Because the body is complex, energy is required to ensure proper functioning. Adenosine triphosphate, or ATP, is the energy source for use and storage at cellular level. ATP’s structure is a nucleoside triophosphate. It consists of a ribose glucose, a nitrogenous base (adenine) and three serially bonded phosphate group. ATP is often referred to by the term “energy currency” because it can be readily releasable in the bond between the third and second phosphate groups. Hydrolysis, which is the process of reducing ATP to energy, serves a wide range of cell functions including signaling and DNA/RNA synthesis. ATP synthesis uses energy from many catabolic mechanisms including cell respiration, betaoxidation, ketosis, and cellular metabolism.
The majority of ATP synthesis takes place in cellular respiration, which generates approximately 32 ATP molecules for every molecule of glucose that has been oxidized. ATP is used for energy in ion transport and muscle contraction. It also helps to propagate nerve impulses, phosphorylate substrates, and chemical synthesis. These and other processes create a high demand of ATP. To ensure proper functioning, the cells of the human body rely on the hydrolysis of 100-150 moles of ATP each day. In the next sections, ATP’s role in daily cell functioning will be further examined.
Definition of Adenosine triphosphate (ATP)
Adenosine triphosphate, also known as ATP is an energy-carrying molecule that can be found in all cells. ATP is a chemical energy-carrying molecule that captures chemical energy from the breakdown of food molecules and then releases it to fuel other cellular functions.
- Three types of chemical energy are required by cells: to drive metabolic reactions that don’t occur automatically; to transport necessary substances across membranes, or to perform mechanical work such as moving muscle.
- ATP is not a storage molecule of chemical energy. That is the job of carbohydrates like glycogen and fats.
- When energy is required by the cell it converts from storage molecules to ATP.
- ATP acts as a shuttle and delivers energy to cells where it is needed.
- ATP can be used to power cellular processes through the transfer of a phosphate group from one molecule to another (a process known as phosphorylation). Special enzymes are responsible for this transfer. They release energy from ATP and then provide energy to the cellular activities that need it.
- Although cells constantly break down ATP in order to get energy, ATP is also continually being made from ADP or phosphate by cellular respiration.
- The enzyme ATP synthase is responsible for producing most of the ATP found in cells. It converts ADP to phosphate into ATP.
- The membrane of the cellular structures known as mitochondria is where ATP synthase can be found. In plant cells, it is also found in chloroplasts.
- Fritz Albert Lipmann, Herman Kalckar and others discovered the central role of ATP for energy metabolism in 1941.
Structure of ATP
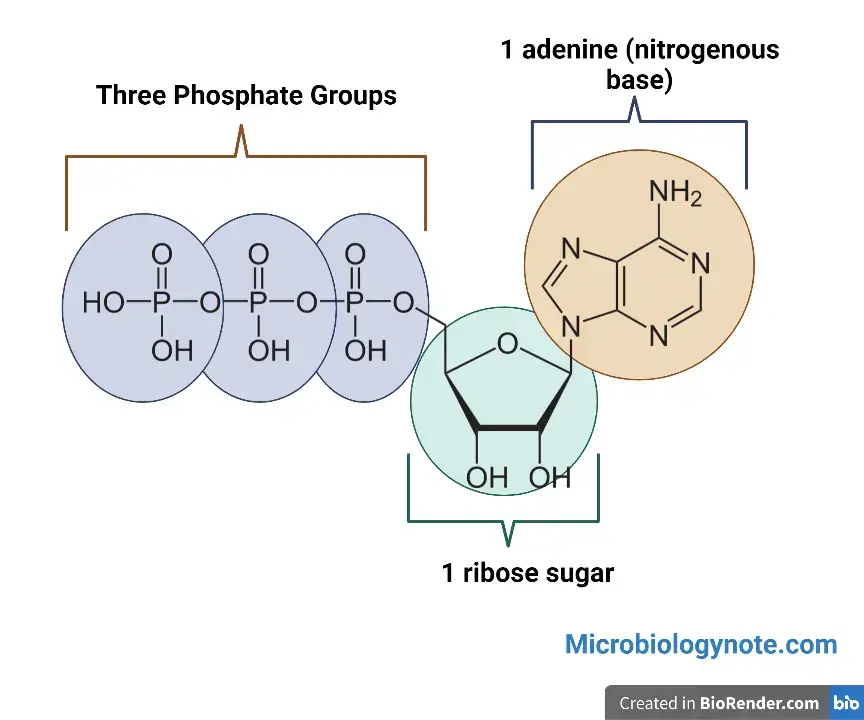
- ATP is a nucleotide which consists of three major structures: the nitrogenous base, adenine, and the sugar, ribose. It also has a chain of three phosphate group that are bound to ribose.
