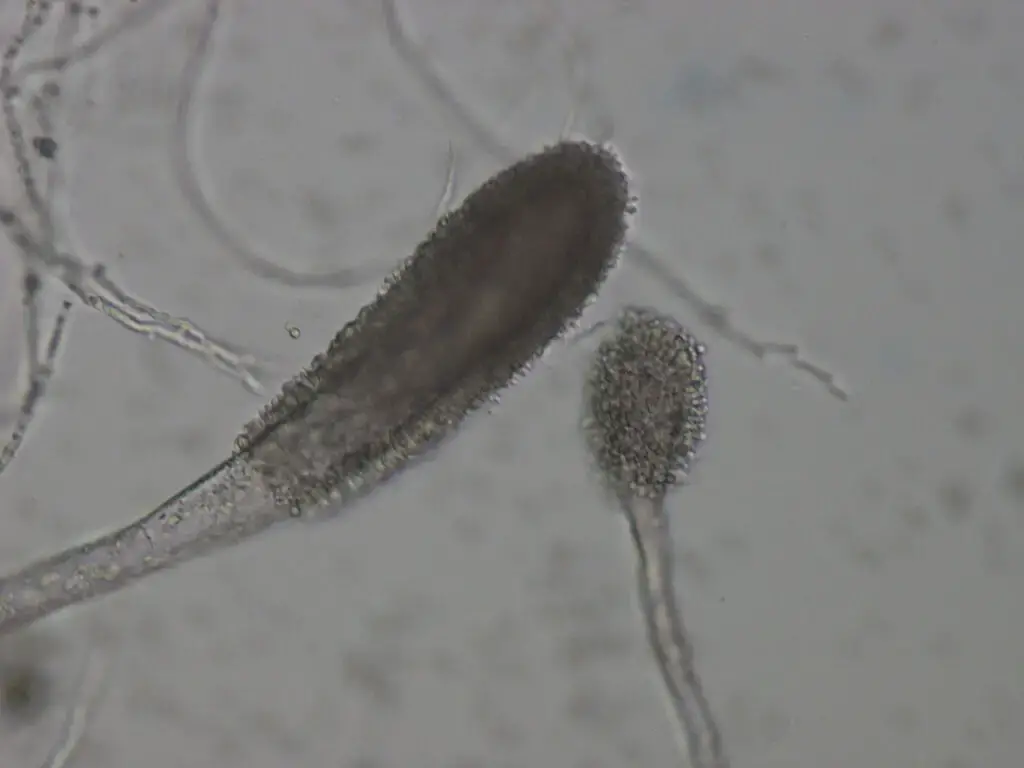
- Aspergillus clavatus is composed of septate, hyaline hyphae which are multicellular and branching.
- The conidiophores are long, erect, smooth‐walled stalks (1.5–3.0 mm in many strains) that arise from the vegetative mycelium.
- At the apex of each conidiophore a clavate (club-shaped) vesicle is formed (i.e. swelling) which is thicker toward its apex.
- The phialides (sterigmata) are borne uniseriately (in a single row) over the entire surface of the vesicle.
- The sterigmata are numerous, crowded, and each supports a conidium (i.e. conidial chains).
- The conidia are elliptical (or broadly ellipsoidal), smooth, relatively thick‐walled.
- Conidial dimensions are about 3.0 – 4.5 × 2.5 – 3.5 µm in many isolates.
- In young state, conidial heads are large and clavate, but with maturity they often split into two or more divergent columns of chains.
- Colony (macroscopic) appearance: a velvety to felted texture, often bluish-green in color, with a white periphery in many culture media.
- Growth rate on e.g. Czapek’s agar: reaches ~3.0–3.5 cm in 10 days at ~24-26 °C in many strains.
- The reverse side of the colony (underside) is usually uncolored initially but may turn brown in some strains over time.
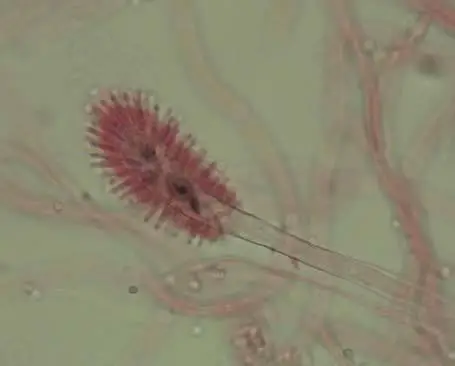
- Some isolates produce a sexual structure (cleistothecia / asci / ascospores) under appropriate conditions (after several weeks) in crosses.
