What is Action Potential?
- Action potential is a fundamental physiological process in neurons that facilitates the transmission of electrical signals. This phenomenon represents a rapid and transient alteration in the electrical membrane potential, enabling communication across considerable distances within the nervous system.
- The process begins at the resting potential, typically around -65 mV. During this state, the neuron maintains a stable, negative charge relative to the external environment. However, upon receiving an adequate stimulus, the membrane potential undergoes depolarization. If the stimulus is sufficient to reach a critical threshold, voltage-gated sodium channels open, permitting sodium ions (Na⁺) to influx into the cell. This influx results in a further decrease in the negativity of the membrane potential.
- As depolarization progresses, the membrane potential ascends and ultimately peaks during the overshoot phase, reaching approximately +40 mV. At this point, the interior of the neuron becomes positively charged compared to the exterior. Following this peak, the neuron transitions into repolarization. The sodium channels close, and voltage-gated potassium channels open, allowing potassium ions (K⁺) to exit the cell. This efflux of potassium ions contributes to the return of the membrane potential toward its resting state.
- The final phase, known as undershoot, occurs when the membrane potential dips below the resting potential before gradually stabilizing back to -65 mV. This sequence of events encapsulates the action potential’s characteristic “all-or-none” response; once the threshold is surpassed, the action potential occurs fully, maintaining a consistent magnitude regardless of the stimulus strength.
- The action potential serves as a crucial mechanism for neuronal communication. It enables the rapid relay of information across neurons, ensuring effective signaling throughout the nervous system. Understanding this process is essential for comprehending how neurons interact and coordinate responses, highlighting the intricate nature of neural activity.
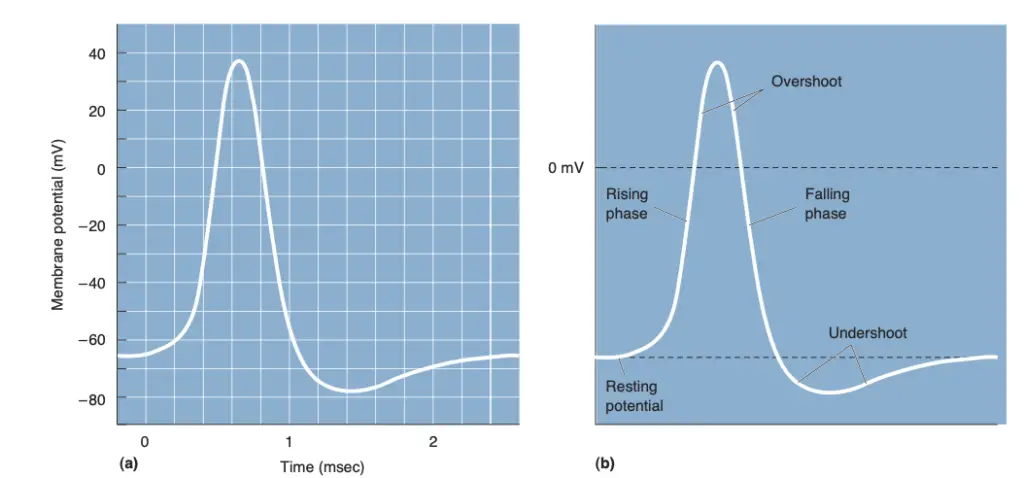
Definition of action potential
An action potential is defined as a rapid, transient change in the electrical membrane potential of a neuron, characterized by a brief depolarization followed by repolarization. This process allows neurons to transmit signals over long distances. It occurs when the membrane potential reaches a critical threshold, leading to the opening of voltage-gated sodium channels, resulting in an influx of sodium ions, followed by the efflux of potassium ions to restore the resting potential. Action potentials are essential for neural communication and are often referred to as nerve impulses or spikes.
Properties of Action Potentials
Action potentials possess several critical properties that underpin their essential role in neural communication. Understanding these properties helps elucidate how neurons transmit signals effectively over distances. The following points outline the key characteristics of action potentials:
- All-or-None Principle: Action potentials manifest in a binary manner; they either occur in full or not at all.
- The generation of an action potential requires the membrane potential to surpass a specific threshold.