- The actual power source that the cell taps is the ATP phosphate tail.
- The bonds between phosphates contain energy and are released when they break. This is done by adding a water molecule to the mixture (a process known as hydrolysis).
- ATP usually only removes the outer phosphate to produce energy. When this happens, ATP is transformed to adenosine triphosphate (ADP), which is a form of the nucleotide with only two phosphates.

Phosphate residues in ATP Structure
- ATP is a chain of three phosphate residues that are linked to the nucleoside adenosine’s 5′-OH group.
- These phosphate residues can be referred to as a, b and y.
- A phosphoric acid ester bonds bind the phosphate to ribose.
- On the other hand, the linkages between the three Phosphate Residues involve more unstable phosphoric Acid Anhydride bonds.
Role of Mg++
- Active coenzyme is a combination of ATP and an Mg2+ ion. It is co-ordinatively bound with the ss and y phosphates (Mg2+ ATP4).
Chemical properties
- You can isolate salts of ATP as colorless solids.
- In the absence of catalysts, ATP is stable at pH 6.8 to 7.4.
- It rapidly hydrolyses to ADP or phosphate at higher pH levels.
- Living cells keep the ratio of ATP and ADP at a point ten times greater than equilibrium. The concentrations of ATP are fivefold higher that those of ADP.
- The P-O-P bonds are often referred to in biochemical reactions as high-energy bonds.
ATP Production
All cells in the body are responsible for creating ATP. This begins with glucose being digested by the intestines. The glucose is then taken up by the cells and converted into pyruvate. It then travels to mitochondria in the cells. This is where ATP is ultimately produced.
Your body wouldn’t be able to use the energy that ATP provides without this pathway. ATP is the universal energy carrier. It holds all the energy each cell requires to complete its tasks. It can also be reused over and over, just like a rechargeable battery.
The body has many systems that can create ATP, because ATP is so vital. These systems are interconnected in three phases (pathways), namely: Glycolysis, Krebs cycle (citric acids cycle) and Electron transport chain.
Glycolysis
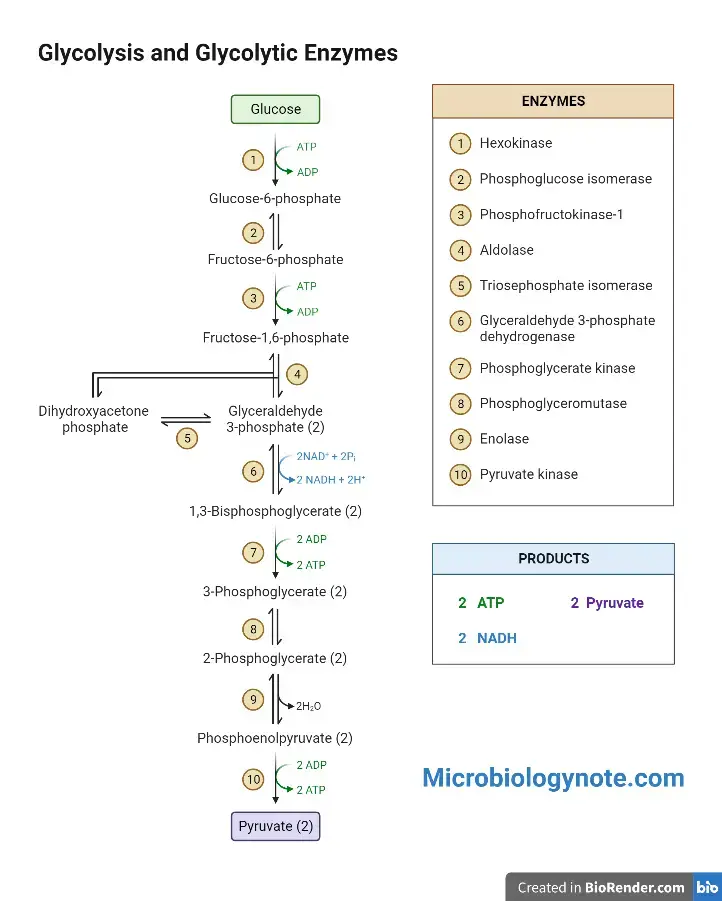
This is the first step in human respiration. Glycolysis is a series of reactions involving the extraction of energy from glucose through the splitting of it into three-carbon molecules called Pyruvates. Glycolysis, an ancient metabolic pathway that evolved long ago, is still found in most organisms today.