- Cleistothecia are often yellowish-brown to dark brown, with a hard outer wall (peridium), diameter ~315-700 µm.
- Ascospores inside the cleistothecia are clear, lenticular, typically ~6.0–7.0 µm in diameter (with ridges).
- The wall composition of conidia / cell walls contains soluble wall carbohydrates such as mannitol and arabitol in many isolates.
- The GC content of DNA (in studied strains) is ~52.5–55 %.
- By TEM / SEM studies it has been shown that first-formed conidium and its phialide may share a continuous wall in early development.
- Some variation is seen among strains: under different media (malt extract agar etc) the abundance, size, and compactness of conidial heads may differ.
- In the taxonomy of section Clavati, A. clavatus and related species share the clavate vesicle trait, but A. clavatus is differentiated by its particular vesicle / conidial features.
Cultural Characteristics of Aspergillus clavatus
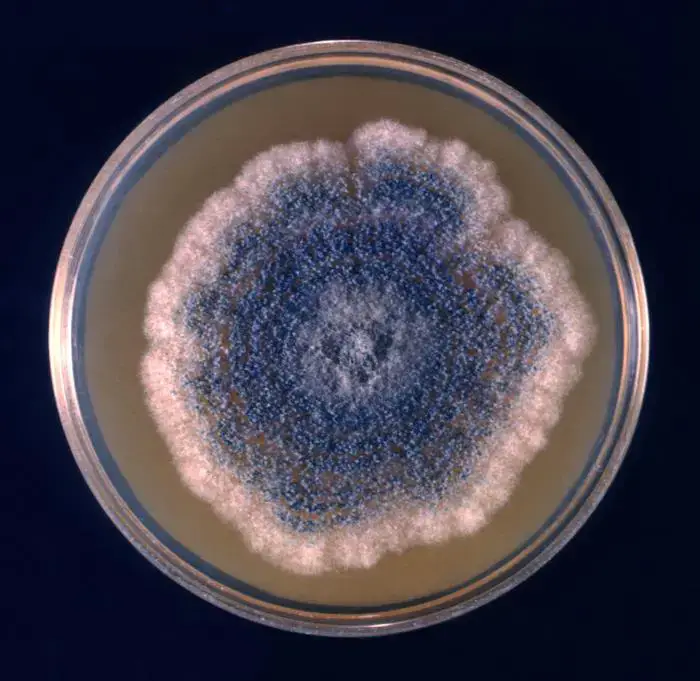
- On Czapek’s solution agar (CYA / Czapek’s agar) Aspergillus clavatus is rapidly grown and colonies of ~ 3.0-3.5 cm diameter are produced in 8–10 days at ~24–26 °C.
- Colony form is plane to slightly furrowed, often floccose (woolly / wool-like) with a thin mycelial felt bearing erect conidiophores.
- The color of the colony surface is bluish-grey green (or blue-green) with a white margin / periphery.
- The underside / reverse of the colony (on agar) is usually uncoloured or pale / white to tan, though in some strains it may turn brown over time.
- On Czapek agar, odor is generally weak or absent, but in some strains an unpleasant smell / odor may develop.
- Conidial heads in early growth (on CYA) are relatively large, club-shaped (clavate), maybe ~300–400 µm × 150–200 µm when young.
- As maturity occurs, conidial heads often split into two or more divergent columns / chains of conidia (divergent heads) reaching lengths ~1.00 mm in some strains.
- Conidiophores (on CZA) reach lengths ~1.5-3.0 mm with diameter ~20–30 µm; stipes are smooth-walled, hyaline, colorless initially.
- Vesicles are clavate (club-shaped), enlarged at apex (200–250 µm long × 40–60 µm width in many strains) as the fertile region supporting phialides.
- Phialides (sterigmata) are uniseriate (single row) over the full surface of the vesicle, numerous and crowded.
- Conidia are elliptical / broadly ellipsoidal, smooth, thick-walled. Their size is ~3.0–4.5 × 2.5–3.5 µm in many cases.