- Once this threshold is achieved, the action potential’s amplitude remains constant, irrespective of the stimulus strength.
- Rapid Depolarization: The onset of an action potential is marked by a swift rise in membrane potential.
- This surge is primarily due to the influx of sodium ions (Na⁺) through voltage-gated sodium channels.
- The rapid depolarization is critical for initiating the signal transmission process.
- Repolarization: Following the peak of depolarization, the neuron undergoes repolarization.
- During this phase, voltage-gated potassium channels open, allowing potassium ions (K⁺) to exit the neuron.
- This efflux of potassium contributes to restoring the membrane potential toward its resting state.
- Overshoot: The action potential reaches a maximum positive charge during this phase.
- The inside of the neuron can attain a potential of approximately +40 mV.
- This overshoot is significant for ensuring effective signal transmission along the neuron.
- Undershoot (After-Hyperpolarization): After repolarization, the membrane potential may dip below the resting level.
- This temporary hyperpolarization enhances the refractory period.
- Eventually, the membrane potential stabilizes back to its resting state.
- Threshold: A specific level of depolarization, known as the threshold, is essential for triggering an action potential.
- This threshold is crucial for the reliable activation of action potentials.
- Failure to reach this level results in no action potential being generated.
- Refractory Periods: Following an action potential, the neuron enters a refractory phase.
- The absolute refractory period is a time when no new action potential can occur, regardless of the stimulus intensity.
- The relative refractory period allows for a new action potential to occur but only in response to a stronger stimulus.
- Propagation: Action potentials propagate along the axon without diminishing in size.
- This property enables long-distance signal transmission, critical for rapid communication in the nervous system.
- The maintenance of amplitude ensures that signals can travel from the neuron’s cell body to its terminals effectively.
- Frequency Coding: The information conveyed by action potentials is encoded in their frequency and pattern.
- A higher frequency of action potentials often indicates a stronger stimulus.
- This mechanism allows for a nuanced representation of sensory input and neural responses.
Mechanisms of Action Potential Generation
The generation of an action potential is a fundamental process in neuronal signaling, driven by the dynamic movement of ions across the neuronal membrane. This process can be broken down into several distinct phases, each characterized by specific ionic events and membrane changes. The following outlines the key mechanisms involved in action potential generation:
- Resting Membrane Potential: Neurons maintain a resting membrane potential of approximately -65 mV. This negative charge results from the unequal distribution of ions, predominantly sodium (Na⁺) and potassium (K⁺). The sodium-potassium pump plays a crucial role by actively transporting Na⁺ out of the cell while bringing K⁺ into the cell, thus establishing the necessary ionic gradients.
- Threshold Potential: A stimulus, such as neurotransmitter binding or mechanical pressure, can cause depolarization of the membrane. When the membrane potential reaches a critical threshold, typically around -55 mV, voltage-gated sodium channels begin to open, allowing Na⁺ to flow into the neuron.
- Depolarization Phase: Following the threshold, a rapid influx of Na⁺ occurs, leading to further depolarization. This phase is characterized by a dramatic increase in membrane potential, which can exceed 0 mV, driven by the high permeability of the membrane to Na⁺.
- Overshoot: The membrane potential continues to rise, reaching a peak of approximately +40 mV. This overshoot results from the substantial concentration of Na⁺ within the neuron and the transient permeability increase of the membrane to sodium ions.
- Repolarization Phase: Shortly after reaching its peak, the voltage-gated sodium channels inactivate, halting the influx of Na⁺. Concurrently, voltage-gated potassium channels open, permitting K⁺ to exit the cell. This efflux of K⁺ leads to a decrease in membrane potential, moving it back toward the resting state.
- Undershoot (After-Hyperpolarization): The outflow of K⁺ may cause the membrane potential to dip below the resting level, a phenomenon known as hyperpolarization. This undershoot occurs before the potassium channels close, resulting in a brief period of increased negativity in the membrane potential.
- Return to Resting Potential: Eventually, the membrane potential stabilizes as the voltage-gated potassium channels close and the sodium-potassium pump resumes its activity, restoring the ion gradients that maintain the resting state.