Glycolysis is the first stage in organisms that use cellular respiration. Glycolysis does not require oxygen. Many anaerobic organisms, which are organisms that don’t use oxygen, also have this pathway.
- Glycolysis is a process that occurs in the cell’s cytosol. It can be divided into two phases: the energy requiring phase and the energy releasing phase.
- Overall, Glycolysis is a six-carbon molecule glucose that converts to two three-carbon pyruvate molecules. This process produces two molecules of ATP, and two molecules NADH.
Krebs Cycle or Citric Acid Cycle
The Krebs Cycle, also known as the citric acid cycling, is the second major step of oxidative phosphorylation. The Krebs Cycle transfers energy from glucose molecules into 3-carbon molecules. This is the process that breaks down glycolysis and produces ATP.
- The inner membrane and the matrix space of mitochondria contain the enzymes that are involved in the Krebs cycle.
- The reactions of the Krebs cycle in the mitochondrial matrix add electrons and protons into a variety of electron carriers. These electron carriers are then used by electron transport chains to make ATP.
- This cycle can only occur under aerobic conditions, since energy-rich molecules such as NAD + or FAD cannot recover from their reduced forms once they transfer electrons into molecular oxygen.
- The common last pathway to oxidation for all biomolecules is through the citric acid cycle. It includes proteins, fatty acids and carbohydrates.
- Citric acid cycle is a sequence of eight enzyme-mediated reactions.
- This cycle is important because it supplies high-energy electrons/molecules to electron transport chains for the production ATP and water.
- After glycolysis, the pyruvate is first oxidized into Acetyl CoA. This then enters the citric acids cycle.
For every 1 molecule glucose, the Krebs cycle creates 2 molecules of ATP. For every molecule of glucose, the Krebs cycle also produces 8 molecules of NADH2 and 2 molecules of FADH2. Later, NADH2 and FADH2 can be used for electron transport phosphorylation to create energy.

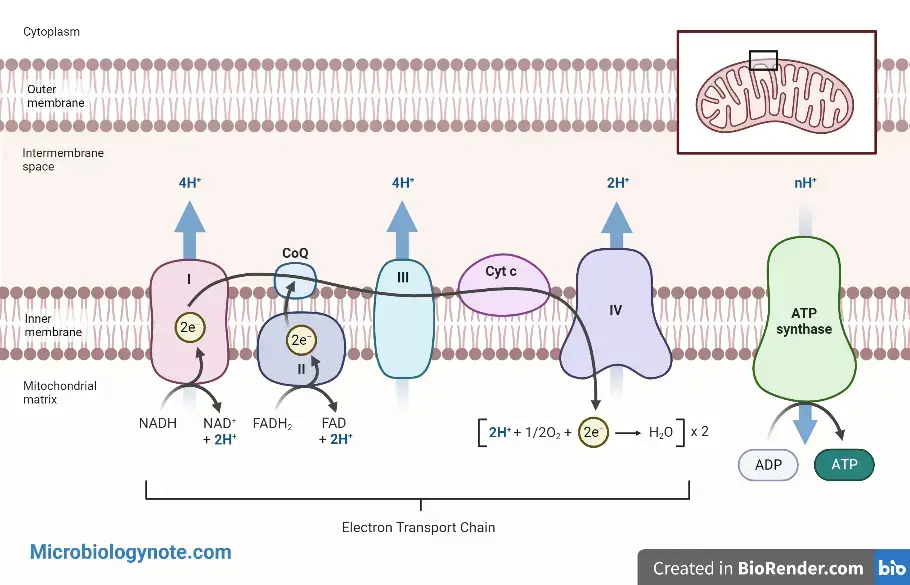
Electron Transport Chain
A series of steps that allow electrons to flow to oxygen, allowing for a gradual decrease in their energy. Sometimes, this stage of oxidative phosphorylation is called the electron transportation chain.
- The electron transport chain is found within mitochondria. The proteins that make up the electron transport chains span the inner mitochondrial membrane. The membrane of mitochondria contains special proteins that are energized with NADH. They continue to produce ATP to power the cells.