- On malt extract agar (MEA / malt agar) the morphology differs: fewer conidial structures are produced, sometimes heads are smaller, conidiophore length / abundance may reduce, and strong odor may be more prominent.
- Colony appearance on malt agar may include more loose, columnar heads, weaker / smaller conidiophores (300–500 µm) in non-typical strains.
- In crosses / sexual culture: cleistothecia are produced after 4–10 weeks (on suitable media, ~25 °C).
- Cleistothecia are yellowish-brown to dark brown, diameter ~315–700 µm, with hard peridium.
- Ascospores (in cleistothecia) are clear, lenticular (with ridges), ~6.0–7.0 µm in diameter.
- Variation is observed among strains / media: size, density, odor, branching, head-splitting patterns differ.
- Growth temperature influences cultural traits: at ~25 °C the described morphology is standard; extremes may reduce sporulation or alter morphology.
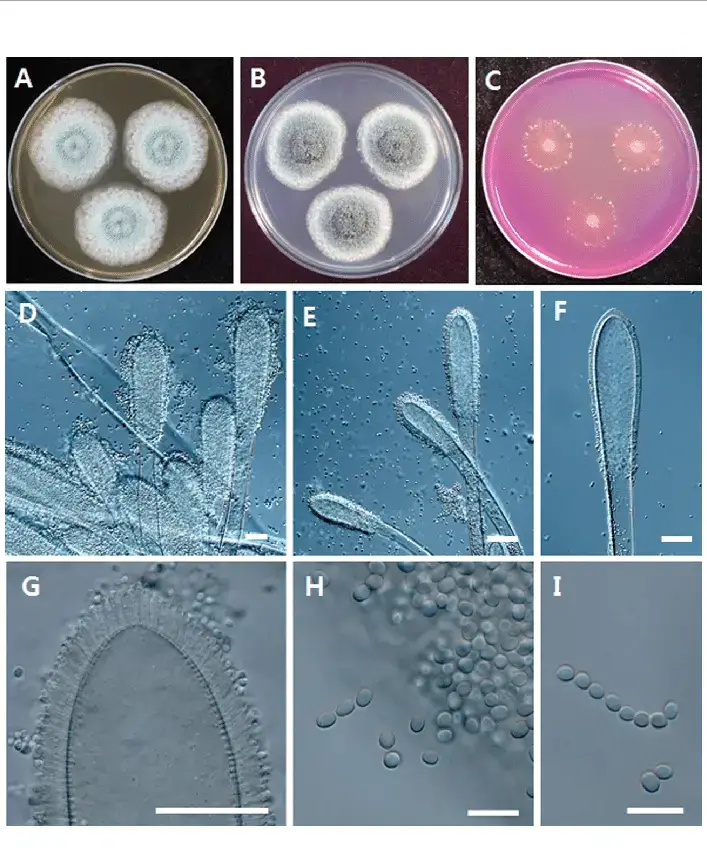
Culture media used for the growth of Aspergillus clavatus
- A common medium used for Aspergillus clavatus is Czapek’s solution agar (Czapek agar / CZA) — on this medium A. clavatus is vigorously grown, and colonies of ~3.0–3.5 cm are formed in ~10 days at 24-26 °C.
- Growth on malt extract agar (MEA) is also employed; on MEA fewer conidial structures may be produced, colony morphology may differ (looser heads, weaker sporulation) compared to Czapek’s medium.
- Use of DG18 agar (dichloran-glycerol agar) has been mentioned as a medium used for environmental Aspergillus (for xerophilic / moisture-limiting growth), in which Aspergillus species like A. clavatus might be recovered under lower water activity conditions.
- Sabouraud’s dextrose agar (SDA / Sabouraud’s medium) is sometimes used especially when fungi are recovered from clinical / mixed samples; Aspergillus colonies (including A. clavatus) may grow on it.
- In pH / selective media like CREA (creatinine sucrose agar) the fungus has been observed to influence / alter pH (by releasing acidic substances), and may grow / survive in such media (used in mold diagnostics) — so CREA is used in some fungal / mold media surveys.