- Refractory Periods: Following an action potential, neurons enter a refractory period. During the absolute refractory period, inactivated sodium channels prevent the initiation of another action potential, ensuring unidirectional signal propagation. The relative refractory period follows, where a stronger-than-normal stimulus is required to generate another action potential, primarily due to lingering hyperpolarization.
The Action Potential Process
This process consists of several well-defined phases, each marked by specific ionic movements and electrical changes within the neuron. The following points outline the action potential process in detail:
- Resting State:
- The neuron maintains a resting membrane potential, typically around -65 mV.
- The membrane exhibits high permeability to potassium ions (K⁺) due to open potassium channels, while sodium channels (Na⁺) remain closed.
- The sodium-potassium pump plays a critical role in maintaining ion gradients, with higher concentrations of Na⁺ outside the cell and K⁺ inside.
- Depolarization:
- A stimulus triggers depolarization, causing the membrane potential to approach the threshold, approximately -55 mV.
- Upon reaching this threshold, voltage-gated sodium channels open, permitting Na⁺ to influx rapidly into the cell.
- This influx generates a more positive membrane potential, leading to further depolarization.
- Rising Phase:
- The continued influx of Na⁺ results in a sharp rise in membrane potential, often peaking at around +40 mV.
- This phase is characterized by the inside of the neuron becoming positively charged relative to the outside, known as the overshoot.
- Repolarization:
- After achieving the peak membrane potential, voltage-gated sodium channels inactivate, halting the influx of Na⁺.
- Concurrently, voltage-gated potassium channels open, allowing K⁺ to exit the cell.
- The efflux of K⁺ contributes to a decrease in membrane potential, moving it back toward the resting level.
- Falling Phase:
- As K⁺ continues to exit, the membrane potential drops further, leading to repolarization.
- The membrane potential may temporarily dip below the resting level, a phase referred to as undershoot or after-hyperpolarization.
- Return to Resting Potential:
- Eventually, voltage-gated potassium channels close, allowing the membrane potential to stabilize back at approximately -65 mV.
- The sodium-potassium pump remains active, restoring the original ion concentrations within the neuron.
- Refractory Periods:
- Absolute Refractory Period: During this phase, no new action potential can be initiated because sodium channels are inactivated.
- Relative Refractory Period: Following the absolute period, a stronger-than-normal stimulus is necessary to generate another action potential, owing to lingering hyperpolarization.
- Propagation:
- The action potential propagates along the axon in a wave-like manner. The depolarization of one segment of the membrane prompts the depolarization of adjacent segments.
- This mechanism is essential for efficient signal transmission over long distances within the nervous system, enabling rapid communication between neurons.
Conduction of Action Potentials
The conduction of action potentials is a critical process that enables neurons to transmit signals over significant distances within the nervous system. This intricate mechanism ensures efficient communication between neurons and plays a fundamental role in various physiological functions. The following points elucidate the process of action potential conduction:
- Initiation of Action Potential:
- The action potential begins at the axon hillock, where the membrane potential reaches the threshold level, typically due to sufficient depolarization from synaptic inputs.
- Voltage-gated sodium channels open, resulting in a rapid influx of sodium ions (Na⁺) and a marked increase in membrane potential.
- Local Current Flow:
- The depolarization at the site of initiation generates a local current that spreads to adjacent segments of the axon.
- This local current further depolarizes neighboring membrane areas, reaching threshold and activating additional voltage-gated sodium channels.
- Propagation of Action Potential:
- As each segment of the axon undergoes depolarization and generates an action potential, the process repeats in subsequent segments, facilitating the forward movement of the action potential.
- This propagation occurs in a wave-like manner, moving away from the axon hillock towards the axon terminals.
- Refractory Periods:
- The absolute refractory period ensures unidirectional propagation of the action potential. During this time, previously activated sodium channels remain inactivated, preventing them from reopening.
- Following this, the relative refractory period emerges, requiring a stronger stimulus to elicit another action potential due to residual hyperpolarization.
- Myelination and Saltatory Conduction:
- In myelinated axons, the conduction of action potentials is accelerated through a process known as saltatory conduction.
- Myelin sheaths, produced by glial cells, insulate sections of the axon and inhibit ion leakage, allowing the action potential to jump from one node of Ranvier (gaps in the myelin) to the next.