- Each molecule of glucose in aerobic respiration leads to approximately 34 molecules of ATP (Adenosine Triphosphate) produced by the electron transportation chain. This is the most productive part in respiration.
- The electron transport chain is a series redox reactions that see electrons being transferred from a donor molecule into an acceptor. These reactions are driven by the free energy of reactants and products. Any reaction that reduces the system’s overall free energy will occur.
- ATP synthase can be found in all domains. It is powered via a transmembrane pron electrochemical gradient. This is due to a series of redox reaction. This gradient is produced by the electron transport chain. It is used to drive ATP synthesis.
- For electron transport phosphorylation, electrons are carried through the NADH generated by glycolysis and citric acids cycle and the FADH2 created by citric acid cycles. This process produces 32 ATP molecules.
- Therefore, the total ATP produced by aerobic respiration is 2 +2 +32 = 36.
Beta-Oxidation
Another mechanism that allows for ATP synthesis is beta-oxidation. Beta-oxidation causes fatty acid chains to be permanently shortened and yields Acetyl CoA molecules. Each cycle of betaoxidation sees the fatty acid being reduced by two carbon lengths. This produces one molecule of Acetyl-CoA which can be oxidized within the citric acid cycle and one each of NADH2 and FADH2, which each transfer their high-energy electrons to the transport chain.
Ketosis
Ketosis refers to a reaction in which ketone bodies catabolly produce ATP. During ketosis, ketone body undergoes catabolism to generate energy. It produces twenty-two ATP molecules as well as two GTP molecules per acetoacetate molecule. These molecules are then oxidized in mitochondria.
Uses of ATP
Many purposes can be served by the ATP molecule. ATP is an important molecule for metabolism because it contains a lot energy that is used in many metabolic processes. eg
- ATP is an essential component of protein synthesis.
- Molecular motors use energy derived from repeated cycles ATP hydrolysis to move.
- It is used to contract muscles
- It’s very useful in transporting molecules through membranes. Active transport is another name for this process.
- Also used to send signals and aid in DNA synthesis.
- Extracellular signaling molecule Adenosine Triphosphate (ATP), is important. ATP is a neurotransmitter that acts in the central and peripheral nervous systems. ATP plays a role in the chemical transmission of chemicals in the peripheral nervous system. ATP is released from synaptic terminals and induces rapid excitatory postsynaptic curents in the central nervous system.
Clinical Significance of ATPs
ATPs Role in Pain Control
- Clinical studies have shown that ATP reduces acute perioperative pain. These patients were given intravenous ATP.
- Injectable adenosine adenosine infusion activates the A1 receptor and initiates a signaling cascade which ultimately aids in pain relief.
- Research has shown that moderate amounts of adenosine compound have a positive effect on hyperalgesia and allodynia.
- Effective pain intervention is possible through A1 adenosine receptor stimulation. This results in a slow onset of pain and a prolonged duration of action that can last for several weeks in some cases.
Anesthesia
- Positive outcomes were achieved during anesthesia with ATP supplementation
- Evidence suggests that low doses adenosine can reduce neuropathic and ischemic pains, as well as hyperalgesia, to levels comparable to morphine.
- Postoperative opioid use was reduced by Adenosine, which suggests a long-lasting activation of the A1 adenosine receptor.
Cardiology and Surgery
- ATP has been shown to be a safe and effective pulmonary vasodilator for patients with pulmonary hypertension.
- Inducing hypotension in patients can also be done during surgery using adenosine or ATP.
Concentration of ATP
Normal cell ATP concentrations are maintained between 1 and 10 mmol/L with an average ratio of ATP/ADP around 1000.
- Adults have approximately 0.10 mol/L of ATP.
- Each day, approximately 100 to 150 mol/L ATP is required. This means that each ATP molecule must be recycled between 1000 and 1500 times per day.
- The human body basically changes its weight in ATP every day.
References
- Dunn J, Grider MH. Physiology, Adenosine Triphosphate. [Updated 2022 Feb 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553175/
- https://www.britannica.com/science/adenosine-triphosphate
- https://www.news-medical.net/life-sciences/Adenosine-Triphosphate-(ATP)-Function-in-Cells.aspx
- https://www.physio-pedia.com/Adenosine_triphosphate_(ATP)
- https://www.acs.org/content/acs/en/molecule-of-the-week/archive/a/adenosine-triphosphate.html
- https://en.wikipedia.org/wiki/Adenosine_triphosphate
- https://www.slideshare.net/namarta28/atp-the-universal-energy-currency-of-cell