- In selective / inhibitory media (i.e. media containing antibiotics or dyes) A. clavatus may be isolated when bacterial growth is suppressed (for example, MEA + chloramphenicol is often used in environmental fungal culture) (this is general for Aspergillus spp.)
Life Cycle of Aspergillus clavatus
- The life cycle of Aspergillus clavatus is dominated by asexual reproduction, though a functional sexual cycle has been discovered.
- Asexual reproduction is accomplished by formation of conidia (asexual spores) on conidiophores; these conidia are dispersed (by air, dust) to new substrates.
- Upon landing on a suitable substrate (nutrient, moisture, temperature favorable), the conidium is germinated; a germ tube is formed and hyphal growth begins (vegetative mycelium).
- The mycelium (hyphae network) spreads by apical extension and branching, and substrate mycelium is formed.
- Under favorable conditions, aerial hyphae differentiate to form conidiophores which develop vesicles, phialides, sterigmata, and conidial chains (i.e. the asexual fruiting).
- In A. clavatus, the conidiophore length is often ~1.5-3.0 mm, vesicle is clavate (club-shaped), phialides uniseriate over entire vesicle surface, conidia ~3.0–4.5 × 2.5–3.5 µm.
- The asexual cycle continues as conidia are produced repeatedly, and the fungus colonizes new niches.
- Sexual reproduction (in A. clavatus) is achieved via the formation of cleistothecia (closed fruiting bodies) under specific cultured conditions (e.g. oatmeal agar, sealed plates, darkness, ~25-28 °C) over several weeks (≈4 weeks).
- Inside cleistothecia, karyogamy (nuclear fusion) + meiosis occur, leading to ascospores which are borne in asci. The ascospores are heat-resistant, hyaline, often with two equatorial ridges.
- After ascospores mature, they are released (or liberated when cleistothecia rupture) into the environment; they then germinate, restarting the cycle.
- Genetic recombination is enabled via the sexual cycle: isolates of MAT1-1 and MAT1-2 mating types have been observed and crossing (outcrossing) was confirmed by molecular markers in ascospore progeny.
- The relative frequency of sexual vs asexual reproduction is skewed: asexual reproduction dominates under most natural conditions, while sexual cycle is induced under special lab or stress conditions.
- A parasexual cycle may be possible (as in other Aspergillus spp), where hyphal fusion / heterokaryosis / mitotic recombination can lead to genetic variation without a full sexual cycle.
- Variation among strains in capacity / ease to undergo sexual cycle is observed; not all isolates will readily form cleistothecia under given conditions.
- The life cycle phases (asexual spore → germination → vegetative growth → conidiation; sexual mating → cleistothecia → ascospores) ensure both propagation and potential for genetic diversity.
Pathogenesis of Aspergillus clavatus
- Exposure is initiated when conidia (asexual spores) of Aspergillus clavatus are inhaled into the respiratory tract (nose, bronchi, alveoli).
- The conidia, being small and airborne, are deposited on airway surfaces (bronchioles, alveoli) where mucosal / alveolar surfaces are contacted.
- Under favorable conditions, the conidia are germinated, and germ tubes emerge, penetrating the epithelial barriers or colonizing the mucosal surface.
- Hyphal growth proceeds by apical extension and branching, forming invasive hyphae that may breach tissue barriers (epithelium, basement membrane).
- Fungal adhesion factors / cell wall components assist in attachment to host cells or matrix; host surfaces are colonized.
- Tissue damage is mediated by enzymes / toxins / metabolites secreted by the fungus (e.g. proteases, ribotoxins, mycotoxins like patulin).
- Inflammatory / immune responses are triggered: neutrophils, macrophages attempt phagocytosis and oxidative killing of the fungal elements.
- In susceptible / immunocompromised hosts, immune defenses are impaired, so hyphal invasion is unchecked, and tissue necrosis / infarction may result.
- Hematogenous dissemination is possible: hyphae or fragments may invade blood vessels (angioinvasion), thereby spreading to distant organs (kidney, brain, skin).
- The fungus is often opportunistic: it is rarely pathogenic in healthy hosts, and strong host immunity usually prevents deep invasion.
- Allergic / hypersensitivity reactions may also occur (non-invasive form): inhaled antigens / spores provoke immune sensitization (extrinsic allergic alveolitis / “malt-worker’s lung”) in exposed persons.