- This mechanism significantly enhances conduction velocity compared to unmyelinated axons.
- Factors Influencing Conduction Velocity:
- Conduction velocity in neurons is a critical aspect of neurophysiology, influenced by various structural and functional factors of axons. Understanding these influences provides insight into how signals are transmitted in the nervous system.
- Axonal Diameter
- The diameter of an axon significantly impacts conduction velocity.
- Larger diameters reduce internal resistance, allowing for faster propagation of action potentials.
- In contrast, smaller diameters increase resistance, slowing the conduction speed.
- This principle is illustrated by the giant axon of the squid, which is remarkably large (up to 1 mm), facilitating rapid escape reflexes.
- Membrane Permeability
- The number and type of ion channels in the axonal membrane also play a crucial role.
- A higher density of voltage-gated sodium channels enhances excitability, permitting quicker depolarization.
- Conversely, axons with fewer channels require more significant depolarization to reach the action potential threshold, affecting overall conduction speed.
- This is especially pertinent for smaller axons, which are more sensitive to depolarization levels.
- The number and type of ion channels in the axonal membrane also play a crucial role.
- Myelination
- The presence of myelin sheaths greatly influences conduction velocity.
- Myelinated axons exhibit saltatory conduction, where action potentials jump between nodes of Ranvier, significantly increasing speed.
- Unmyelinated axons, lacking this adaptation, conduct signals more slowly as they rely on continuous propagation.
- Myelination not only enhances speed but also conserves energy, as fewer ions need to be exchanged across the membrane.
- The presence of myelin sheaths greatly influences conduction velocity.
- Temperature
- Temperature affects ion channel kinetics, which in turn influences conduction velocity.
- Higher temperatures generally increase the speed of conduction by enhancing the activity of voltage-gated channels.
- Conversely, lower temperatures can slow down conduction, potentially leading to dysfunction in neural pathways.
- This is relevant in various physiological and pathological states, such as during hypothermia or fever.
- Temperature affects ion channel kinetics, which in turn influences conduction velocity.
- Electrical Properties of the Axon
- The intrinsic electrical properties of the axonal membrane, including capacitance and resistance, determine how charge spreads.
- Lower membrane capacitance facilitates faster changes in voltage across the membrane, aiding rapid signal transmission.
- Higher membrane resistance supports the flow of current along the axon rather than through the membrane, which is essential for efficient conduction.
- The intrinsic electrical properties of the axonal membrane, including capacitance and resistance, determine how charge spreads.
- Role of Ion Channels:
- Voltage-gated sodium and potassium channels are essential for action potential conduction.
- The precise opening and closing of these channels during the action potential phases enable the rapid alterations in membrane potential necessary for effective signal propagation.
Synaptic Transmission
Synaptic transmission is the process by which neurons communicate with each other at synapses, the junctions where one neuron connects to another. This process is crucial for the functioning of the nervous system, allowing for the transfer of information and the integration of signals. Here’s an overview of the key steps involved in synaptic transmission:
- Action Potential Arrival:
- The process begins when an action potential travels down the axon of the presynaptic neuron and reaches the axon terminal (synaptic terminal).
- Calcium Influx:
- The arrival of the action potential causes voltage-gated calcium (Ca²⁺) channels in the presynaptic membrane to open.
- Calcium ions flow into the neuron due to the concentration gradient, leading to an increase in intracellular calcium levels.
- Neurotransmitter Release:
- The influx of calcium triggers synaptic vesicles, which contain neurotransmitters, to move toward and fuse with the presynaptic membrane.
- This fusion results in the exocytosis of neurotransmitters into the synaptic cleft, the small gap between the presynaptic and postsynaptic neurons.
- Binding to Receptors:
- The released neurotransmitters diffuse across the synaptic cleft and bind to specific receptors on the postsynaptic membrane.
- This binding can lead to various effects depending on the type of neurotransmitter and receptor involved.
- Postsynaptic Response:
- The binding of neurotransmitters to receptors can cause ion channels in the postsynaptic membrane to open or close, leading to changes in the postsynaptic membrane potential.
- If the postsynaptic potential is depolarizing and reaches the threshold, it can generate an action potential in the postsynaptic neuron.