- In animals, ingestion of contaminated food / feed (with spores / toxins) may lead to mycotoxicosis and secondary lesions (e.g. neurotoxic effects).
- In pulmonary infection, alveolar damage, hemorrhage, and granulomatous inflammation may be found; fungal hyphae are seen invading tissues in histological sections.
- Ultimately clearance or control may be achieved by immune mechanisms (phagocytosis, antifungal responses) or the infection may worsen, depending on host status.
Diseases caused by Aspergillus clavatus
- Aspergillus clavatus is known to cause hypersensitivity pneumonitis (extrinsic allergic alveolitis) in humans — especially in malt workers (“malt-worker’s lung”) — by repeated inhalation of spores / antigens.
- In such allergic disease, immune complexes / Type I / III hypersensitivity are involved, and lung parenchyma inflammation with microgranulomas is produced.
- Pulmonary aspergillosis (invasive or chronic) has occasionally been associated with A. clavatus in immunocompromised individuals.
- Mycotoxicosis is a disease risk, because A. clavatus produces patulin (a mycotoxin) and other secondary metabolites which can cause toxicity in animals / humans if ingested / inhaled in high amounts.
- In animals (e.g. sheep), neurotoxicosis has been reported when contaminated feeds (with spores, toxins) are consumed.
- Cases of otomycosis (ear fungal infection) have also been ascribed to A. clavatus in some reports.
- Hyperkeratosis in calves has been linked to exposure to A. clavatus spore derivatives / extracts in experimental settings.
- Pulmonary mycotoxicosis / lung inflammation / tumor induction has been shown in animal experiments where A. clavatus extracts / spore wall components were given to mice (causing lung reaction, inflammatory lesions, occasional tumor increase) in unimmunized mice.
Treatment of Aspergillus clavatus diseases
- Treatment of disease caused by Aspergillus clavatus is not well standardized because the organism is only occasionally pathogenic, and data are limited.
- In pulmonary aspergillosis or invasive aspergillus infections (general Aspergillus therapy), azoles such as voriconazole, isavuconazole or itraconazole are often used as first-line antifungals.
- Amphotericin B (lipid or deoxycholate formulations) is applied as alternative therapy when azoles cannot be used or in severe invasive cases.
- In allergic forms (such as hypersensitivity / pneumonitis, e.g. “malt-worker’s lung”) corticosteroids (systemic immunosuppression) are employed to reduce inflammation.
- Antifungal agents (e.g. itraconazole) may be used adjunctively along with steroids in allergic forms to reduce fungal burden / antigen load.
- For ocular / keratitis / surface infections with Aspergillus species, topical irrigation with voriconazole, amphotericin B, or pimaricin has been described. A. clavatus may respond similarly.
- Supportive care (management of respiratory failure, immunosuppression reversal) is critical in severe disease.
- Duration of therapy is usually prolonged (weeks to months), and treatment must often continue until resolution of lesions and control of underlying risk factors.
- In allergic disease monitoring of IgE levels, imaging and symptoms is done during therapy.
- Preventive measure (minimizing exposure to mold, protective masks) is considered adjunct to therapy to reduce recurrence.
- Because A. clavatus is rare as a pathogen, susceptibility testing and individualized therapy may be required (i.e. adjusting antifungal drug choice/dose).
- In severe refractory cases, combination antifungal therapy or surgical resection (if focal lesion) might be considered (by analogy with invasive aspergillosis guidelines).
- Care must be taken with drug toxicity, drug interactions (especially with azoles) and host immune status in therapy planning.
Prevention and control of Aspergillus clavatus
- Exposure to spores / conidia of Aspergillus clavatus is minimized by removal / reduction of all possible mold sources (walls, damp surfaces, stored grains).
- Moisture control is central: humidity levels are maintained low, leaks and condensation are repaired promptly so that growth is discouraged.
- In indoor or storage environments material (wood, paper, grains) should be kept dry, ventilated, with good air circulation so mold growth is inhibited.
- Use of protective masks / respirators (e.g. N95) is advised for individuals (farm workers, malt workers) in high-spore exposure zones.
- Protective clothing, gloves, boots are recommended so that skin / clothing contamination is limited.
- Surfaces suspected of having mold should be cleaned / decontaminated using appropriate antiseptics / fungicides / biocides and physical removal of biofilm / spores.
- In buildings / hospitals, air filtration, HEPA filters, and positive pressure rooms may be used to prevent spore ingress into sensitive areas (especially immunocompromised patients).
- During construction / renovation in healthcare or residential settings, dust control, wetting down surfaces, sealing off areas are done so mold spread is prevented.
- For high-risk patients (immunocompromised), prophylactic antifungal medication may in some protocols be prescribed to reduce risk of invasive Aspergillus infections.
- Regular monitoring / inspections of buildings, storage facilities, ventilation systems are carried out to detect early mold colonization / humid zones.
- In grain storage, controlling moisture content, aeration, prompt drying after harvest are practiced to prevent A. clavatus proliferation.
- In occupational / industrial settings, worker education (on risks, exposure) and routine environmental surveillance (air sampling, spore counts) are used as control measures.
- Barrier methods (e.g., sealed containers, impermeable packaging) are used for susceptible materials / products to avoid contamination.
FAQ
What is Aspergillus clavatus?
Aspergillus clavatus is a species of fungus that can cause a variety of diseases in humans, ranging from mild allergic reactions to severe invasive infections.
What are the symptoms of Aspergillus clavatus infection?
The symptoms of Aspergillus clavatus infection depend on the type and severity of the infection, but can include fever, cough, shortness of breath, chest pain, skin lesions, and ear pain and discharge.
How is Aspergillus clavatus infection treated?
Treatment of Aspergillus clavatus infections involves antifungal medications, surgery, and supportive care. The choice of treatment depends on the type and severity of the infection.
What are the risk factors for Aspergillus clavatus infection?
Individuals who are immunocompromised, such as those with HIV/AIDS, cancer, or organ transplants, are at higher risk for Aspergillus clavatus infections. However, healthy individuals can also develop infections with this fungus.
How can I prevent Aspergillus clavatus infection?
Prevention of Aspergillus clavatus infections involves infection control practices, air filtration, antifungal prophylaxis, environmental control, and patient education.
What are the complications of Aspergillus clavatus infection?
Complications of Aspergillus clavatus infection can include tissue damage, organ failure, and death, especially in severe or invasive infections.
What is the prognosis for Aspergillus clavatus infection?
The prognosis for Aspergillus clavatus infection depends on the type and severity of the infection, as well as the underlying health of the affected individual. In general, early diagnosis and treatment can improve outcomes.
What is the research on Aspergillus clavatus?
Research on Aspergillus clavatus focuses on improving diagnostic techniques, developing new antifungal therapies, and understanding the molecular mechanisms of the fungus.
What are the latest developments in the treatment of Aspergillus clavatus infection?
Recent developments in the treatment of Aspergillus clavatus infection include the use of combination antifungal therapy, new formulations of existing antifungal drugs, and the development of novel antifungal agents.
What are the latest developments in the prevention of Aspergillus clavatus infection?
Recent developments in the prevention of Aspergillus clavatus infection include the use of prophylactic antifungal agents, the implementation of infection control measures, and the development of new air filtration technologies.
- Seye F, Faye O, Ndiaye M, Njie E, Marie Afoutou J. Pathogenicity of the Fungus, Aspergillus clavatus, isolated from the locust, Oedaleus senegalensis, against larvae of the mosquitoes Aedes aegypti, Anopheles gambiae and Culex quinquefasciatus. J Insect Sci. 2009;9:1-7. doi: 10.1673/031.009.5301. PMID: 20050773; PMCID: PMC3011963.
- Samson, R.A., et al. (2014). Aspergillus systematics in the genomic era. Studies in Mycology, 78, 49-71.
- Vesper, S.J., et al. (2007). Quantitative PCR analysis of molds in the dust from homes of asthmatic children in North Carolina. Journal of Environmental Monitoring, 9, 826-830.
- Dehghan, P., et al. (2017). Investigation of Aspergillus fumigatus and Aspergillus clavatus in indoor air and dust from hospitals in Kerman, Iran. Journal of Hospital Infection, 96(1), 78-83.
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/aspergillus-clavatus
- https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=info&id=5057
- https://en.wikipedia.org/wiki/Aspergillus_clavatus
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/aspergillus-clavatus
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.