- Termination of Signal:
- The action of neurotransmitters is terminated through several mechanisms:
- Reuptake: Neurotransmitters are taken back into the presynaptic neuron through transporter proteins.
- Enzymatic Degradation: Enzymes in the synaptic cleft break down neurotransmitters (e.g., acetylcholinesterase breaks down acetylcholine).
- Diffusion: Some neurotransmitters may simply diffuse away from the synaptic cleft.
- The action of neurotransmitters is terminated through several mechanisms:
- Types of Synapses:
- Synaptic transmission can occur at different types of synapses:
- Chemical Synapses: Involve the release of neurotransmitters and are the most common type of synapse.
- Electrical Synapses: Involve direct electrical connections between neurons through gap junctions, allowing for faster transmission.
- Synaptic transmission can occur at different types of synapses:
- Modulation:
- Synaptic transmission can be modulated by various factors, including the presence of neuromodulators, which can enhance or inhibit the effects of neurotransmitters.
The action potential, in reality
The action potential is a fundamental electrical phenomenon in neurons that allows for rapid signal transmission. This process involves precise changes in ion conductance, primarily through voltage-gated sodium and potassium channels. Understanding the intricacies of the action potential can enhance comprehension of neuronal function and communication.
- Initiation of Action Potential
- The action potential begins when the neuronal membrane is depolarized to a threshold level.
- At this threshold, there is a transient increase in sodium conductance (gNa), allowing Na⁺ ions to flood into the neuron.
- This influx rapidly depolarizes the membrane potential towards the equilibrium potential for sodium (E_Na).
- A brief increase in potassium conductance (gK) assists in restoring the membrane potential during repolarization.
- The action potential begins when the neuronal membrane is depolarized to a threshold level.
- Role of Voltage-Gated Sodium Channels
- The voltage-gated sodium channel is crucial for the action potential’s rising phase.
- This channel consists of a single polypeptide with four domains, each contributing to a pore that selectively allows Na⁺ ions to pass.
- Upon depolarization, specific segments of the channel (notably S4) sense the voltage change and trigger conformational shifts that open the channel.
- The channels open rapidly but remain open for only about 1 millisecond before inactivating, preventing further depolarization until the membrane potential returns to a negative value.
- The voltage-gated sodium channel is crucial for the action potential’s rising phase.
- Voltage-Gated Potassium Channels
- These channels open with a delay compared to sodium channels, contributing to the falling phase of the action potential.
- Upon depolarization, potassium channels gradually open, allowing K⁺ ions to exit the neuron.
- This efflux is essential for repolarizing the membrane and often causes a brief hyperpolarization, or undershoot, beyond the resting membrane potential.
- These channels open with a delay compared to sodium channels, contributing to the falling phase of the action potential.
- Key Phases of the Action Potential
- Threshold: The membrane potential at which sufficient sodium channels open, leading to a predominance of sodium permeability.
- Rising Phase: Rapid influx of Na⁺ ions causes swift depolarization of the membrane.
- Overshoot: Membrane potential briefly exceeds 0 mV due to high sodium permeability.
- Falling Phase: Inactivation of sodium channels combined with the opening of potassium channels causes rapid repolarization.
- Undershoot: Membrane potential dips below resting levels due to prolonged potassium conductance.
- Refractory Periods: The absolute refractory period occurs as sodium channels remain inactivated, preventing another action potential. The relative refractory period follows, requiring a stronger stimulus to generate a new action potential.
- Influence of Toxins
- Various toxins can selectively disrupt sodium channel function, providing insights into action potential dynamics.
- Tetrodotoxin (TTX): Blocks sodium channels, halting action potentials and leading to potential lethality if ingested.
- Batrachotoxin: Causes channels to open inappropriately, leading to prolonged depolarization and disrupted signaling.
- Understanding these toxins aids in elucidating the structural and functional characteristics of sodium channels.
- Various toxins can selectively disrupt sodium channel function, providing insights into action potential dynamics.
- Research Contributions
- The foundational work of Hodgkin and Huxley in the mid-20th century, utilizing techniques like voltage clamping and patch clamping, allowed for direct measurement of ionic conductance during action potentials.
- Their hypothesis regarding sodium and potassium gates paved the way for later discoveries about voltage-gated ion channels.
- The foundational work of Hodgkin and Huxley in the mid-20th century, utilizing techniques like voltage clamping and patch clamping, allowed for direct measurement of ionic conductance during action potentials.
Influence of External Factors
The influence of external factors on synaptic transmission and action potentials can significantly affect neuronal communication and overall nervous system function. Here are some key external factors that can impact these processes:
- Neurotoxins:
- Certain substances, such as tetrodotoxin (TTX) and saxitoxin, can block voltage-gated sodium channels, preventing the generation of action potentials. This leads to paralysis and can be fatal.
- Other neurotoxins, like botulinum toxin, inhibit neurotransmitter release at the presynaptic terminal, disrupting synaptic transmission.
- Drugs and Medications:
- Many drugs can modulate synaptic transmission. For example, antidepressants like selective serotonin reuptake inhibitors (SSRIs) increase the availability of serotonin in the synaptic cleft by inhibiting its reuptake.
- Anesthetics can block sodium channels, preventing action potentials and leading to loss of sensation.
- Ionic Concentrations:
- Changes in the concentrations of ions (e.g., sodium, potassium, calcium) in the extracellular environment can affect the resting membrane potential and the generation of action potentials.
- For instance, hyperkalemia (high potassium levels) can lead to depolarization of the membrane, making it easier to reach the threshold for action potentials.
- Temperature:
- Temperature can influence the kinetics of ion channels and the overall metabolic activity of neurons. Higher temperatures generally increase the rate of action potentials, while lower temperatures can slow down neuronal activity.
- pH Levels:
- Alterations in pH can affect the function of ion channels and neurotransmitter receptors. For example, acidosis (low pH) can impair synaptic transmission and neuronal excitability.
- Hormones:
- Hormones such as adrenaline (epinephrine) and cortisol can modulate synaptic transmission by influencing neurotransmitter release and receptor sensitivity, thereby affecting mood, stress responses, and overall brain function.
- Environmental Stressors:
- Stressful experiences can lead to changes in neurotransmitter systems, particularly those involving dopamine and serotonin, which can affect mood and behavior.
- Diet and Nutrition:
- Nutritional factors, such as the availability of essential fatty acids and amino acids, can influence neurotransmitter synthesis and function. For example, omega-3 fatty acids are important for maintaining neuronal membrane integrity and function.
- Electromagnetic Fields:
- Some studies suggest that exposure to electromagnetic fields (e.g., from mobile phones) may influence neuronal activity and synaptic transmission, although the evidence is still inconclusive.
- Age and Development:
- The influence of external factors can vary with age. For instance, the developing brain is particularly sensitive to environmental toxins, which can disrupt synaptic development and function.
- https://nba.uth.tmc.edu/neuroscience/m/s1/chapter01.html
- https://ksumsc.com/download_center/Archive/1st/436/2-%20Musculoskeletal%20block/Team%20Work/Physiology/2%263-%20Action%20Potential%20and%20Resting%20Membrane.pdf
- https://www.bristol.ac.uk/phys-pharm-neuro/media/teaching/actionpotential_vetsci2010.pdf
- https://ksumsc.com/download_center/Archive/1st/439/2.%20Muscloskeletal%20Block/Team%20Work/Physiology/2-Action%20potential%20.pdf
- https://home.csulb.edu/~cwallis/382/readings/482/Larkmanaction.pot.07.pdf
- https://www.kenhub.com/en/library/physiology/action-potential
- https://healthacademy-web.radboudumc.nl/coo/ipweb7/misc/assignmentfiles/nervous/Action_Potential.pdf
- https://www.eolss.net/sample-chapters/c03/E6-54-09-04.pdf
- https://teachmephysiology.com/nervous-system/synapses/action-potential/
- https://people.musc.edu/~woodward/Action%20Potential%20Lecture.pdf
- https://www.cuyamaca.edu/student-support/tutoring-center/files/student-resources/action-potential-physiology-worksheet.pdf
- https://www.moleculardevices.com/applications/patch-clamp-electrophysiology/what-action-potential
- https://www.ncbi.nlm.nih.gov/books/NBK538143/